Abstract
The monitoring of human exposures to diesel exhaust continues to be a vexing problem for specialists seeking information on the potential health effects of this ubiquitous combustion product. Exposure biomarkers have yielded a potential solution to this problem by providing a direct measure of an individual's contact with key components in the exhaust stream. Spurred by the advent of new, highly sensitive, analytical methods capable of detecting substances at very low levels, there have been numerous attempts at identifying a stable and specific biomarker. Despite these new techniques, there is currently no foolproof method for unambiguously separating diesel exhaust exposures from those arising from other combustion sources.
Diesel exhaust is a highly complex mixture of solid, liquid, and gaseous components whose exact composition can be affected by many variables, including engine technology, fuel composition, operating conditions, and photochemical aging. These factors together with those related to exposure methodology, epidemiological necessity, and regulatory reform can have a decided impact on the success or failure of future research aimed at identifying a suitable biomarker of exposure. The objective of this review is to examine existing information on exposure biomarkers for diesel exhaust and to identify those factors and trends that have had an impact on the successful identification of metrics for both occupational and community settings. The information will provide interested parties with a template for more thoroughly understanding those factors affecting diesel exhaust emissions and for identifying those substances and research approaches holding the greatest promise for future success.
I. BACKGROUND
Interest in human biomonitoring has undergone a renaissance in recent years with the application of new innovative analytical techniques for measuring small incremental differences in body burden or receptor binding. This has led to an evolution in the methodological, procedural, and ethical guidelines used to guarantee the relevance, reliability, and confidentiality of any new initiative (Boogaard, Citation2007). The advantages of using an internal measure of absorbed dose rather than an external exposure concentration are widely acknowledged and well accepted. These include the ability of internal dosimeters to factor all routes of exposure into consideration and to simultaneously account for individual differences in the uptake, distribution, and elimination of a substance. Perhaps the greatest utility of biomonitoring is, however, its ability to account for intraindividual changes in pharmacokinetics as a function of a person's age, lifestyle, diet, and activity level. Although methods currently exist for the ambient monitoring of diesel exhaust exposure, particularly in occupational environments, there is no uniform agreement on the best approach, and this has led to a confusing array of methods and models that are not intercomparable. This has resulted in the widespread use of exposure reconstruction techniques and surrogate exposure models in the dosimetry studies that accompany retrospective examinations of estimated health effects in exposed populations. These methods, however, are often highly suspect and may yield a distorted picture of past exhaust exposures. The logical way out of this conundrum is the identification of a robust and sensitive biomarker of diesel exhaust exposure.
Human biomonitoring has become an essential tool for answering many questions regarding exposure and effect (Budnik and Baur, Citation2009). A successfully developed biomonitoring program using established and corroborated biomarkers can help (1) establish exposure trends, (2) identify highly exposed subgroups, (3) determine reference ranges for acceptable exposures, and (4) verify human health risk. For diesel exhaust, the first step, however, is the identification a reliable and specific biomarker that can be utilized in either an occupational or a community setting. The ideal solution, therefore, is the identification of a biomarker that can be linked forward to potential effects and backward to known sources of exposure (Needham et al., Citation2007). In this context, it is essential that any biomarker of diesel exhaust exposure possesses the following characteristics (Gil and Pla, Citation2001):
A consistent quantitative association with external exposure at all relevant air concentrations.
A robust relationship to external exposure despite the presence of confounding factors or background exposures.
High sensitivity and specificity at low levels of exposure.
The availability of a highly accurate analytical method with low inter and intra laboratory variability.
In the case of diesel exhaust, these are particularly critical considerations, given its compositional complexity and the existence of many potentially confounding substances from other combustion sources. As will be shown in the following sections, the issue of specificity has been the single greatest impediment to the identification of a distinctive biomarker for diesel exhaust and the primary reason why most attempts have only met with moderate success.
As depicted in , a biomarker can focus on any or all of the stages in an exposure-response continuum. Although three types of biomarkers (exposure, effect, and susceptibility) are generally recognized, traditional large-scale biomonitoring programs have often focused on the use of a single unambiguous biomarker for assessing either exposure or effect in a particular setting. These values are often evaluated relative to a reference concentration applicable to an occupational or community environment (Boogaard, Citation2009). In the absence of such a reference concentration, a biomarker cannot be used to assess potential health risk. This limitation does not, however, vitiate its value in a biomonitoring program. In the case of diesel exhaust, where health-based reference concentrations do not exist outside of the workplace, an exposure biomarker would be a valuable tool for the following:
Establishing baseline levels of exposures in the general public;
Comparing exposures among different populations;
Assessing the effectiveness of regulatory and environmental risk management actions intended to reduce exposure; and
Supporting epidemiological research on potential links between exposure and specific health effects.
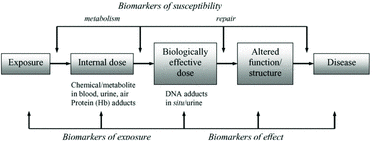
These benefits, however, cannot be fully realized until the biomarker has been validated in a laboratory that has an established quality control program that includes participation in available proficiency evaluations (ECETOC, Citation2005).
II. FACTORS AFFECTING DIESEL BIOMARKER IDENTIFICATION
A. Exposure Considerations
Diesel exhaust exposures can occur in any developed society where diesel engines are used in the power, transportation, or manufacturing sectors. Although diesel exhaust is commonplace in both rural and urban settings, it is often very difficult to parcel out from the myriad of other fuel combustion exposures that coexist in ambient air. There are several situations, however, where diesel exhaust exposures predominate and provide a semi-controlled environment for evaluating exposure magnitude and the probability of harm. Foremost among these are the mining, trucking, and transportation industries where diesel exhaust is an ever present concern for health professionals looking to assess and control emissions relative to published exposure standards. Occupational monitoring in these environments has provided the best opportunity for developing the sampling and analytical methods needed to assess personal exposure minus the interferences from secondary combustion products. Even here, however, cross-contamination from other nondiesel exhaust sources is a potential concern that needs to be evaluated. Although numerous exposure surrogates have been identified for use in these environments, the results are not intercomparable due to differing analytical procedures, emission characteristics, or operational definitions.
The most common approach for measuring diesel exhaust exposure has been the determination of elemental carbon (EC) or total carbon (TC) in the particulate fraction. Use of these surrogate exposure metrics is driven largely by the fact that most occupational exposure limits have been expressed in terms of the carbon content in diesel particulates. The American Conference of Governmental Industrial Hygienists (ACGIH) first added diesel exhaust to its list of intended changes in 1995 and recommended an 8-hour threshold limit value (TLV) of 0.15 mg/m3 as total diesel particulate matter (DPM). The ACGIH then lowered the value to 0.05 mg/m3 in 2001. This value was replaced with an equivalent TLV of 0.02 mg/m3 for EC by assuming an EC/TC ratio of 40% (Ono-Ogasawara and Smith, Citation2004). The ACGIH value was ultimately withdrawn in 2004 after the United States Environmental Protection Agency (USEPA) issued a health assessment report, which contained information that conflicted with the ACGIH standard (ACGIH, Citation2005). The US Mine Safety and Health Administration (MSHA) issued an occupational exposure standard for DPM in 2001 that has also undergone considerable revision since its initial promulgation (MSHA, Citation2001). Initially, a value of 0.4 mg/m3 was proposed for TC, which was then modified to 0.308 using a TC to EC ratio of 1.3 (EC/TC = 0.77) as a conversion factor (MSHA, Citation2005). As of 2008, the MHSA standard stands at 0.16 mg/m3 TC, which will be adjusted to an EC-based limit in an upcoming regulation (Belle, Citation2010). The primary impediment for implementing the change has been the magnitude of the carbon conversion factor (i.e., the EC/TC ratio), which can be affected by many factors including fuel type and engine load (Noll et al., Citation2005). In contrast to particulate-based surrogates, the Occupational Safety and Health Administration (OSHA) has, since the mid-1980s, promoted the monitoring of hydrocarbon vapors or inorganic gases such as carbon monoxide as the best surrogates for diesel exhaust exposure (OSHA, Citation2005).
There are few explicit exposure limits for the diesel exhaust in ambient air. The USEPA established a DPM reference concentration of 5 μg/m3 that was predicted to be protective of non-cancer health effects (Ris, Citation2007). This limit is backed up by a PM2.5 National Ambient Air Quality Standard of 35 μg/m3 (24-hr) and 15 μg/m3 (annual) that will likely be the subject of proposed reduction within the next several years given recent International Agency for Research on Cancer (IARC) findings that re-labeled diesel exhaust as a group I carcinogen for humans (Simcox, Citation2009; Scheepers and Vermeulen, Citation2012). The European Union has no exposure guideline for ambient diesel exhaust or DPM, but a general PM2.5 limit value of 25 μg/m3 will take effect in 2015, and a more stringent indicative limit value of 20 μg/m3 will need to be met by 2020.
Use of particle-based surrogates has hindered the identification of a suitable exposure biomarker for diesel exhaust, because particulates above the nano range are not absorbed and systemically circulated following inhalation. Consequently, it is not possible to measure a dosimetrically equivalent internal and external exposure concentration. There are, however, alternatives to the carbon measurements, but they are not routinely employed, and their relationship to EC and TC levels is, for most part, unknown. Any de novo identification and use of an exposure biomarker for diesel exhaust will ultimately require a thorough examination of its relation to progenitor concentrations in ambient air to ensure that the biomarker is sensitive to changes in diesel exhaust levels. These concentrations can then, with appropriate knowledge, be related back to the EC and TC concentrations that have historically been used to track exposures. This assumes, of course, that changes in the composition of diesel exhaust will not disproportionately affect the relative amount of each metric, which may be true over a short time frame but highly unlikely for periods extending beyond a few years. Perhaps, the best surrogates for diesel exhaust exposure from a biomarker perspective are the volatile and semivolatile organics that exist in either the vapor phase or adsorbed onto respirable particulates. These substances may be absorbed following inhalation then sequestered or excreted in a form that can be measured analytically with a high degree of accuracy. Although these volatile and semivolatile chemicals include hundreds of possible candidates, they have been largely ignored as a basis for setting exposure limits since their role in the exposure-response continuum is more uncertain than the overall particulate burden to the lung. This is partly due to the ubiquitous nature of these substances and their release from a variety sources that are often difficult to identify or associate with a particular emission source.
The preferred method for measuring DPM concentration in both an occupational and community environment uses the EC fraction as a selective surrogate for the total particulate mass, which is composed of approximately 80% carbon. EC comprises roughly 40% of the TC content with the remainder consisting of the organic carbon (OC) from adsorbed volatile and semivolatile organic compounds. EC can be measured by any of several different standardized methods that employ size selective sampling along with thermal optical detection. In an occupational environment, NIOSH method 5040 and the European standard EN 14530 methods are the most commonly employed techniques (Birch, Citation2002). Both methods measure EC and OC independently, and the values are summed to arrive at a TC determination. The analysis has limitations, however, as these methods take no account of particle source and the positive bias produced by wood and cigarette smoke, if present in appreciable quantities. In addition, if these methods are used to assess the TC concentration as recommended by MSHA, separate steps need to be taken to control for sampling artifacts in the OC fraction, which can be affected by both nonspecific particle adsorption and the elution of volatile substances from the surface of the filter as sampling progresses (Cheung et al., Citation2009).
Diesel exhaust concentrations in ambient air can be monitored using either a batch sampling technique or a semicontinuous approach. Unlike the goals for occupational monitoring however, ambient air assessments have different constraints and somewhat different objectives. Not only are the levels of diesel exhaust in ambient air far lower than those observed in the workplace, interferences from other anthropogenic and biogenic combustion sources are almost always present. Consequently, the sampling and analysis methodology needs to be tailored to the specific needs of the program and rigorously controlled to guarantee reliable results (Sarigiannis and Saisana, Citation2008). Two basic analytical methods have been developed for the semicontinuous analysis of the EC, OC, and TC content of ambient air samples. These are the thermal-optical transmittance (TOT) method, standardized by NIOSH for use in underground mines, and the thermal-optical reflectance (TOR) method developed by the USEPA for use in ambient air monitoring programs (Bae et al., Citation2009). Although both methods are capable of accurately measuring TC content, differences in the thermal profile and optical measurements result in different operational definitions for the EC that is measured. Consequently, EC levels determined by the TOT method can be less than half of those observed using the TOR method (Chow et al., Citation2001).
This potpourri of sampling and analytical measurements for diesel exhaust demonstrates the problems currently faced by regulators seeking to ensure compliance with recommended exposure standards. Likewise, health specialists wishing to investigate the nature and degree of exposure in different emission environments have to contend with a confusing array of surrogate measurements. This patchwork of methods underscores the need for a more unified approach for documenting exposures in a variety of diverse environments. One solution to this complex problem hinges on the development of an agreed upon approach for assessing the actual body burden of a key ingredient in diesel exhaust. Whereas, most attention has been on the EC content of the particulate fraction, relatively little consideration has been given to the semivolatile substances that constitute the OC fraction. Although some progress has been made on identifying a unique OC marker of diesel exhaust exposure, much more effort is needed before a suitable method can be identified for routine use.
Any biomarker for diesel exhaust ultimately needs to be associated either directly or indirectly with a potential health effect to be of any value in a risk evaluation. This requirement is particularly problematic for complex mixtures such as diesel exhaust since the particular agent(s) responsible for any decrements in morbidity or mortality remain largely unknown. Still, this linkage should not be viewed as an insurmountable impediment since multiple mechanisms and modes of action may exist for the range volatile and nonvolatile substances in diesel exhaust. Another more basic requirement is the identification of a reliable and well-validated analytical method for measuring internal and external exposure concentrations. The specificity, sensitivity, and reproducibility of the method will need to be fully examined and well documented. Similarly, a sampling method will be needed to collect the analyte of interest with high efficiency and recovery.
B. Epidemiological Considerations
Few of the epidemiology studies conducted outside an occupational setting have adequately documented the magnitude of current or past exposures to diesel exhaust (Gamble, Citation2010). Some have relied on ambient air monitoring of total aerosol mass or, alternatively, the particulate levels within a specific size fraction. Others have used surrogates such as EC, carbon monoxide, nitrogen dioxide, or benzene (Janssen et al., Citation2001; McCreanor et al., Citation2007). Oftentimes, the use of these chemical measures has resulted in considerable measurement error, particularly when the values are based on area monitoring versus personal exposure assessments (Van Roosbroeck et al., Citation2008). Typically, the measurements taken at local area-monitoring stations are applied to receptor populations residing within an area of interest. This area-monitoring approach to exposure assessment does not, however, adequately represent the multitude of microenvironments that an individual can occupy in the course of a day. The measurements also suffer from a lack of temporal resolution for humans whose activities change constantly throughout the day (Adgate et al., Citation2002).
Since particulate matter (PM) is composed of many source fractions each capable of eliciting some of the same health effects as diesel exhaust, there is a need to separate the influence of individual particulate types to assess their contribution to the overall health risk. However, few epidemiological studies have been able to adequately sort out the contribution of various PM source types to overall incidence rates, regardless of the techniques employed (Lewtas, Citation2007). Some investigators have attempted to use physical or statistical models to apportion the total PM exposure to individual sources, but these techniques often lead to large uncertainties in the actual magnitude of the exposure. Another approach, gaining greater acceptance, relies on measures of traffic density and vehicle type as indirect indicators of exposure intensity for residents living in close proximity to a road or highway. These so-called land use regression models have been used to estimate diesel exhaust exposures as a function of wind direction, elevation, and distance from a roadway, truck density, and other geographic- or traffic-related variables (Ryan et al., Citation2008). As before, however, exposure misclassification can be particularly problematic, leading to either an underestimate or overestimate of the actual risk.
Other investigators have, with varying degrees of success, developed alternative spatial and temporal air pollution models to characterize traffic-related exposures. The most commonly employed approaches have been categorized as either proximity models, dispersion models, interpolation models, integrated meteorological-emission models, or hybrid models (Jerrett et al., Citation2005). Of these, proximity models are generally considered to be least effective since they are based on the overarching assumption that exposures closer to a traffic source are automatically greater than those further from the source (Zou et al., Citation2009). Alternatively, hybrid models, which are an amalgamation of modeling approaches systematically applied in some logical fashion, hold greater promise for estimating individual exposure-based personal or area monitoring data. Whereas other modeling techniques exist, such as inhalation models that take into consideration human activity patterns, they have not been utilized to any great degree because of the intense data needs. Perhaps the single greatest issue with many of these modeling techniques has been the absence of any independent validation to reduce the possibility of exposure misclassification (Baxter et al., Citation2010). The underlying assumptions associated with many of these methods have also constrained their utility and limited the possibilities for data intercomparison. In fact, differences in exposure measurement accuracy have been cited as the single most important cause of conflicting results in epidemiology studies focusing on vehicular exhaust (Grahame, Citation2009). These issues have led to a strong and pressing demand for a metric that could help segregate the true health risks of diesel exhaust exposure from those associated with other influential background contaminants such as cigarette smoke, wood smoke, and gasoline exhaust (Mauderly and Chow, Citation2008).
The need has only intensified with the creation of biomonitoring programs on a regional and national level to assess population-related exposures in a more unambiguous fashion (Schulz et al., Citation2007). Identification of a viable exposure biomarker has the potential to overcome many of the inaccuracies associated with the use of exposure surrogates and spatial/temporal models (Scheepers, Citation2008). Identifying a sensitive and specific biomarker for diesel exhaust is not an easy matter, however, since any candidate needs to be associated with a well-vetted and suitably validated analytical method that is free of the inaccuracies caused by matrix effects, analyte instability, or chemical interference (Calafat and Needham, Citation2008). Once developed and suitably evaluated, an exposure biomarker would also provide an avenue for assessing aggregate exposures to diesel exhaust from a variety of nonvehicle-related sources. Although issues of cost, reliability, and specificity may exist, exposure biomarkers have clearly become a highly desirable method for reducing the uncertainties and exposure misclassifications associated with more conventional approaches to diesel exposure assessment (Briggs et al., Citation2009).
C. Aging Considerations
Nucleation, condensation, and adsorption are all important processes that take place as freshly emitted diesel exhaust cools and ages. The exhaust aging process results in the formation of new liquid aerosols and the deposition of volatile and semivolatile organics onto particle surfaces. The first step in the aging process, nucleation, occurs when inorganic substances are oxidized and transformed to very small microparticulates that serve as condensation sites for further particle growth (Vaaraslahti et al., Citation2004). The condensation nuclei found in DPM are composed of sulfate, nitrate, and ammonium particles that include both low-volatility organics and semivolatile organics that are deposited as the temperature declines. Precursors to nuclei formation include gases such as sulfur dioxide, nitric oxide, and nitrogen oxides; however sulfuric acid is postulated to be the primary source (Ning and Sioutas, Citation2010). The degree of nucleation is dependent on the engine load and the temperature of the exhaust gas at the tailpipe (Giechaskiel et al., Citation2005).
Shortly after their formation in the combustion cylinder, semivolatiles partition onto the surfaces of both the solid soot particles and the liquid aerosols created through condensation. In addition, agglomeration can occur as the carbon spherules clump together to form larger particles of soot. So as cooling proceeds, the mass concentration of PM increases as does the organic fraction deposited on the soot (Ning et al., Citation2004). This is accompanied by a decrease in the concentration of volatiles in the vapor phase as well as the total number of particulates. Once emitted, the organic constituents of diesel exhaust can also undergo a wide range of photooxidation reactions that will result in the formation of secondary products. In some cases, these products will be identical to those already present in the exhaust, and in other cases new and novel substances will be created. Since a variety of factors can affect the rate of these oxidation reactions, including temperature, humidity, oxidant radical concentration, and sunlight intensity, it is not surprising that atmospheric photooxidation reactions show wide spatial and temporal variability. Likewise, the lifetimes of the various reaction products in the atmosphere may range from minutes to months (Zielinska, Citation2005). Three oxidants, in particular, control the transformations of diesel organics; these are hydroxyl radicals, nitrate radicals, and ozone. Hydroxyl radicals and ozone control the daytime photooxidation reactions of diesel organics, whereas nitrate radicals and ozone affect the nighttime reactions. In addition to these chemically induced oxidation reactions, sunlight can photolyze some diesel-related organics such as carbonyls that contain photo-labile functional groups.
Numerous photochemical reactions were observed to occur in smog chamber tests with diesel exhaust samples aged in the dark with nitrate radicals or in the daylight with hydroxyl radicals (Zielinska et al., Citation2010). Chief among these were the oxidation of C14–C40 normal and branched alkanes and the formation of nitro-naphthalenes from polycyclic aromatic hydrocarbon (PAH) precursors. There was a notable increase in alkanedioic acids and aromatic diacids associated with the hydroxyl radical-initiated reactions. Substituted ethyl- and dimethyl-naphthalenes, in particular, have been shown to rapidly react with nitrate and hydroxyl radicals to yield the corresponding nitro- and hydroxy-naphthalenes (Wang et al., Citation2010). These atmospheric nitration reactions have also been shown to occur with several monocyclic and bicyclic gas phase hydrocarbons such as toluene and biphenyl (Nishino et al., Citation2008). The hopanes and steranes in the gas phase of diesel exhaust were also shown to be oxidized to more polar compounds at rates that produced atmospheric lifetimes of a day or less (Lambe et al., Citation2009).
Photooxidation reactions in the atmosphere can not only alter the chemical composition of diesel exhaust, but also change its physical form as well. This is because the reaction products are often less volatile than the precursors, leading to condensation and the formation of secondary organic aerosols (SOA). Experimentally, the photooxidation of diesel exhaust led to rapid formation of SOA, which contributed 20–60% of the overall aerosol mass after only 3 hr (Miracolo et al., Citation2010). The source of the SOA could not be solely attributed to the photooxidation of light aromatics and was speculated to arise from the oxidation of low volatility polycyclics as well.
Other studies have shown that PAHs are major contributors to the SOA that appears following the emission of diesel exhaust. Aerosol yields ranging from 2–22% were observed when naphthalene, 1-methylnaphthalene, 2-methylnaphthalene, acenaphthylene, and acenaphthene were reacted with a hydroxyl radical source in a smog chamber (Shakya and Griffin, Citation2010). These results have been independently confirmed and extended to show that PAHs can potentially account for up to 54% of the aerosols from diesel exhaust emissions (Chan et al., Citation2009). When exposed to sunlight, soot-absorbed PAHs with two or three rings were shown to disappear rapidly at a bi-exponential rate (Kim et al., Citation2009). The first phase of this loss process typically had a half-life of 5 hr or less, whereas the half-life of second phase was generally 14–50 hrs. The degradation of PAHs with 4–6 rings was mono-exponential and occurred at a far slower rate.
These studies suggest that many of the hydrocarbons found in freshly formed diesel exhaust may be subsequently photooxidized in air to more polar substances. The measurement of these oxidized and nitrated substances in diesel exhaust complicates their use as potential biomarkers, since their levels will vary as a function of the oxidizing capacity of the ambient air as well as their residence time in the atmosphere. Structure activity models are available, however, that allow the photooxidation rate and atmospheric lifetime of a chemical to be predicted with a reasonable degree of certainty. Effective use of these models allows a potential biomarker to be screened on the basis of their susceptibility to atmospheric oxidation.
Another important factor to be considered is the inability of chassis dynamometer testing to realistically cool, dilute, and photooxidize diesel exhaust prior to emission factor determination. Since most evaluations of diesel exhaust composition are performed with chassis dynamometers, their results may be biased depending on the design and construction of the dilution tunnel, which cools the exhaust from a high of about 300°C to a low of 15°C. Since dilution tunnels can only achieve a fraction of the sample dilution that is observed in the ambient environment, reliance on these data as a basis for identifying potential biomarkers needs to be tempered by the fact that diesel exhaust undergoes two separate dilution stages under real-world conditions. The (1) tailpipe to road and (2) road to ambient air dilution stages results in an overall dilution of 1:10,000 within a 10 min timeframe (Zhang and Wexler, Citation2004). In contrast, the dilution tunnels used with chassis dynamometers only achieve an average dilution of 1:20–1:200. This testing artifact has been shown to affect the particle partitioning of semivolatile organics and artificially increase the amount of PM2.5 and OC that is collected, but the extent depends to a large degree on the engine load and the amount of organics present in the exhaust (Lipsky and Robinson, Citation2006). Unlike the partitioning processes in a dilution tunnel, the soot-absorbed semivolatile organics in ambient air will repartition into the gas phase as the exhaust ages. This has broad implications for the identification of biomarker candidates, since the compositional information from dynamometer testing may not be as reliable as the data obtained from on-road or roadway tunnel studies.
D. Regulatory Considerations
Any discussion of diesel exhaust biomarkers and composition needs to be prefaced with a few facts regarding the impact of clean air regulations on the future of diesel exhaust emissions. In both the US and Europe, a plethora of new diesel engine emission control standards has led to an everchanging description of DPM and its attendant gases and vapors (Johnson, Citation2006). Since 1992, there has been a staged introduction of new regulations affecting the emission of nitrogen oxides, hydrocarbons, and PM in on-road diesel-powered vehicles. The values presented in show that the allowable emission of DPM has declined steadily in both passenger cars and commercial vehicles. The emission of hydrocarbons and nitrogen dioxide in diesel exhaust from passenger and commercial vehicles was also projected to decline by more than 5-fold for the 12 year period beginning in 1992. The USEPA has developed a similar set of emission limits that have been promulgated in two separate phases lasting 5 years each. A third tier of regulations was initiated in 2010 and is scheduled to end in 2016. The promulgation of these increasingly stringent regulations has required engine manufacturers to make dramatic changes in the combustion characteristics of new engines and introduce new pollution control measures, such as fuel reformulation, diesel particulate filters (DPFs), and oxidation catalysts (Tschoeke et al., Citation2010). Since these technologies will continue to be modified and improved to meet future restrictions on DPM and hydrocarbon emissions, the diesel emissions of today will undergo further change with the types and quantities of each pollutant shifting with time. Despite these improvements, the dramatic rise in diesel engine use, especially in developing countries may offset the gains realized by the use of more advanced pollution control devices. This sweeping statement, however, contains many assumptions regarding the pace and extent of change. Air quality improvements in developing countries will be greatly influenced by the rate of turnover in the vehicle fleet and by the degree of retro-fitting with improved abatement technologies. Likewise, the passage and implementation of local emission standards and the mandated use of newer fuel types may also have a decided impact on future air quality changes arising from the increased use of diesel-powered vehicles.
TABLE 1 European emission standards for nitrogen oxides, hydrocarbons, and PM in diesel exhaust
Perhaps the greatest innovation in diesel emission control has been the creation of DPFs. DPFs came into prominence with the institution of the 2009 EURO V regulations and the Tier 2 USEPA regulations phased in from 2004 to 2009. Their popularity stems in part from their ability to be used as retrofit devices on existing vehicles (Burgard and Provinsal, Citation2009). The filters are typically employed as part of a two-stage control process that also involves the use of a diesel oxidation catalyst (DOC) that reduces hydrocarbon emissions and promotes the formation of nitrogen dioxide. In the first stage, NO is catalytically oxidized to NO2, which is needed to lower the oxidation temperature of the particulates trapped in the second stage. This lower temperature helps to reduce filter maintenance by promoting self-cleaning of the DPM. Continuously regenerating DPFs have been shown to reduce the emissions of CO, hydrocarbons, and DPM in transit buses by over 90% (Lanni et al., Citation2001). Likewise, DPFs also resulted in carbonyl reductions greater than 99%, PAH reductions up to 80%, and nitrated-PAH reductions of 94%. In some cases, a 3-fold reduction in DPM has been noted in heavy-duty transit vehicles equipped with DPF (Tzamkiozis et al., Citation2010). Under more controlled conditions, dynamometer testing of a heavy-duty diesel vehicle equipped with a DOC and DPF showed an average reduction of 96% in the emission of eight PAHs (Shibata et al., Citation2010). Under some operating conditions, use of DPFs can result in the emission of large numbers of nanoparticulates in the nuclei mode of 30 nm or less; but this finding is the subject of considerable debate, with numerous studies showing a decline in nanoparticulate formation (Kittelson et al., Citation2006; Hesterberg et al., Citation2011).
DOCs often utilize a platinum catalyst to promote the removal of CO and hydrocarbons. The DPM removal efficiency with this catalyst generally ranges between 30% and 50%, but some catalysts such as zeolite and vanadium can achieve greater reductions. The amount of catalyst in a DOC helps determine its overall oxidation potential for hydrocarbons and DPM (Herner et al., Citation2009). Ultimately, however, it is the balance between exhaust temperature and catalyst loading that determines the reduction efficiencies for regulated pollutants. Studies also suggest that a DOC can be used in conjunction with a reformulated low-sulfur fuel to augment the reduction in particle numbers over what is observed with just a single control technique (Corro, Citation2002). The use of low-sulfur or no-sulfur fuel along with DPF has not, however, provided an appreciable improvement in DPM emissions due to the large gains that can be attained with particulate filters alone. The use of low sulfur fuel along with a DPF has been shown to reduce the toxicity associated with New Technology Diesel Exhaust (CONCAWE, Citation2005; McClellan et al., Citation2012).
A wide range of new pollution control devices are under development to help meet current and anticipated new pollution control regulations (Bauner et al., Citation2009). Technologies such as selective catalytic reduction, nonthermal plasma after-treatment, and nitrogen oxide storage catalysis will result in further qualitative and quantitative changes in the composition of diesel exhaust, but their exact impact cannot be predicted with any degree of accuracy. It is imperative, however, that any methodical search for potential diesel biomarkers evaluates the likely impact of pollution control on the emission rates under real-world conditions.
E. Fuel Considerations
Fuel changes alone can dramatically affect the composition of diesel exhaust. The introduction of low-sulfur/low-aromatic fuels has been shown to decrease the OC/EC ratio by affecting the percentage of PAHs in the OC fraction (Alander et al., Citation2004). Dynamometer testing with a range of light- to heavy-duty diesel vehicles showed that fuels with low sulfur and aromatic content did not affect the emissions of BTEX (benzene, toluene, ethylbenzene, and xylene) chemicals or simple aldehydes, but did reduce the emission of larger PAHs (Nelson et al., Citation2008). A similar examination of a bus fleet equipped with heavy-duty diesel engines revealed that the use of low-sulfur diesel fuel (50 ppm sulfur) could result in the reduction of PAH emissions by more than 90% as compared to low-sulfur fuel (500 ppm sulfur) (Lim et al., Citation2005). In other studies, reducing the fuel sulfur content from 1473 ppm to 47 ppm was shown to result in an appreciable reduction of total hydrocarbon emissions from a diesel-powered vehicle tested under a full range of load conditions (Tan et al., Citation2009). Although not used commercially to a large degree, there has been considerable interest in the emissions profile of alcohol-containing diesel fuel blends. Methanol-supplemented diesel fuels did not, however, provide the same benefits as low-sulfur fuels and caused an increase rather than a decrease in hydrocarbon emissions (Zhang et al., Citation2010). The same is true for ethanol-supplemented fuels, with the emission of benzene, toluene, and xylene from a light-duty diesel engine increasing at blending concentrations greater than 18% (Di et al., Citation2009). These results, however, were highly dependent on the engine load conditions, with decreases rather than increases observed at higher loads. Synthetic gas-to-liquid diesel fuels have recently been introduced in some areas of the world, and their emissions profile compare favorably with more traditional diesel fuels with lower aldehyde and aromatic levels in the exhaust (Bermudez et al., Citation2011).
Use of biofuels has attracted considerable attention by many since they hold promise as both a renewable energy resource and an alternative approach to emissions control. Although modern diesel engines are capable of using hydrotreated vegetable oils as a fuel, the most commonly encountered biodiesels are produced by the transesterification of fatty acids derived from any of several animal or vegetable oil sources such as tallow, sunflower seeds, or rapeseeds (Murugesan et al., Citation2009). The resulting biodiesel fuel contains fatty acid methyl esters (FAME) that can vary in chain length from C1 to C3. Biodiesel fuels are named according to the percentage of biodiesel added to conventional diesel fuel, with B20 (20% biodiesel) being one of the most common. As the biodiesel content of a fuel mixture increases, there is a disproportionate decrease in DPM emissions, with reductions in excess of 90% observed in some cases with B100. There has, however, been some disagreement about the degree of DPM reductions that can be achieved with biodiesel, with some researchers suggesting that that there are no benefits whatsoever (Turrio-Baldassarri et al., Citation2004; Durbin et al., Citation2007). To the contrary, most researchers find that reductions in the order of 20–40% can be reliably achieved.
Higher relative reductions of PM are generally obtained with a B20 blend than with blends containing a higher proportion of biodiesel (Haas et al., Citation2001). Quantitatively greater reductions are also generally observed at higher engine load conditions (Leung et al., Citation2006). An examination of the testing results from 49 studies with a B20 blend revealed DPM reductions in the order of 10–20% (Yanowitz and McCormick, Citation2009). Occupational exposures to PM2.5, formaldehyde, and acetaldehyde were shown to be reduced up to 5-fold when heavy equipment operators at a waste recovery facility switched from using diesel fuel to a soy-based B20 blend (Traviss et al., Citation2010). Although there are particulate mass reductions with biodiesel, there can be, under some conditions, an increase in the number of ultrafine particulates less than 40 nm in size (Krahl et al., Citation2003; Lapuerta et al., Citation2008). The results, however, are inconsistent and seem to be related to the sulfur content of the biodiesel or the degree of exhaust gas dilution that occurs. Whereas the use of biodiesel blends was shown to cause a reduction in PM mass emissions in the fine and ultrafine range on the order of 41–45%, there was a shift in the size distribution with greater formation of nanoparticulates as the blending percentage increased (Chien et al., Citation2009). The overall air pollution benefits of biodiesel have been attributed to the high oxygen content of these fuels, which allows more complete combustion of the soot particles (Rakopoulos et al., Citation2008).
Biodiesel use has also been associated with a decline in PAH and nitrated-PAH emissions, but an increase in the emissions of several aromatic compounds including benzene and toluene. The magnitude of these emission changes was found to be highly dependent on the size of the engine, the compression and injection timing, and the engine load (Raheman and Ghadge, Citation2008). In some cases, a decrease of up to 20% was noted for some BTEX chemicals (Ferreira et al., Citation2008); but these declines were not consistently observed in all circumstances. When engines were operated using an urban driving cycle that maximized the percentage of hydrocarbons emitted, the concentration of semivolatile aromatics, esters, paraffins, oxygenated aromatics, naphthalenes, and acids declined as the biodiesel ratio was increased (Ballesteros et al., Citation2008). The only hydrocarbon category that increased with biodiesel use was the fraction that included aromatic aldehydes and ketones. Most studies have also shown an increase in the emission of carbonyls such as formaldehyde and acrolein with the use of biodiesel blends, especially at low engine loads (He et al., Citation2009; Shah et al., Citation2009). The relative emission of aliphatic carbonyls can vary according to the biodiesel source with the emission factors from animal and vegetable sources showing no particular relationship (Graboski et al., Citation2003). The increased emission of aldehydes has been associated with the short chain esters in biodiesel, which can lead to formation of low molecular weight aldehydes during the combustion process (Guarieiro et al., Citation2009). Compared to pure diesel fuel, a B20 or B50 biodiesel blend from the transesterification of used frying oil has also been shown to produce a substantial increase in the release of C2, C4, and C8 alkanes (Payri et al., Citation2009).
Most studies have shown that the emission of aromatic and polyaromatic hydrocarbons decreases with the use of biodiesel, but the degree of change is highly dependent on the engine operating characteristics. Reductions of individual PAH and nitrated-PAHs as high as 80–90% can be observed under some circumstances, but more modest reductions of 30–50% are more common. Bench testing of a light-duty diesel engine with a B10 blend made from soybean oil revealed a dramatic reduction in benzene and toluene emissions at all load conditions, but a far more modest effect on ethylbenzene and the three isomers of xylene (Ferreira et al., Citation2008). When a B20 blend from transesterified castor oil was examined in a heavy-duty engine, the average reduction in 8 monocyclic and 10 polycyclic aromatics was 21.1% and 17.2%, respectively (Correa and Arbilla, Citation2006). Several aromatics, however, such as phenanthrene, ethyl benzene, and the trimethyl-benzenes, showed an emission increase with use of the B20 blend.
In separate experiments, dynamometer testing of seven different biodiesel blends in a diesel passenger car equipped with a DPF showed a variable effect on the PAH emissions (Karavalakis et al., Citation2010). The PAH emissions from biodiesel blends of B5–B20 prepared from transesterified rapeseed oil, palm oil, and coconut oil were tested in light-duty diesel passenger cars using the New European Driving Cycle (NEDC) (Karavalakis et al., Citation2009). Emission reductions for the 15 PAHs ranged from near zero to 90% for the B10 blends. A comparison of the reductions for the B10 blend prepared from palm/coconut oil failed to show a consistent advantage over rapeseed oil for all of the PAHs examined. In contrast, blends using frying oil methyl esters showed an increase in the emission of PAHs relative to pure diesel, whereas the emissions decreased with soy-based methyl esters. A similar result was seen for 1-nitropyrene with the frying oil-based biodiesel producing the greatest emissions. The emission of four oxygenated-PAHs all decreased with the use biodiesel-based fuels. Testing with a B20 biodiesel from transesterified waste cooking oil in a heavy-duty diesel engine resulted in a 2- to 9-fold reduction in the emission factors of PAHs with 4–7 rings (Yang et al., Citation2007). Likewise, PAH emission factors relative to diesel fuel decreased by an average of 13.1% for a B20 blend and 19.4% for a B100 biodiesel fuel prepared from soybean oil (He et al., Citation2010). The PAHs adsorbed to PM were affected to a much larger degree than those in the gas phase.
The preceding results suggest that there is a decided advantage to using biodiesel fuels to reduce the emissions of volatile and semivolatile organics, but the changes are quite variable and difficult to predict simply on the basis of the type of biodiesel used. Oftentimes, the engine operating conditions are the deciding factor dictating the nature and magnitude of the reductions. The dramatic increase in the emission of some aldehydes such as acrolein is noteworthy, however, since the results suggest that some biodiesel-specific aldehydes may yet be identified. To date, there has not been any concerted effort to compile a biofuels emissions database, despite the large degree of testing that has been performed in recent years. Given the growth in biodiesel use, construction of an unregulated emissions database would be a very useful exercise that could aid in biomarker identification.
The upswing in biodiesel fuel use poses yet another challenge to biomarker development that needs careful consideration. Although many of the changes resulting from biodiesel combustion appear to be quantitative in nature there may be key qualitative changes in the emission profile that could be used as a fingerprint to help normalize measurements and account for the percentage of diesel exhaust emissions from a biodiesel source.
F. Biochemical Considerations
One of the more important considerations in biomarker development is the rate of clearance from the body. Understanding the metabolic and pharmacokinetic properties of an exposure biomarker is critical to interpreting the results from field measurements. The rate of elimination of a biomarker can, under some nonsteady state conditions, decidedly influence how exposure fluctuations affect biomarker concentrations. Key to this consideration is the relationship between exposure frequency and the biological half-life for a putative biomarker, since these two processes will affect the magnitude of any fluctuations in biomarker concentration (Hays et al., Citation2007). If exposures are relatively infrequent and short in duration, then the oscillations in biomarker concentration will be large for those substances with a relatively short elimination half-life. These large fluctuations in body burden will lead to a greater degree of interindividual variance in biomarker concentration since the postexposure sample collection time cannot be tightly controlled for all individuals. The selection of biomarkers with a long biological half-life will help dampen the oscillations in whole body concentration by retarding the clearance and minimizing the impact of changes in exposure frequency (Watson and Mutti, Citation2004). As a result, biomarkers with a long biological half-life are generally preferred over those where the clearance rate is relatively rapid. This is less of an issue in an occupational setting, however, where specimen collection can immediately follow exposure termination.
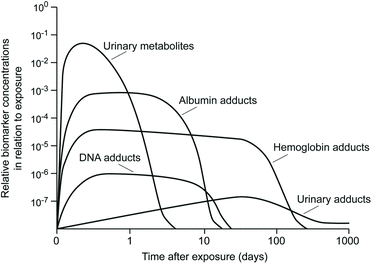
For diesel exhaust, this means that an exposure biomarker with a clearance half-life of 10–12 hrs may provide perfectly acceptable results in an occupational setting, but fail in a community environment where the time interval between exposure termination and sample collection can be quite variable, especially when the study population involves subjects who move between numerous microenvironments. When longer postexposure time intervals are anticipated, it is important to use a biomarker with a long biological half-life that can be retained in the body for days rather than hours. Where long time intervals may exist before sample collection, it is essential that the biomarker possesses a biological half-life that is as long as possible so that the temporal impact is minimized and interindividual variance kept to a minimum. The relative half-life for different types of biomarkers is depicted in , which shows that urinary metabolites possess far shorter residence times within the body than albumin or hemoglobin adducts (Henderson et al., Citation1989). Although the lack of persistence for rapidly eliminated substances is partially compensated for by the sensitivity gains that come with higher concentrations in the body, this advantage can only be capitalized on if the sample is collected shortly after exposure termination. The ideal biomarker for diesel exhaust in a population study would, therefore, focus on a macromolecular adduct rather than a parent chemical or metabolite.
III. RESEARCH TRENDS
A. Past Efforts
There has been no shortage of attempts at developing a reliable and effective biomarker for diesel exhaust exposure. Because diesel exhaust is a complex mixture of substances containing a myriad of widely found substances, the identification of a biomarker with adequate specificity has been difficult to achieve thus far. Although generally well validated, currently available biomarkers of exposure for diesel exhaust often show high interindividual variability due to the confounding effects of secondary source exposures. These encumbrances have limited the usefulness of most methods and prevented their widespread use in population-based biomonitoring programs. Despite these complications, researchers have endeavored to use some diesel biomarkers in specific occupational settings where the uncertainties were far less than those associated with the general population.
Three chemical categories have attracted the most attention as exposure biomarkers for diesel exhaust. These are the PAHs, oxygenated-PAHs, and nitrated-PAHs. The vast majority of research on these chemicals has focused on the development of a biomarker suitable for use in an occupational setting. Because occupational settings provide a more controlled and uniform environment for examining whether a biomarker can consistently and reliably be related to an airborne exposure measurement, this has been the starting point for most field trials. Once vetted and fully examined in an occupational setting, a biomarker can be examined for broader use at the population level.
1. Polycyclic Aromatic Hydrocarbons
Although hundreds of PAHs can be identified in diesel exhaust, only a few have been targeted as potential biomarkers. Once absorbed in the body, most PAHs are extensively oxidized and excreted in the urine as their respective hydroxylated metabolite; the concentration of the unmetabolized parent in the urine is, therefore, relatively low. Despite these drawbacks, there are some advantages in using the unmetabolized parent compound since the levels are less affected by interindividual differences in oxidative metabolism by cytochrome P450. PAH exposures in the workplace can vary greatly depending on the occupation involved and the specific PAH being monitored. The level of individual PAHs rarely exceeds 100 ng/m3, except in high-hazard occupations such as carbon black manufacturing (Choosong et al., Citation2010). Workplace concentrations in underground mines are more typically in the range of 0.2–5.4 ng/m3 for the individual PAHs commonly found in diesel exhaust (Sauvain et al., Citation2003).
Although a limited number of studies are available, the measurement of PAHs in urine has recently been evaluated as a biomarker to track exposures in occupations with low airborne concentrations of diesel exhaust. Naphthalene, phenanthrene, and pyrene have attracted the most attention in this regard. Interest was piqued by a preliminary report from the USEPA on chamber measurements performed in conjunction with controlled exposures of human volunteers to 100 μg/m3 of a diluted diesel exhaust (Sobus et al., Citation2008). The measurements revealed that the chamber air contained appreciable quantities of eight separate 2-ring and 4-ring PAHs in both the gas and particulate phases. Two PAHs, in particular, naphthalene and phenanthrene, were found in the diluted exhaust at respective average concentrations of 2600 ng/m3 and 765 ng/m3, which prompted the authors to speculate whether these compounds would be useful as biomarkers. Although biological specimens were collected as part of this investigation, the authors, as yet, have not published the results from the urinalysis.
In a subsequent study, however, the same group of authors evaluated employees working in occupations with high (coke-oven workers), medium (asphalt workers), and low (diesel-exposed loading dock workers) exposures to airborne PAHs (Sobus et al., Citation2009). Whereas many of the volunteers in the medium and high PAH-exposed groups could be segregated based on the preshift/postshift ratio of unmetabolized naphthalene and phenanthrene in their urine, the loading dock workers in the low “diesel exposure” group displayed a ratio that was slightly less than one (0.96–0.99) for naphthalene and phenanthrene, indicating that the inhalation of diesel exhaust had no impact on their excretion. Although the authors of this study assumed that the primary source of PAHs in the medium exposure group was related to the use of hot asphalt, studies indicate that these workers may also be exposed to sizable amount of diesel exhaust as well (Elihn et al., Citation2008). The impact of this confounding factor on the results and conclusions from this study requires further investigation. In addition, the authors noted that the ratio of urinary naphthalene to phenanthrene decreased with increasing exposure, which suggested that phenanthrene levels were a more robust measure, perhaps because of a higher excretion rate as a function of exposure concentration.
Despite the disappointing results, evidence from other studies involving occupational PAH exposures from other nondiesel sources suggest that the urinary excretion of some nonmetabolized PAHs may have utility in sites such as underground mines where the diesel exhaust concentrations can be relatively high. For example, using a highly sensitive isotope dilution mass spectrometry method, coke-oven workers exposed to a wide range of PAHs were shown to excrete 10 of 13 measurable PAHs in postshift urine specimens (Campo et al., Citation2009; Campo et al., Citation2010). The median levels in the urine specimens were generally highest for naphthalene, phenanthrene, and fluoranthene, whereas those PAHs possessing 5-rings were below the detection limit in most cases. The urinary recovery of these three parent substances was far less than the amounts for corresponding hydroxylated metabolites, which were nearly 20-fold higher.
In a separate study, unmetabolized benzo(a)pyrene was not detected in the urine of coke-oven workers exposed to a variety of PAHs, but the concentrations of naphthalene, phenanthrene, and pyrene were strongly correlated with the exposures expected in three different job categories (Waidyanatha et al., Citation2003). Although these results are intriguing, they are not likely to apply to workers exposed to diesel exhaust since the PAH concentrations are far lower than those observed for coke-oven workers. Likewise, PAHs such as these are commonly detected in a host of emission sources, so their utility as a selective biomarker of diesel exhaust is likely to be limited.
The measurement of DNA bulky adducts is another commonly encountered biomarker of PAH exposure in high-risk populations; however there are surprisingly few examples of its use in groups specifically exposed to diesel exhaust (Scherer, Citation2005). Although the categorization of this measurement has been the subject of some debate, many consider it to be an exposure biomarker since the relationship between adduct formation and the onset of a toxic response can only be surmised (Angerer et al., Citation2007).
The specimen of choice for measuring bulky PAH adducts in workplace studies is often blood lymphocytes or buccal cells collected with mouth swabs (Cavallo et al., Citation2009). The preferred method for quantitating bulky PAH adducts in occupationally exposed individuals has been 32P postlabeling; however, this technique is regarded by many to lack specificity, and has been largely supplanted by more accurate mass spectrometry methods that have the ability to measure the lower levels of binding observed in the general public (Angerer et al., Citation1997a; Himmelstein et al., Citation2009). In a study of adduct levels in workers exposed to high levels of vehicular exhaust, the levels of bulky DNA adducts tended to be higher for those groups with the highest exhaust exposures, but there was no statistically significant difference in the levels observed for the six job categories (bus drivers, taxi drivers, garbage collectors, policemen, street vendors, automotive mechanics/attendant) examined (Palli et al., Citation2001). Lymphocytes from a group of bus garage workers and mechanics employed in a high diesel exhaust environment contained bulky DNA adducts levels that were on average 8-fold higher than the values from a control group (Nielsen et al., Citation1996).
The DNA adduct levels in peripheral blood mononuclear cells from workers employed in two separate coal mines yielded mixed results (Qu et al., Citation1997). In the first mine, where the workmen were divided into low-, medium-, and high-exposure groups, there was no apparent relationship between adduct levels and the estimated exposures to diesel exhaust. There was, however, a 2-fold increase in adduct levels when the below ground exposure groups were compared to a surface group that had no diesel exhaust exposures. Similar results were observed in a second mine where the adduct levels showed no apparent relationship with diesel exposure, smoking status, job category, or length of employment. An increase in bulky adduct levels was observed, however, in those miners who had a particularly intense exposure to diesel exhaust below ground. Considerable caution is necessary when evaluating these findings since there was no attempt to control for the PAH exposures resulting from coal dust, which can under some circumstances be laden with alkylated 2–3 ring polyaromatics (Laumann et al., Citation2011).
Other studies have noted inconsistencies in the use of DNA adducts as a surrogate measure of exposure to the PAHs in diesel exhaust. Very small differences in lymphocyte DNA adduct levels were observed in a study comparing diesel-exposed taxi and rural bus drivers with a group of nonsmoking controls (Hemminki et al., Citation1994). In a particularly well-controlled study that involved actual personal monitoring of PAH concentrations in the gas and particulate phases of diesel exhaust, lymphocyte DNA adduct levels were measured in groups of workers from bus garages, waste collection stations, and garbage truck driving operations (Pohjola et al., Citation2004). A good relationship was observed between adduct levels and total PAH airborne concentrations across the exposure groups, but the correlation was only noted during the wintertime and not the summertime measurement periods.
Considering the contradictions observed in these studies, bulky DNA adducts are concluded to be a poor marker of diesel exhaust exposure. This is due in part to the ubiquitous presence of PAHs from a host of biogenic and anthropogenic sources. Charcoal-broiled food, as well as wood, coal, and cigarette smoke, all contain an abundance of PAHs that can result in high background exposures with an associated elevation in adduct formation from non-diesel-related sources (Schoket, Citation1999). Despite their relatively long half-life in the body of 10–12 weeks, PAH adducts are not a particularly useful metric to measure diesel exhaust exposures in either an occupational or a residential population.
2. Oxygenated Polycyclics
A widely used approach for biomonitoring PAH exposures in the workplace relies on the measurement of hydroxylated metabolites in urine specimens. Whereas several analytical methods have been developed to accurately quantitate these biotransformation products, the most sensitive has a limit of detection in the low pg/L range and involves gas chromatography followed by isotope dilution mass spectrometry (Li et al., Citation2006). The most commonly examined oxygenated polycyclic in this category has been 1-hydroxypyrene, which has often been used as a surrogate for the entire class of oxygenated PAHs. This particular pyrene metabolite possesses some favorable properties that make it a highly attractive exposure biomarker. These include a strong relation between exposure concentration and urinary elimination and a long half-life for urinary excretion on the order of about 29 hrs (Huang et al., Citation2007). Urinary 1-hydroxypyrene can also account for PAH uptake by all three routes of exposure, including dermal, inhalation, and ingestion. A recent review of 1-hydroxypyrene use as a biomarker for occupational PAH exposures documented over 130 different studies involving its use, mostly in metal foundries and petrochemical plants (Hansen et al., Citation2008). Postshift urine concentrations in workers employed in jobs involving high exposure to vehicle exhaust typically ranged from 0.01 to 1.60 μmol/mol of creatinine. Some employees, like toll both attendants and bus drivers, excreted appreciably higher amounts ranging as high as 4.68 μmol/mol of creatinine (Tsai et al., Citation2004). Workplace determinations are typically interpreted through the use of a urinary reference value that is linked to an occupational exposure limit for pyrene (Jongeneelen, Citation2001). Alternatively, the concentration in urine can be related back to the total PAH exposure as a benzo(a)pyrene equivalent concentration using the benzo(a)pyrene to pyrene ratio in the workroom air as an adjustment factor (Bouchard and Viau, Citation1999).
The value of using 1-hydroxypyrene as a biomarker in workplaces where diesel exhaust is the primary source of PAH exposure is, however, not entirely clear. Several studies failed to show a good correlation between exposure concentration and urinary excretion once all confounding influences were factored into the analysis. A comparison of the urinary 1-hydroxypyrene levels in shale-oil miners employed above ground and below ground showed only a small difference, despite known differences in the level of diesel exhaust (Scheepers et al., Citation2004). Cigarette smoking, grilled food consumption, and the use of a wood-burning fire place were found to be important confounding variables. Likewise, there was no correlation between urinary 1-hydroxypyrene levels in garbage collectors and the pyrene levels in diesel exhaust particulates from personal air samples (Hara et al., Citation1997). A large impact was also observed for those who smoked cigarettes, leading the researchers to conclude that 1-hydroxypyrene measurements were not suitable when low-level occupational exposures were associated with high background levels from secondary sources.
In contrast to these studies, some have noted a distinct relationship between exposure and urinary excretion. For instance, the levels of 1-hydroxypyrene in postshift urine specimens from workers in a bus garage where diesel exhaust exposures were commonplace were about 2-fold higher than those from a control group (Karahalil et al., Citation1998; Kuusimaki et al., Citation2004). A 4-fold increase in urinary excretion was also observed in workers from an engine repair shop relative to a matched control group, and the results did not reveal any apparent impact from cigarette smoking (Karahalil et al., Citation1998).
An examination of the results for 2000 subjects participating in the US National Health and Nutrition Examination Survey revealed that 99% of the participants excreted measurable amounts of 1-hydroxypyrene levels in their urine with the average median value equaling 0.04 μmol/mol of creatinine. Cigarette smokers were found to have 3-fold higher levels than nonsmokers (Huang et al., Citation2004). In general, workplace studies have not demonstrated a universal correlation between urinary 1-hydroxypyrene levels and airborne levels of pyrene or total PAHs. For this reason, and because of the influence of age, gender, diet, alcohol, medicine intake, and activity patterns, a standard biological exposure index has not been established for this PAH metabolite (Maina et al., Citation2007).
Advances in the use of urinary 1-hydroxypyrene levels as a biomarker have inspired research on other hydroxylated PAH metabolites showing less background interference and less interindividual variability. Five metabolites, in particular, have been examined for use in an occupational setting: 1-hydroxynaphthalene, 2-hydroxynaphthalene, 3-hydroxyphenanthrene, 4-hydroxyphenanthrene, and 9-hydroxyphenanthrene. Because of its presence in the gas phase of diesel exhaust at high concentrations, naphthalene is an enticing target for investigation. Studies initially revealed that urinary levels of the parent compound correlated well with the total concentration of PAHs in airborne samples from coke production facilities, iron foundries, and aluminum production factories (Rappaport et al., Citation2004). In addition, postshift urinary levels of 1-hydroxynaphthalene and 2-hydroxynaphthalene were found to correlate well with the urinary excretion of 1-hydroxypyrene. Other studies have suggested, however, that cigarette smoking can have a measurable impact on the excretion of these napthols. For instance, the urinary levels of 2-hydroxynaphthalene in workers from aircraft maintenance facilities and shipyards were shown to have mean values ranging from 3.74 to 4.44 μmol/mol of creatinine for smokers and 1.16 to 2.53 μmol/mol of creatinine for nonsmokers (Kim et al., Citation2001; Lee et al., Citation2001). The high naphthalene levels in cigarette smoke highlight potential problems with the use of urinary 2-hydroxynaphthalene as a biomarker under low exposure conditions involving only diesel exhaust. These concerns were borne out in a study of urinary 2-hydroxynaphthalene levels in nonsmoking bus garage workers and garbage truck drivers exposed to diesel exhaust on a daily basis (Kuusimaki et al., Citation2004). The average value for these workers ranged between 3.34 and 4.85 μmol/mol of creatinine, whereas the range for a nonexposed control population was 2.51–2.58 μmol/mol of creatinine. Although some differences were noted for these two groups, the disparity was not sufficient to outweigh the impact of cigarette smoking on the excretion of this metabolite. Some investigators have, in fact, ruled out using 2-hydroxynaphthalene as a biomarker for any type of PAH exposure because of the large contribution from cigarette smoking (Serdar et al., Citation2003).
The hydroxylated metabolites of phenanthrene provide yet another potential exposure metric, but they too suffer from low sensitivity and specificity. Measurement of the hydroxylated phenanthrenes excreted in the urine of workers at a graphite-electrode production plant revealed that the total urinary concentration of the 1, 2 + 9 (co-eluted), 3, and 4 isomers showed some correlation (r = 0.6) with the airborne concentration of phenanthrene. In this instance, the urinary concentration of the 3-hydroxy isomer predominated, accounting for 43% of the total hydroxyphenanthrene concentration (Angerer et al., Citation1997b). A stronger relationship was observed in the excretion of 3-hydroxyphenanthrene and 9-hydroxyphenanthrene in the urine of coke-oven workers (Serdar et al., Citation2003). Whereas the urinary levels from control subjects were below the detection limits, the average urinary concentration of the 3 and 9 isomers in the occupationally exposed group ranged from 1.04 to 1.38 μg/L and 4.55 to 7.35 μg/L, respectively. The differences were not as great for a group of diesel-exposed bus garage mechanics and garbage truck drivers (Kuusimaki et al., Citation2004). In this instance, no correlation was observed between the urinary excretion of 1 + 9 (co-eluted), 2, 3, and 4-hydroxyphenanthrene and the concentration of the parent PAH in workplace air. A small but significant improvement was observed when the total postshift concentration for all hydroxylated phenanthrenes was compared to a group of control subjects. The differences, however, were less than 2-fold and highly variable. Importantly, the preshift and postshift concentrations of the individual isomers were not appreciably different, suggesting a long elimination half-life with a sizable potential for day-to-day accumulation. The urinary elimination of the 2, 3, and 4 isomers of hydroxyphenanthrene was all statistically higher in female cigarette smokers than in nonsmokers, but a smoking-related difference was not detected for the urinary excretion of the 1-hydroxyphenanthrene isomer (Gundel et al., Citation1996).
Despite the sensitivity losses caused by high background exposure from nondiesel sources of PAHs in the environment, research has continued on the excretion of other marker metabolites. Although excreted in far smaller amounts than hydroxy isomers at the C2 and C4 position, 3-hydroxybenzo(a)pyrene has offered an alternative to the more traditional urinary metabolites. Whereas this metabolite has not been the focus of any diesel-related research, the feasibility of using this metric has been demonstrated in other occupational environments. Some studies have shown that that there is a long lag time in the excretion of 3-hydroxybenzo(a)pyrene in the urine of workers at seven different types of factories where PAHs are present (Gendre et al., Citation2004). The excretion of this metabolite took place over a period of days with the highest urinary concentration observed 25 hr after the beginning of a work shift. These findings suggest that the best time for collecting a spot urine specimen was on the day following an exposure. Despite the relatively short half-life of approximately 9 hr, investigators have calculated a reference value equating the urinary concentration of 3-hydroxybenzo(a)pyrene with the occupational exposure limit for benzo(a)pyrene (Lafontaine et al., Citation2004).
A more detailed examination of the relationship between airborne exposures to benzo(a)pyrene and the urinary concentration of 3-hydroxybenzo(a)pyrene showed a poor association between the two exposure metrics (Forster et al., Citation2008). Investigators postulated that the poor correlation with airborne measurements for workers in coke plants and graphite electrode manufacturing facilities was due to dermal absorption. Further, the urinary concentration and total mass of 3-hydroxybenzo(a)pyrene were less than 2-fold higher in smokers than in nonsmokers, and there was no relationship with the number of cigarettes smoked (Lafontaine et al., Citation2006). These data allowed for the calculation of a population reference value of 0.014 nmol/mol of creatinine for nonsmoking and nonoccupationally exposed individuals and 0.03 nmol/mol of creatinine for smoking nonoccupationally exposed individuals.
Although some of the techniques described above may be suitable for routine use in special environments such as underground mines where diesel exhaust is the chief contaminant, the abundance of secondary PAH sources in most occupations and community environments precludes the use of PAH or its primary hydroxylated metabolites as measures of diesel exposure in the vast majority of situations. The high background levels of these substances in the environment would dramatically reduce the sensitivity of any biomarker measurements for diesel exhaust and obscure the association with diesel exhaust exposure.
3. Nitrated Polycyclics
There are a variety of semivolatile nitrated-PAHs adsorbed onto DPM. The relative stability of these substances and their potential contribution to the overall toxicity of diesel exhaust have attracted the attention of researchers looking to establish an exposure biomarker suitable for use in occupational environments. Early research with 1-nitropyrene demonstrated that this compound, when administered orally to rats, could form small amounts of chemically stable hemoglobin adducts that persist up to 24 hr after treatment (van Bekkum et al., Citation1997). Similarly, metabolism studies in rats using C14-labelled 1-nitropyrene showed high levels of plasma protein binding along with the urinary excretion of 2-aminopyrene, which was determined to be the metabolite responsible for the observed protein adduct formation (van Bekkum et al., Citation1999). These latter studies also demonstrated that adduct level in blood and plasma were still elevated when the last sample was collected at 36 hr postexposure. Extending these studies to humans, investigators found that workers in a bus garage with suspected, but not measured, exposures to diesel exhaust did not possess 1-nitropyrene or 2-nitrofluorene hemoglobin adducts levels that were appreciably higher than controls living in either an urban or rural location (Zwirner-Baier and Neumann, Citation1999). Adduct levels for three other nitrated-PAHs, 3-nitrofluoranthrene, 9-nitrophenanthrene, and 6-nitrochrysene were below analytical detection limits for both the exposed and control populations. A difference in hemoglobin adduct levels could be detected between garage workers and controls using the sum total of all five nitrated-PAHs; but the general conclusion was that high background exposures to these substances limited the usefulness of this quantitative approach.
In more recent years, attention has shifted to other potential measures of nitrated PAH exposure. The BIOMODEM (Biomarkers for Occupational Diesel Exhaust Exposure Monitoring) study was established with funding from the European Union to explore the relationship between diesel exhaust exposure and various biomarkers of exposure and effect in underground miners (Scheepers et al., Citation2002). These studies have shown that personal exposures to the 1-nitropyrene found on respirable particulates in a shale-oil mine were 8-fold higher in workers inside the mine than in those above ground. Similarly, the 1-nitropyrene levels in respirable dust fractions for a large group of coal miners were shown to be 2- to 3-fold higher for those employed below ground than for those at the surface (Scheepers et al., Citation2003). Despite these differences in exposure concentration, however, there was no appreciable change in the level of 1-nitropyrene DNA adducts observed between miners underground and those at the surface (Knudsen et al., Citation2005).
Urinary levels of 1-nitropyrene metabolites have also been proposed as potential biomarkers for diesel exhaust exposure. Controlled human exposures to 300 μg/m3 of the PM10 fraction from diesel exhaust for 1 hr resulted in a 6-fold increase in the urinary excretion of 1-aminopyrene relative to control subjects (Laumbach et al., Citation2009). There were, however, large interindividual variations in the 24-hr integrated urinary concentrations from a clean air control group that sometimes exceeded the values observed in the test subjects. The time to maximum urinary excretion also varied for the test subjects with two distinct subgroups identified using pharmacokinetic modeling techniques (Huyck et al., Citation2010). The maximum elimination in the fast excretors (63% of the test subjects) occurred after a median interval of 5.4 hr, whereas the slow excretors (30% of the test subjects) displayed a maximum excretion time of more than 24 hr. The variability was presumed to be associated with either physiological (i.e., ventilation rate) or biochemical (i.e., metabolism rate) differences in the two subpopulations. In a very cursory study involving a small number of underground salt miners exposed to 1-nitropyrene from diesel exhaust at a concentration ranging from 0.5 to 3.0 ng/m3, the urinary elimination of 1-aminopyrene in a 24 hr specimen ranged from 2 to 200 ng (Seidel et al., Citation2002). By comparison, the ambient air concentration of 1-nitropyrene inside school buses burning regular diesel fuel was found to be in the range of 1–2 pg/m3 (Zielinska et al., Citation2008), which calls into question the utility of this method when the exposures approximate those found in the general public.
Using a highly sensitive analytical method that included a sophisticated sample handling technique, four minor metabolites of 1-nitropyrene could be detected in the urine of baseline control subjects who were not intentionally exposed to diesel exhaust (Toriba et al., Citation2007). By this method, 6-hydroxy-N-acetyl-1-aminopyrene, 8-hydroxy-N-acetyl-1-aminopyrene, 6-hydroxy-1-nitropyrene, and 8-hydroxy-1-nitropyrene could all be isolated from a large urine specimen as either their glucuronide or sulfate conjugates. The value of this approach is yet to be demonstrated in a large-scale study using a diesel-exposed population; however, given the sophisticated nature of the analytical method, routine use in population-based biomonitoring programs is highly unlikely. With further analytical refinement, however, these biomarkers could prove to be useful in an occupational setting.
Another potential biomarker generating considerable interest in both an occupational and community setting is 3-nitrobenzanthrone. This potent mutagen has been the subject of numerous studies detailing its toxic potential and mode of action. 3-Nitrobenzanthrone is found in substantial quantities in both DPM and ambient aerosols and is formed during fossil fuel combustion or as a result of the atmospheric nitration of benzanthrone (Enya et al., Citation1997). It has repeatedly been shown to interact with DNA and form stable adducts that are felt to be responsible for its genotoxicity (Arlt et al., Citation2006). Surprisingly, although the macromolecular binding of 3-nitrobenzanthrone has been thoroughly explored in rodents, there is little information on DNA adduct formation in humans (Arlt, Citation2005). Those studies that have been performed are generally restricted to human cell cultures where extensive binding was observed under a variety of treatment conditions (Nagy et al., Citation2007). Likewise, the propensity for 3-nitrobenzanthrone to form hemoglobin adducts following metabolism to the corresponding amine is largely unknown. This is an area of research worth pursuing, however, given the relative ease of collecting hemoglobin via a routine blood specimen.
A different scenario has unfolded for 2-nitrobenzanthrone, which is found in DPM at concentrations that are 70-fold higher than 3-nitrobenxanthrone (Phousongphouang and Arey, Citation2003). The binding and mutagenicity observed with this isomer were far less than 3-nitrobenzanthrone due to its slower rate of reduction and activation to a reactive hydroxylamine metabolite (Arlt et al., Citation2007a, Citation2007b; Stiborova et al., Citation2010). Research on the use of 3-nitobenzanthrone as a biomarker is limited to a single occupational exposure study, where detectable levels of 3-aminobenanthrone were detected in 24-hr urine specimens from a small group of salt miners. Personal exposure measurements to DPM showed small trace airborne quantities of 3-nitrobenzanthrone that did not exceed 0.06 ng/m3 (Seidel et al., Citation2002). Although research on the nitrobenzanthrones is still in its infancy, there is concern that high background concentrations resulting from atmospheric nitration reactions will limit the value of this approach (Inazu et al., Citation2008).
Of the three biomarker categories listed above, the nitrated polycyclics hold the greatest promise for yielding an exposure marker that meets the sensitivity requirements of diesel exhaust. However, because many of these substances can be formed secondarily in the atmosphere, the vast majority are liable to have inadequate specificity in many exposure situations. The exception to this finding is 1-nitropyrene, which is not formed through atmospheric nitration reactions and is reasonably stable to further oxidative change following air dispersion. This substance also possesses other favorable characteristics including its emission following the use of both diesel and biodiesel blends and its relatively good resilience to exhaust stream removal by pollution control devices. Before 1-nitropyrene or its metabolites can be used on a routine basis, however, more research is needed to establish the exact relationship between external and internal exposure under a range of occupational conditions. This would necessitate additional validation studies under both controlled laboratory conditions and actual occupational operations. Although attractive as a biomarker for occupational exposure, 1-nitropyrene is unlikely to be suitable in a community environment because of its short biological half-life. In this setting, a more stable protein or hemoglobin adduct will need to be found that is representative of exposures periods lasting days rather than hours.
B. Current Endeavors
The challenges facing researchers seeking to identify an exposure biomarker for diesel exhaust are daunting. Not only are there numerous sources of carbonaceous soot in the environment that possess physical and chemical characteristics similar to DPM, the compositional characteristics of diesel exhaust are constantly changing due to environmental influences and technological change. Ideally, any biomarker of exposure needs to be consistently present in DPM and absent from other common emission sources such as coal, wood, agricultural crops, trash, tobacco products, and gasoline exhaust. Although inroads have been made on several different fronts, to date there is no universally accepted approach to measuring the internal dose or body burden from diesel exhaust exposures. Research efforts have not abated, however, and there continues to be intense interest in this subject by groups and institutions throughout the world. There is a pervasive sense that continued research will lead to a solution in much the same way that cotinine was identified as a biomarker for cigarette smoke and levoglucosan for wood smoke (Benowitz, Citation1999; Migliaccio et al., Citation2009). The question remains, however: what avenues of research show the greatest likelihood of success given what is known about the nebulous qualities of diesel exhaust in general and its individual constituents in particular? This review was performed, in part, to answer this question and to provide a blueprint for identifying both existing and emerging areas of research that could yield an approach for measuring internal dose in both an occupational and community setting.
1. Nanoparticulates
The mandated switch to more advanced pollution control devices has had a dramatic impact on the emissions DPM. Use of DPFs has been shown to decrease the mass emissions of PM by over 95% in heavy-duty vehicles (Herner et al., Citation2009). Although these devices have not yet come into widespread use within the US, retrofit rules are beginning to take effect in states such as California (Millstein and Harley, Citation2010). In contrast, DPFs are common in Europe where the requirements of EURO IV have mandated large reductions in the emission of PM (Prasad and Bella, Citation2010). One consequence of using these new pollution control devices is the emission of nanoparticulates with aerodynamic diameters of 50 nm or less (Holmen and Ayala, Citation2002). The carbon content of these nanoparticulates is, however, very low, and their composition is dominated by the presence of inorganic sulfates (Hesterberg et al., Citation2011).
In diesel vehicles operating without advanced aftertreatment devices, over 90% of the particle numbers in the exhaust are emitted as carbon-based nanoparticulates (Kittelson, Citation1998). The soot nanoparticulates from diesel engines possess some unique structural characteristics that are unlike those emitted from a standard gasoline engine or a new technology diesel engine (Su et al., Citation2004). Using transmission electron microscopy, these nanoparticulates were observed to have a very rough surface with an onion-like structure that possesses multiple shells or lamella. Imaging techniques allow a direct measurement of the length, spacing, and orientation of these lamellae within the nanoparticle. Using this method, the lamella for nanoparticles from diesel soot were shown to be distinguishable from those generated by burning vegetation, residential and industrial boilers, and jet engines (Wal et al., Citation2010). Furthermore, a comparison of the structural and physical properties of nanoparticulates from engines fueled with conventional diesel did not reveal any substantial difference with those from biodiesel blends (Happonen et al., Citation2010).
Since much of the research on the structural characteristics of diesel nanoparticulates is still in its infancy, there are bound to be many new revelations in the coming years. Meanwhile, research on nanoparticulate exposures has intensified within the European Union, with six different organizations developing work groups to evaluate and control potential occupational health risks (Park et al., Citation2009). Chief among these is the OECD Working Party on Manufactured Nanomaterials (WPMN) that was created in 2006 and has eight steering groups addressing specific issues regarding the safety and use of nanomaterials (Murashov et al., Citation2009). The Exposure Measurement and Exposure Mitigation (EMEM) steering group, charged with identifying and compiling guidance information on manufactured nanomaterials, recently prepared a preliminary analysis that included a list of specifically recommended projects (OECD, Citation2009). Of the six projects outlined, the identification of exposure biomarkers is the most pertinent for diesel exhaust. Unlike the nanoparticle-related research activities of other groups, the work of this committee stands out because exposure, rather than effect, biomarkers are specifically being targeted. Although there are numerous challenges to developing a selective biomarker, not the least of which is first identifying a suitable method for sample collection and exposure monitoring, the organizational structure is now in place to promote the requisite research (Seaton et al., Citation2010).
2. Nitropyrene
As noted previously, interest in the use of nitrated-PAHs as potential exposure biomarkers has been tempered by the fact that many of these substances can be formed in the atmosphere when unsubstituted PAHs react with nitrate radicals. Regardless, several nitrated-PAHs in diesel exhaust still hold considerable promise as exposure biomarkers. This is particularly true for 1-nitropyrene, which is found in diesel and biodiesel exhaust in relatively high amounts with small background contributions from photochemical nitration reactions (Miller-Schulze et al., Citation2007). In addition, the use of DPFs for emission control did not affect 1-nitropyrne emission factors to as great a degree as other PAHs (Ratcliff et al., Citation2010). This has stimulated research into the use of urinary 1-nitropyrene and its metabolites as biomarkers of occupational exposure to diesel exhaust. Although partially validated, the urinary measurement of 1-nitropyrene or its metabolite, 2-aminopyrene, needs to undergo further evaluation before it can be used on a broad scale. Researchers at the University of Washington Simpson Laboratory and the Pharmaceutical Division at Kanazawa University have an established track record of involvement with 1-nitropyrene and have been actively involved in the development of new analytical approaches and monitoring techniques for this substance (Toriba et al., Citation2007). Given their past success, there is a distinct possibility that new findings may be forthcoming from these research teams that will expand and improve on the current state of the science. Likewise, it is equally possible that other researchers have taken notice of the advantages offered by 1-nitropyrene and will refocus their efforts on this promising area of study.
The University of Washington, in particular, has one unique asset that could be of great value in any collaborative effort at biomarker identification. The Department of Environmental and Occupational Health Science has recently completed construction of a diesel exhaust exposure facility suitable for use with human volunteers (Gould et al., Citation2008). This state-of-the-art exposure chamber is capable of providing controlled exposures to freshly generated diesel exhaust from a heavy-duty diesel engine. The exposure facility is capable of delivering exposures under a range of engine operating conditions using advanced dilution control systems to provide a well-mixed exhaust stream. Although the analytical capabilities associated with the routine operation of the chamber appear to be limited to elemental and organic carbon as well as some trace elements, this is an easily resolved problem that would not hinder use of the chamber for validation experiments. The availability of this resource provides an excellent opportunity to link new analytical developments to a controlled laboratory setting where the relationship between internal dose and external exposure can be fully explored.
3. Protein/Hemoglobin Adducts
A significant difficulty in identifying and validating a reliable biomarker for diesel exposure is the relatively short half-life that most parent chemicals and metabolites have in the human body. Since the time interval between exposure termination and specimen collection in most large-scale biomonitoring programs is rarely known with any certainty, the clearance rate of a targeted biomarker can have a big influence on the amount detected in a specimen at any particular point of time (Jakubowski and Trzcinka-Ochocka, Citation2005). One approach to reducing the large interindividual differences caused by variations in the postexposure body burden is to select a biomarker with a long biological half-life.
Given the complex and often variable nature of diesel exhaust exposures, protein adducts provide a very attractive alternative for overcoming the high variance associated with more traditional measures such as parent or metabolite levels in a blood or urine specimen. Measurement of hemoglobin adducts is an extremely powerful biomonitoring approach since they persist for months in the bloodstream, providing exposure assessors with a historical record of exposures over a period of weeks rather than hours or days. This feature has been exploited and used to identify many useful exposure biomarkers for agents such as ethylene oxide, butadiene, epichlorohydrin, and styrene (Boogaard, Citation2002; Ogawa et al., Citation2006). Monocyclic and polycyclic quinones, such as those present in diesel exhaust, are generally highly reactive and capable of forming albumin and hemoglobin adducts that are stable for periods of a month to four months, respectively (Granath et al., Citation1992). Although some polycyclic quinones are too reactive to be absorbed intact through the lungs without first being scavenged by other nucleophilic receptors, diesel exhaust contains several unique quinones, such as 9,10-phenanthroquinone and 2-methyl-9,10-anthraquinone, whose reactivity may be sufficiently low that blood protein adducts may be detected following an exposure (Mirivel et al., Citation2010). An example of a recent success in this regard is the use of 1,2-naphthoquinone albumin adduct as a biomarker for occupational naphthalene exposure in coke-oven workers (Waidyanatha et al., Citation2004).
Another potential source of hemoglobin or protein adducts are aromatic and aliphatic aldehydes. Diesel exhaust has been shown to contain several reactive aldehydes, including t-cinnamaldehyde, 2-ethylbenzaldehyde, and 4-biphenylcarboxaldehyde that are not found in gasoline exhaust to any appreciable degree (Jakober et al., Citation2008). Aldehydes of this nature have been shown to form adducts with the N-terminal valine residue of hemoglobin (Tornqvist and Kautiainen, Citation1993). Although research has yet to be conducted with any of the higher molecular weight aldehydes found in diesel exhaust, the methodological approaches for examining these substances are not substantially different from those currently in use. Given the relative abundance of European laboratories with expertise in the identification hemoglobin adducts for biomonitoring purposes, expertise is in place to search for a distinctive adduct that accompanies diesel exhaust exposures (Jones et al., Citation2006). Although there is no records to show that any of any these laboratories is actively pursuing research with diesel exhaust, the possibility cannot be overlooked.
4. Phthalic Acid
It has been known for nearly 20 years that aromatic and aliphatic dicarboxylic acids in the C2–C10 range are common in atmospheric aerosols from urban areas (Kawamura and Kaplan, Citation1987). The primary source of these dicarboxylic acids was initially presumed to be the exhaust of gasoline- and diesel-powered engines since their emission was many fold higher than other urban sources. Further research revealed, however, that these substances had many direct and indirect sources including their release during wood burning and meat cooking (Schauer et al., Citation2002). Because of their polarity, these substances possess some peculiar characteristics that distinguish them from other more hydrophobic substances. First, polycarboxylic acids, keto acids, and dicarbonyls are all associated with the water-soluble organic fraction (WSOC) that can be extracted from urban aerosols. Second, WSOC materials are thought to be intimately associated with the formation of SOAs in the troposphere through their condensation on atmospheric nuclei (Hallquist et al., Citation2009). Finally, once formed, these substances are relatively stable to further atmospheric transformation processes that can alter their oxidation state.
Research has shown that low molecular weight aliphatic dicarboxylic acids such as butanedioic, hexanedioic, and propanedioic acid are formed through the photooxidation of airborne precursors, whereas high molecular weight aromatic polycarboxylic acids are emitted directly from vehicle exhaust as well as other combustion sources (Fraser et al., Citation2003). Although there has been an intensive effort at identifying potential dicarboxylic markers for source attribution studies, most investigations have revealed that the majority of polycarboxylic and ketocarboxylic acids are either emitted by nonspecific sources or formed secondarily through photooxidation reactions (Wang et al., Citation2006). Still, a few studies suggest that some polycarboxylic acids, such as oxalic and phthalic, may be uniquely associated with vehicle exhaust emissions (Ho et al., Citation2010; Jung et al., Citation2010).
A research program currently underway at Environment Canada has yielded some very intriguing findings that may hold promise for the identification of an exclusive biomarker for diesel exhaust exposure. Using a highly sensitive capillary electrophoresis/electrospray ionization mass spectrometry method, a select group of polycarboxylic acids were detected in the water-soluble extract of aerosol samples (Dabek-Zlotorzynska and Piechowski, Citation2007). The analysis showed the presence of a single substance, 4-hydroxyphthalic acid, in the PM2.5 from diesel exhaust that was absent when same particulate fraction was isolated from gasoline exhaust (Yassine and Dabek-Zlotorzynska, Citation2010). Since this substance was also detected in urban aerosol, the authors concluded that it may be a useful tracer for diesel exhaust emissions. Although these studies are preliminary and failed to include a full array of particulates from other source types, they are worth looking at more closely since 4-hydroxyphthalic acid possesses several of the preferred characteristics for a good exposure biomarker. For instance, its high aqueous solubility favors rapid and extensive pulmonary absorption and the high oxidation state limits further metabolism and helps maintain high measurable levels in blood or urine specimens. These properties are supported by pharmacokinetic research with an analogous chemical, phthalic acid, which is excreted unchanged in the urine of treated rats in substantial amounts (i.e., 20–25% of the dose) within 8 hr of treatment (Williams and Blanchfield, Citation1974). Scaling these results to humans would imply a sufficiently long half-life for routine detection in the urine of exposed individuals. Some studies suggest, however, that the diesel exhaust emissions of dicarboxylic acids may be quite variable, depending on the type of fuel being used (Samy and Zielinska, Citation2010). Still, the initial findings are tantalizing and worth closer scrutiny.
C. Future Opportunities
Research into the chemical composition of diesel exhaust has evolved steadily as new analytical methodologies have been developed. Hundreds of papers have been published in the last 2–3 years that contain relevant information helping to elucidate its chemical and physical nature. Perhaps the greatest achievement during this period has been the creation of a Diesel Speciation Database (DSD) that contains a compilation of all emissions test results for a variety of engine categories, model years, and fuel types (Hsu and Mullen, Citation2007). This truly ambitious project has resulted in the first-ever collection of findings from nearly 60,000 different test cycles. The information has been segregated in such a fashion that the average emission factors for 928 individual chemical substances can be extracted and tracked as a function of vehicle age (Mullen, Citation2010). Although created primarily as an aid to the development of emission inventories and mass balance models, the database also provides an opportunity to systematically search for potential biomarkers that meet many of the longevity, specificity, and mass abundance criteria associated with an ideal exposure metric.
For the purposes of this report, the DSD was not specifically mined for biomarker candidates. Instead, the database was used to list several substance categories worthy of a more thorough evaluation. The list does not represent a comprehensive and exhaustive look at all of the chemical categories found in diesel exhaust, but instead represents a targeted examination of those chemical substances that could potentially lead to fruitful avenues of research. Despite all of the research conducted to date, a large number of unidentified chemicals still remain in diesel exhaust (Fraser et al., 1999). Hundreds of substances may have escaped identification and quantitation simply because they failed to fall into one of the targeted categories. For instance, mercaptans, aminocarbolines, nitrophenols, and sulfur heterocyclics have all been detected in diesel exhaust, but the extent of their presence under various operating conditions is largely unknown (Manabe et al., Citation1991; Liang et al., Citation2006; Correa and Arbilla, Citation2008b). New substances will continue to be identified as analytical methods improve and knowledge of the combustion process advances, but for now the following substance categories have been highlighted in the hopes of stimulating a more advanced analysis of their potential utility.
1. Carbonyls
The carbonyl category provides a particularly attractive source of potential biomarkers for monitoring diesel exhaust exposure. Not only do aromatic and aliphatic carbonyls account for a sizable percentage of the organic compounds emitted in diesel exhaust, but their emissions per unit of distance traveled are substantially greater than for gasoline-powered vehicles (Legreid et al., Citation2007). Detectable quantities of aldehydes, ketones, and dicarbonyls can all be measured in both dynamometer testing and roadway tunnel studies. Up to 60% of the gas-phase mass emissions from medium-duty diesel trucks were found to be C1–C13 carbonyls in dynamometer tests (Schauer et al., Citation1999). The most commonly detected carbonyls included formaldehyde, acetaldehyde, acetone, propanal, butanone, and crotonaldehyde, which account for over half of the carbonyl mass emissions. Tunnel studies have shown that carbonyl emission factors for light-duty vehicles fueled with gasoline is 6.4 mg/km, whereas the value for heavy-duty diesel trucks is 26.1 mg/km (Grosjean et al., Citation2001). Although not true for all carbonyls, the average emission of formaldehyde has steadily declined with the introduction of new engine technologies and more advanced pollution control devices (Mullen, Citation2010). In contrast, the emission of many low molecular weight aliphatic aldehydes has increased with the use of biodiesel blends containing progressively higher amounts of FAME biofuels (Correa and Arbilla, Citation2008a).
The carbonyl compounds isolated from diesel exhaust include saturated and unsaturated aliphatic aldehydes, aromatic and alicyclic aldehydes, aliphatic and aromatic ketones, and aliphatic and aromatic dicarbonyls. Tunnel estimates of exhaust emission factors have shown that the diesel to gasoline ratio generally ranges from 3 to 10 for the most common carbonyls with the highest ratios observed with saturated aliphatic aldehydes (Ban-Weiss et al., Citation2008). The carbonyl emission factors observed in tunnel studies can be orders of magnitude higher than the values from chassis dynamometer testing, but the difference is highly dependent on the engine operating mode with the idling and creep conditions producing higher factors than the cruise mode (Sawant et al., Citation2007).
Tunnel studies have also yielded some important clues regarding the nature and variety of carbonyls emitted in diesel exhaust. In fact, some studies have shown that over 100 different carbonyls can be identified and quantified inside busy roadway tunnels (Gertler et al., Citation2002). In general, however, the list of notable carbonyls in diesel exhaust usually numbers between 40 and 50 (Hsu and Mullen, Citation2007). Although methods exist to separately calculate the emission factors for diesel- and gasoline-fueled vehicles by keeping track of the number of light- and heavy-duty vehicles passing through a tunnel, this approach is less precise than the independent dynamometer testing of each vehicle type. Dynamometer testing provides the best data for identifying chemical substances that are uniquely associated with diesel engine exhaust. As noted earlier, however, the emission factor measurements from dynamometer testing may be biased because the degree of exhaust dilution in this test system is substantially less than what is observed under actual driving conditions.
Dynamometer testing has shown that exhaust carbonyls are emitted in both the gas and particle phases (Jakober et al., Citation2006). As shown in , the carbonyl levels in the gas phase are often many times higher than the levels found on PM, and that the mass recovered from diesel-generated particulates is substantially greater than the amount from gasoline (Jakober et al., Citation2008). Carbonyls also comprise approximately 19% of the particulate OC from low-emission light-duty vehicles and 37% of the OC from light-duty vehicles equipped with an oxidation catalyst, so these pollution control devices increase the emission of carbonyls through the oxidation of the precursor hydrocarbons. The individual emission factors for heavy-duty vehicles were substantially greater than light-duty vehicles with idling heavy-duty trucks showing the highest emissions. Of the 59 carbonyls identified, 10 substances (bold type) were uniquely identified in the gas or particle phase of diesel exhaust with no comparable presence in gasoline exhaust. The ability of these substances to form characteristic protein adducts following systemic absorption would qualify their selection as potentially attractive exposure biomarkers. In addition, there is an abundance of analytical approaches for measuring ambient air exposures to these chemicals, which would greatly aid the validation process. A more exhaustive survey of other combustion sources is needed to confirm the specificity of these substances for diesel exhaust.
TABLE 2 Emission factors for carbonyls in the gas and particle phases of diesel and gasoline exhaust (Jakober et al., Citation2008)
2. Quinones
The oxygenated PAHs associated with diesel exhaust have two sources: the fuel combustion process itself and the photochemical oxidation of soot adsorbed PAHs by reaction with atmospheric hydroxyl radicals (Kojima et al., Citation2010). Although oxygenated PAHs can be traced to the incomplete combustion of many materials including wood, coal, and agricultural debris, there is relatively little information on the contribution of these alternative sources to the overall atmospheric burden (Walgraeve et al., Citation2010). For convenience, this category of substances has been narrowed down to those oxygenated-PAHs containing carboxylic oxygen atoms in the form of a quinone or ketone. These compounds have a strong affinity for diesel soot and are preferentially bound to DPM when they possess three or more aromatic rings (Murillo-Tovar et al., Citation2010). In contrast, simpler quinones such as 1,4-benzoquinone exist primarily in the gas phase due to their high vapor pressure (Jakober et al., Citation2007).
Two of the more commonly encountered oxygenated-PAHs, 9-fluorenone and anthraquinone, have been detected in diesel fuel and oil as well as diesel exhaust (Zielinska et al., Citation2004). Beyond these rather ubiquitous oxygenated-PAHs, a limited group of high molecular weight substances have also been isolated from diesel particulates. lists some of the oxygenated-PAHs that have been isolated and identified in separate evaluations of diesel exhaust emissions. The list shows that a range of high molecular weight aldehydes and quinones have been detected in exhaust streams, but the comparison suggests that there is little agreement across test conditions. This is due in part to the capabilities of the analytical methodologies employed and the specific goals of the study. The aim of some studies was to compare the relative abundance of individual oxygenated PAHs in PM from a small select group of combustion products without any attempt at covering the full range of possible sources. For instance, the study by Cho et al. revealed that quinone concentrations were 20- to 70-fold lower in urban dust samples than in diesel soot (Cho et al., Citation2004). These same studies also found that fine PM from Los Angeles air samples contained 1,4-nahthoquinone and 9,10-phenanthroquinone at levels that were consistent with those from diluted vehicle exhaust. Other studies have demonstrated that nine of the oxygenated-PAHs in diesel soot from a passenger car were present at concentrations less than or equal to the levels emitted by a gasoline engine (Oda et al., Citation1998). In a separate study performed with passenger automobiles, 9,10-anthraquinone, 9,10-phenanthroquinone, and aceanthrenequinone were uniquely associated with diesel particulates with no measurable amounts found in the exhaust particulates from gasoline-powered vehicles (Jakober et al., Citation2007). A comparison of oxygenated-PAHs from diesel particulate with those found in sediment and urban dust revealed that the concentrations in DPM were 2- to 6-fold higher for five of the nine chemicals, with a particularly high difference observed for 4H-cyclopenta[def]phenanthrene-4-one (Layshock et al., Citation2010). Although these findings are notable, testing with a more complete range of combustion sources is needed to verify the relevance of these results.
TABLE 3 Studies determining oxygenated PAH concentrations in diesel exhaust PM
Regardless, the information from these studies provides tantalizing clues on the types of substances uniquely associated with diesel exhaust. In this regard, the recently published work of Mirivel et al. is perhaps the most significant and worthy of the closest scrutiny (Mirivel et al., Citation2010). As shown in , when the levels of individual oxygenated-PAHs from diesel soot are compared with other potential sources, including kerosene soot as well as reference PM for urban air, indoor air, and vehicle exhaust, an interesting pattern emerges. Two substances, 9,10-phenanthroquinone and 2-methyl-9,10-anthraquinone, display a relatively high abundance in diesel particulates relative to other particle sources. These data suggest that 9,10-phenanthroquinone and 2-methyl-9,10-anthraquinone may meet the specificity requirements for a diesel exhaust biomarker. Amongst other factors, the relative abundance of secondarily formed quinones needs closer scrutiny since atmospheric photooxidation has been shown to be an important source of these substances in ambient air (Kojima et al., Citation2010). For instance, relatively high levels of 9,10-phenanthroquinone and 2-methyl-9,10-anthraquinone have been detected in urban air samples from the city of Athens (Andreou and Rapsomanikis, Citation2009). In addition, an examination of 9,10-phenanthroquinone concentrations in an air parcel moving across the Los Angeles metropolitan suggested that approximately 90% of the mass was the result of photochemical formation (Eiguren-Fernandez et al., Citation2008). These data underscore the need to proceed with a good understanding of the fate processes affecting the environmental degradation of freshly emitted diesel exhaust. This effort can be aided by the use of multimedia and structure-activity models to evaluate the environmental distribution and atmospheric photooxidation potential of candidate substances using tools such as the EPI (estimation program interface) Suite developed by the US EPA (USEPA, Citation2011).
TABLE 4 Quinones adsorbed to PM isolated from different sources (Mirivel et al., Citation2010)
3. Nitrated Polycyclics
The nitrated PAH category contains a somewhat less attractive source of potential biomarkers because of the secondary influences from atmospheric processing. Nitrated-PAHs are formed both by incomplete combustion and by transformation reactions in the atmosphere catalyzed by hydroxyl radicals during the daytime and nitrate radicals at night (Atkinson and Arey, Citation1994). The relative amounts formed by these different processes are affected by the radical source concentrations found at different times of the day and seasons of the year (Reisen and Arey, Citation2005). Ambient air measurements of nitrated-PAHs indicate that most are formed secondarily in the gas phase from their analogous PAH parent, then adsorbed onto soot to some degree (Bamford and Baker, Citation2003). Nitrated-PAHs containing two rings are generally found in the gas phase, whereas the 3–4 ring substances are found in urban particulates at gas-to-particle ratios ranging from 0.1 to 1.7 (Cecinato, Citation2003). Concentrations of individual nitrated-PAHs can differ widely between regions with countries in Eastern Europe having higher concentrations than those in the west (Soderstrom et al., Citation2005). Research suggests that 2-nitropyrene and 2-nitrophenanthene are formed solely following photochemical oxidation, and that 1-nitropyrene is perhaps the best marker for diesel exhaust since it is formed mostly during the combustion cycle (Miller-Schulze et al., Citation2007). The ratio of 2-nitropyrene to 1-nitropyrene is therefore a good indicator of the diesel source contribution under a particular set of circumstances (Miller-Schulze et al., Citation2010).
Nearly 30 different nitrated and dinitrated PAHs have been identified in diesel exhaust. A wide variety of extraction and analytical methods have been developed to separate and quantify individual nitrated-PAHs (Zielinska and Samy, Citation2006). These include both gas and liquid chromatographic methods using chemical ionization detection in conjunction with mass spectrometry or tandem mass spectrometry for speciation. provides a compendium of studies focusing on the isolation and identification of nitrated-PAHs from diesel particulates collected from vehicle exhaust streams or supplied as a standard reference material (SRM) by the US National Institute of Standards. A comparison of the results from the various studies underscores the high proportion of 1-nitropyrene in diesel exhaust and suggests that opportunities may exist with several other biomarker candidates. High concentrations of 9-nitroanthracene and 6-nitrobenz[a]pyrene were consistently detected in the various diesel exhaust samples. Both chemicals were also identified in urban air samples with 9-nitroanthracene (3-ring) existing primarily, but not exclusively, in the gas-phase and 6-nitrobenz[a]pyrene (4-ring) occurring wholly in the particle phase (Araki et al., Citation2009). Particulates from four Brazilian cities typically showed the presence of both 9-nitroanthracene and 6-nitrobenz[a]pyrene in airborne samples with maximum concentrations of 191 pg/m3 and 116 pg/m3, respectively (Vasconcellos et al., Citation2008). The level of both chemicals was approximately 2- to 4-fold higher in urban PM collected in the winter compared to the summer (Delhomme et al., Citation2007). Assuming diesel as the predominant winter fuel and that seasonal biomass burning is not a factor, these data tend to suggest that the source of these substances was primarily from fuel combustion rather than atmospheric photooxidation. If this fact holds true, then 9-nitroanthracene and 6-nitrobenz[a]pyrene may have value as potential markers of diesel exhaust exposure.
TABLE 5 Studies determining nitrated-PAH concentrations in diesel exhaust PM
Use of pollution control devices has been shown to impact the emission of some nitrated-PAHs such as 9-nitroanthracene, which is emitted in appreciably higher amounts when DPFs are used in conjunction with some fuel types (Heeb et al., Citation2008). On the contrary, use of low-sulfur fuel in a modern heavy-duty diesel engine has been shown to dramatically decrease the emission of 9-nitroanthracene along with many other nitrated-PAHs, but modestly increase the emission of 9-nitrophenanthracene (Liu et al., Citation2010). Since the nitrated-PAHs found in cigarette smoke are restricted to substances possessing less than 3-rings, the ambient air concentration of these two potential candidates is not affected by this commonly found interferent (Havey et al., Citation2009).
A detailed examination of the nitrated-PAHs in SRMs supplied by several recognized government agencies revealed the presence of several other potential biomarkers of diesel exhaust (Bamford et al., Citation2003). The list of nitrated-PAHs shown in were identified in standardized test samples for urban air and diesel exhaust using an improved extraction and cleanup procedure prior to analysis by gas chromatography/tandem mass spectrometry. Of particular interest is the prevalence of the three dinitropyrenes in the diesel exhaust samples. Although there is a limited amount of information available on these substances, some urban air measurements have been performed. Two of the three dinitropyrenes, 1,3-dinitropyrene and 1,8-dinitropyrene, were detected in winter, but not summer, air samples from the city of Strasbourg, Germany, at concentrations ranging as high as 21 pg/m3 (Delhomme et al., Citation2007). As noted in , studies have shown that these three compounds are not found in appreciable quantities in gasoline exhaust, tire debris, or asphalt paste (Ozaki et al., Citation2010). The concentration of 1,3-dinitropyrene and 1,8-dinitropyrene in aerosol samples has been shown to decrease in expected fashion with urban particulates > suburban particulates > rural particulates (Albinet et al., Citation2007). None of these dinitropyrenes could be detected in street runoff samples from a busy highway in Japan (Murakami et al., Citation2008).
TABLE 6 Concentration (mean ± SD) of nitrated-PAHs in standard reference materials and urban air samples (Bamford et al., Citation2003)
TABLE 7 Nitrated-PAHs in commonly found roadway particulates (Ozaki et al., Citation2010)
The preceding information indicates that there is a strong possibility of locating at least a few candidate biomarkers from this class of nitrated substances. Great care must be taken with the nitrated-PAHs because of the strong possibility that confounding may occur as the result of secondary nitration processes or contact with co-combustion sources. The primary advantage of targeting this class of substances stems from test data showing that they are not severely impacted by the use of advanced pollution control devices.
IV. CONCLUSIONS
Diesel engines have become a mainstay of modern life and an essential means of transporting goods, generating power, and applying mechanical force. Trucks, locomotives, ships, and marine vessels all rely on diesel engines to provide the power and reliability needed to transport goods across long distances. Manufacturers of passenger vehicles have also increasingly turned to the diesel engine as an alternative to traditional gasoline-powered engine because of the dramatic fuel savings it affords. From the year 1980 to 2000, the number of new diesel-powered automobiles sold in Europe quadrupled to the point where one in every three vehicles sold contained a diesel engine (Lloyd and Cackette, Citation2001).
Because of its lean combustion characteristics, however, diesel exhaust is an inherently polluting emission that engineers have endeavored to control since the mid-1970s when the first emission standards went into effect. These efforts have led to tremendous reductions in PM, nitrogen oxides (NOx), and volatile organic compounds (VOCs) through a combination of engine redesign, fuel alternatives, and new types of pollution control devices. Despite these improvements, however, the emissions of NOx and fine PM in the United States from on-road vehicle use have not declined greatly in part because of the increase in diesel fuel use (Dallmann and Harley, Citation2010). In Europe, the results are much the same with modest emission reductions offset by the large projected increases in diesel vehicle use (Giannouli et al., Citation2011).
The upswing in diesel-powered vehicles has also intensified concerns about the potential health and environmental effects of diesel exhaust. Most competent authorities now consider the components of diesel exhaust to pose acute and chronic health risks in humans (Ris, Citation2007; Zhang et al., Citation2009; Jarvholm and Reuterwall, Citation2012). The issue, however, is not clear-cut since major questions persist regarding potency and the relative impact of other nonspecific background sources on any aggregate measure of risk. Without a clearly defined measure of diesel exhaust exposure, the strength of any association between exposure and health risk remains unknown or highly uncertain. Despite the uncertainties, however, use trends and potential health effects have necessitated an ever-changing array of pollution control measures for limiting exhaust emissions.
Past, present, and future emission control regulations continue to have a huge impact on the chemical and physical characteristics of diesel exhaust and have forced the design of new technologies for limiting the emission of potentially harmful gases, vapors, and particulates (Burtscher, Citation2005; Hesterberg et al., Citation2011). In fact, current regulations in effect within the United States and Europe virtually guarantee that the composition of diesel exhaust will continue to undergo dramatic change in coming years with the diesel exhaust of today bearing small resemblance qualitatively or quantitatively to the exhaust of tomorrow. Modifications in engine design and pollution control device efficiency are having a dramatic impact on the emission characteristics of diesel exhaust. Results from the Advanced Collaborative Emission Study have shown that modern heavy-duty diesel engines equipped with an oxidation catalyst and particulate filter generally emit 90–99% less polycyclics and volatile organics than do older, pre-2004 engines (Khalek et al., Citation2011). As this technology improves further and is applied to a wider variety of vehicle types, the concentration of diesel-related volatile and semivolatile substances in urban air may fall to negligible levels, but this is not likely to occur for many decades due to the impact of nonroad sources.
In addition to the variable impact of aftertreatment devices, other factors need to be considered since they can also dramatically influence the concentration, detectability, and usability of a candidate biomarker. As shown in , the impact of extraneous variables on the suitability of biomarker candidates from past, present, and future research will vary appreciably depending on the target substance or chemical category. Perhaps the most difficult problem with the identification of a diesel biomarker usable under a variety of exposure conditions is that of specificity. Past attempts at identifying a biomarker have largely taken place in occupational environments where cross-contamination issues were not a serious problem. In reality, however, many of the previously examined biomarkers can be found in combustion products from a variety of sources. To overcome this problem, future research will need to take a more creative and systematic approach that considers those substances unique to diesel exhaust. Another confounding variable that needs careful consideration, especially for those substances that have a long residence time in air, is atmospheric aging. The high propensity of PAHs to undergo atmospheric oxidation virtually guarantees that this category will not yield a viable candidate suitable for use in large-scale population studies. Other possibilities exist, however, particularly with those substances having a high oxidation state upon initial emission from the tail pipe. These same substances, however, have a relatively short half-life in the body due to the ease of excretion following phase 2 metabolism. This is partially offset by the tendency of some substances to form protein adducts, which have a far longer residence time in the body. For example, quinones such as those in diesel exhaust generally have a short half-life in the body on the order of 10–15 min, but their protein adducts can persist for days and exhibit a half-life of nearly two weeks in humans (Rappaport et al., Citation2002; Lin et al., Citation2009). By comparison, the half-life for representative PAH metabolites has been reported to average 18 hr, whereas the value for nitrated PAH metabolites is even shorter with an estimated value of 12 hr or less (Buchet et al., Citation1992; Miller-Schulze et al., Citation2013). The clearance of bulky DNA adducts is somewhat longer, as might be expected, with half-lives lasting up to 10–12 weeks; but again there are issues of specificity with these biomarkers (Godschalk et al., Citation2003). From a regulatory perspective, future legislation will likely affect all of the candidate substances similarly since emissions are restricted on the basis of total hydrocarbon content rather than an individual component or chemical class. When all of these confounding variables are factored into consideration, three substances emerge as the most viable for further study: nanoparticulates, 1-nitropyrene, and quinone adducts. This is not to say that other lines of research are unlikely to yield fruitful results, but rather that the three substances identified above are less likely to be affected by confounding influences.
TABLE 8 Impact of confounding variables on the utility of putative biomarkers for diesel exhaust exposure
Despite intense interest in the availability of a suitable and reasonably well-vetted biomarker for diesel exposure, progress has been relatively slow. To date most research has been bottom-up and inductive in nature, focusing on exploratory studies and observational finding to assess the usefulness and dependability of a particular technique. Although this inductive approach has its advantages in terms of focusing limited resources on those issues posing the greatest challenge, it tends to be rather unstructured and fragmented with no clear hypothesis-driven objectives. An alternative to this scheme and one offering a more systematic method of mining all available information, takes a top-down perspective that is entirely data-driven. A top-down analysis would take advantage of the vast amount of information that has been assembled on the emission of individual substances under various test conditions as well as those factors affecting their airborne concentration. An examination of these data sources in a prescribed and systematic fashion could yield a small select group of substances that possess the characteristics and properties required from an ideal exposure biomarker. The most notable challenge in this regard, and the one that has proved to be the most difficult to overcome, is that of specificity. Since many components of diesel exhaust are also found in wide variety of other emission types such as coal, wood, and cigarette smoke, as well as the exhaust from gasoline-powered engines, background exposures to many biomarker candidates can be unacceptably high. To overcome this problem, more focus needs to be placed on identifying those substances that are absent from secondary emission sources. Although this would entail a detailed examination and cross-comparison of the emissions profile from primary and secondary combustion sources, the potential rewards from this exercise are well worth the effort required.
Whereas this review has attempted to identify a small number of suitable candidates by searching for unique characteristics for a range of different categories, the effort was by no means systematic. A variety of qualitative and quantitative tools now exist to more thoroughly examine the potential for a substance to be (1) absorbed into the body; (2) metabolized and excreted; and (3) eliminated within a period of hours or days (van Veen, Citation2001; Marchant et al., Citation2008). The dosimetry, pharmacokinetic, and structure-activity models needed for this type of analysis currently exist and can be supplemented with others capable of answering questions regarding atmospheric photooxidation potential and environmental fate. The goal of using these modeling techniques would be to identify a subset of substances in diesel exhaust whose properties are conducive to further research on biomarker development. In order to accomplish this task, however, a current and comprehensive list is needed of the chemical substances emitted in diesel exhaust. Until very recently, this information was not available, but creation of the DSD by the Coordinating Research Council has yielded a truly unique and magnificent resource. As noted previously, the DSD contains a compilation of all previously conducted emission studies with diesel-powered engines (Hsu and Mullen, Citation2007). The information has been organized to provide average emission factors for nearly a thousand individual chemical substances as a function of vehicle age, engine size, and fuel type (Mullen, Citation2010). A systematic top-down examination of the chemicals listed in this database using a structured set of rules and requirements could yield a subgroup of substances that have been screened for their specificity. This is merely the starting point, however, for assembling a group of chemicals that can undergo a more detailed examination. Factors such as the removal efficiency by pollution control devices, background concentrations in the environment, and the influence of fuel and engine-related properties would also need to be examined. The successful application of a methodical identification scheme such as this could lead to an orderly escape from the morass that has engulfed the search for a useable biomarker for diesel exhaust exposure.
As an array of ever more sophisticated analytical techniques is applied to the problem of biomarker identification, a breakthrough may occur; but there are many impediments. Although a variety of parent chemicals, urinary metabolites, and macromolecular adducts have been examined as potential biomarkers, none have been unequivocally and uniquely associated with diesel exhaust. There have been some tenuous successes, however, mostly with the measurement of 1-nitropyrene concentrations in the urine. Although this is a particularly intriguing substance that is neither formed secondarily through atmospheric nitration reactions nor excessively impacted by the use of advanced pollution control devices or alternative fuel types, additional validation is needed to establish the performance characteristics of the assay under actual field conditions (Ratcliff et al., Citation2010). Because of its short elimination half-life, however, urinary 1-nitropyrene measurements do not provide an optimal approach for measuring community exposures, which can occur intermittently or with a long lag periods between exposure termination and sample collection. The best approach in these settings would be the measurement of a protein or hemoglobin adduct that has a relatively long biological half-life. Although there are few published studies to date on the feasibility of using hemoglobin adducts to measure diesel exhaust exposure, it remains a promising approach given the known presence of semireactive quinones and aldehydes in the exhaust stream. This is a long-term prospect, however, that will take many years to organize and bring to fruition. In the interim, a more short-term approach, at least for occupational exposures, would be a more thorough vetting of the attributes, interferences, and complexities associated with urinary 1-nitropyrene measurements.
ACKNOWLEDGMENTS
The author would like to acknowledge the assistance of Drs. Pamela Dalton, Gary Minsavage, and Arlean Rohde for their helpful comments during the preparation of the manuscript.
FUNDING
Funding for this project was provided by the Oil Companies’ European Association for Environment, Health and Safety in Refining and Distribution (CONCAWE).
REFERENCES
- ACGIH. (2005). 2005 TLVs and BEIs. American Conference of Governmental Industrial Hygienists. Cincinnati, OH`.
- Adgate, J.L., Ramachandran, G., Pratt, G.C., Waller, L.A., and Sexton, K. (2002). Spatial and temporal variability in outdoor, indoor, and personal PM2.5 exposure. Atmos. Environ., 36, 3255–3265.
- Alander, T.J. A., Leskinen, A.P., Raunemaa, T.M., and Rantanen, L. (2004). Characterization of diesel particles: Effects of fuel reformulation, exhaust aftertreatment, and engine operation on particle carbon composition and volatility. Environ. Sci. Technol., 38, 2707–2714.
- Albinet, A., Leoz-Garziandia, E., Budzinski, H., and ViIlenave, E. (2007). Polycyclic aromatic hydrocarbons (PAHs), nitrated PAHs and oxygenated PAHs in ambient air of the Marseilles area (South of France): Concentrations and sources. Sci. Total Environ., 384, 280–292.
- Andreou, G., and Rapsomanikis, S. (2009). Polycyclic aromatic hydrocarbons and their oxygenated derivatives in the urban atmosphere of Athens. J. Hazard. Mater., 172, 363–373.
- Angerer, J., Ewers, U., and Wilhelm, M. (2007). Human biomonitoring: State of the art. Int. J. Hyg. Environ. Health, 210, 201–228.
- Angerer, J., Mannschreck, C., and Gundel, J. (1997a). Biological monitoring and biochemical effect monitoring of exposure to polycyclic aromatic hydrocarbons. Int. Arch. Occup. Environ. Health, 70, 365–377.
- Angerer, J., Mannschreck, C., and Gundel, J. (1997b). Occupational exposure to polycyclic aromatic hydrocarbons in a graphite-electrode producing plant: Biological monitoring of 1-hydroxypyrene and monohydroxylated metabolites of phenanthrene. Int. Arch. Occup. Environ. Health, 69, 323–331.
- Araki, Y., Tang, N., Ohno, M., Kameda, T., Toriba, A., and Hayakawa, K. (2009). Analysis of atmospheric polycyclic aromatic hydrocarbons and nitropolycyclic aromatic hydrocarbons in gas/particle phases separately collected by a high-volume air sampler equipped with a column packed with XAD-4 resin. J. Health Sci., 55, 77–85.
- Arlt, V.M. (2005). 3-Nitrobenzanthrone, a potential human cancer hazard in diesel exhaust and urban air pollution: A review of the evidence. Mutagenesis, 20, 399–410.
- Arlt, V.M., Glatt, H., Da Costa, G.G., Reynisson, J., Takamura-Enya, T., and Phillips, D.H. (2007a). Mutagenicity and DNA adduct formation by the urban air pollutant 2-nitrobenzanthrone. Mutagenesis, 22, 439.
- Arlt, V.M., Glatt, H., Da Costa, G.G., Reynisson, J., Takamura-Enya, T., and Phillips, D.H. (2007b). Mutagenicity and DNA adduct formation by the urban air pollutant 2-nitrobenzanthrone. Toxicol. Sci., 98, 445–457.
- Arlt, V.M., Schmeiser, H.H., Osborne, M.R., Kawanishi, M., Kanno, T., Yagi, T., Phillips, D.H., and Takamura-Enya, T. (2006). Identification of three major DNA adducts formed by the carcinogenic air pollutant 3-nitrobenzanthrone in rat lung at the C8 and N-2 position of guanine and at the N-6 position of adenine. Int. J. Cancer, 118, 2139–2146.
- Atkinson, R., and Arey, J. (1994). Atmospheric chemistry of gas-phase polycyclic aromatic hydrocarbons: Formation of atmospheric mutagens. Environ. Health Perspect., 102, 117–126.
- Bae, M.S., Schauer, J.J., Turner, J.R., and Hopke, P.K. (2009). Seasonal variations of elemental carbon in urban aerosols as measured by two common thermal-optical carbon methods. Sci. Total Environ., 407, 5176–5183.
- Ballesteros, R., Hernandez, J.J., Lyons, L.L., Cabanas, B., and Tapia, A. (2008). Speciation of the semivolatile hydrocarbon engine emissions from sunflower biodiesel. Fuel, 87, 1835–1843.
- Bamford, H.A., and Baker, J.E. (2003). Nitro-polycyclic aromatic hydrocarbon concentrations and sources in urban and suburban atmospheres of the Mid-Atlantic region. Atmos. Environ., 37, 2077–2091.
- Bamford, H.A., Bezabeh, D.Z., Schantz, M.M., Wise, S.A., and Baker, J.E. (2003). Determination and comparison of nitrated-polycyclic aromatic hydrocarbons measured in air and diesel particulate reference materials. Chemosphere, 50, 575–587.
- Ban-Weiss, G.A., McLaughlin, J.P., Harley, R.A., Kean, A.J., Grosjean, E., and Grosjean, D. (2008). Carbonyl and nitrogen dioxide emissions from gasoline- and diesel-powered motor vehicles. Environ. Sci. Technol., 42, 3944–3950.
- Bauner, D., Laestadius, S., and Iida, N. (2009). Evolving technological systems for diesel engine emission control: balancing GHG and local emissions. Clean Technol. Environ. Policy, 11, 339–365.
- Baxter, L.K., Wright, R.J., Paciorek, C.J., Laden, F., Suh, H.H., and Levy, J.I. (2010). Effects of exposure measurement error in the analysis of health effects from traffic-related air pollution. J. Exp. Sci. Environ. Epidemiol., 20, 101–111.
- Belle, B.K. (2010). Use of baseline personal DPM exposure data for mine ventilation planning - A South African Journey. J. Mine Vent. Soc. S. Afr., April/June, 12–16.
- Benowitz, N.L. (1999). Biomarkers of environmental tobacco smoke exposure. Environ. Health Perspect., 107, 349–355.
- Bermudez, V., Lujan, J.M., Pla, B., and Linares, W.G. (2011). Comparative study of regulated and unregulated gaseous emissions during NEDC in a light-duty diesel engine fuelled with Fischer Tropsch and biodiesel fuels. Biomass Bioenergy, 35, 789–798.
- Birch, M.E. (2002). Occupational monitoring of particulate diesel exhaust by NIOSH method 5040. Appl. Occup. Environ. Hyg., 17, 400–405.
- Boogaard, P.J. (2002). Use of haemoglobin adducts in exposure monitoring and risk assessment. J. Chromatogr. B Anal. Tech., 778, 309–322.
- Boogaard, P.J. (2007). Human biomonitoring activities – programmes by industry. Int. J. Hyg. Environ. Health, 210, 259–261.
- Boogaard, P.J. (2009). Biomonitroing of the worplace environment. In B. Ballantyne, and Marrs, T.S. T. (Eds.), General and applied toxicology (pp. 1–31). Chichester: John Wiley & Sons Ltd.
- Bouchard, M., and Viau, C. (1999). Urinary 1-hydroxypyrene as a biomarker of exposure to polycyclic aromatic hydrocarbons: biological monitoring strategies and methodology for determining biological exposure indices for various work environments. Biomarkers, 4, 159–187.
- Briggs, D.J., Sabel, C.E., and Lee, K. (2009). Uncertainty in epidemiology and health risk and impact assessment. Environ. Geochem. Health, 31, 189–203.
- Buchet, J.P., Gennart, J.P., Mercadocalderon, F., Delavignette, J.P., Cupers, L., and Lauwerys, R. (1992). Evaluation of exposure to polycyclic aromatic-hydrocarbons in a coke production and a graphite electrode manufacturing plant – assessment of urinary-excretion of 1-hydroxypyrene as a biological indicator of exposure. Br. J. Ind. Med., 49, 761–768.
- Budnik, L.T., and Baur, X. (2009). The assessment of environmental and occupational exposure to hazardous substances by biomonitoring. Dtsch. Arztebl. Int., 106, 91–97.
- Burgard, D.A., and Provinsal, M.N. (2009). On-road, in-use gaseous emission measurements by remote sensing of school buses equipped with diesel oxidation catalysts and diesel particulate filters. J. Air Waste Manage. Assoc., 59, 1468–1473.
- Burtscher, H. (2005). Physical characterization of particulate emissions from diesel engines: A review. J. Aerosol Sci., 36, 896–932.
- Calafat, A.M., and Needham, L.L. (2008). Factors affecting the evaluation of biomonitoring data for human exposure assessment. Int. J. Androl., 31, 139–143.
- Campo, L., Mercadante, R., Rossella, F., and Fustinoni, S. (2009). Quantification of 13 priority polycyclic aromatic hydrocarbons in human urine by headspace solid-phase microextraction gas chromatography-isotope dilution mass spectrometry. Anal. Chim. Acta, 631, 196–205.
- Campo, L., Rossella, F., Pavanello, S., Mielzynska, D., Siwinska, E., Kapka, L., Bertazzi, P.A., and Fustinoni, S. (2010). Urinary profiles to assess polycyclic aromatic hydrocarbons exposure in coke-oven workers. Toxicol. Lett., 192, 72–78.
- Cavallo, D., Ursini, C.L., Rondinone, B., and Iavicoli, S. (2009). Evaluation of a suitable DNA damage biomarker for human biomonitoring of exposed workers. Environ. Mol. Mutagen., 50, 781–790.
- Cecinato, A. (2003). Nitrated polynuclear aromatic hydrocarbons in ambient air in Italy. A brief overview. J. Sep. Sci., 26, 402–408.
- Chan, A.W. H., Kautzman, K.E., Chhabra, P.S., Surratt, J.D., Chan, M.N., Crounse, J.D., Kurten, A., Wennberg, P.O., Flagan, R.C., and Seinfeld, J.H. (2009). Secondary organic aerosol formation from photooxidation of naphthalene and alkylnaphthalenes: Implications for oxidation of intermediate volatility organic compounds (IVOCs). Atmos. Chem. Phys., 9, 3049–3060.
- Cheung, C.S., Zhu, L., and Huang, Z. (2009). Regulated and unregulated emissions from a diesel engine fueled with biodiesel and biodiesel blended with methanol. Atmos. Environ., 43, 4865–4872.
- Chien, S.M., Huang, Y.J., Chuang, S.C., and Yang, H.H. (2009). Effects of biodiesel blending on particulate and polycyclic aromatic hydrocarbon emissions in nano/ultrafine/fine/coarse ranges from diesel engine. Aerosol Air Qual. Res., 9, 18–31.
- Cho, A.K., Di Stefano, E., You, Y., Rodriguez, C.E., Schmitz, D.A., Kumagai, Y., Miguel, A.H., Eiguren-Fernandez, A., Kobayashi, T., Avol, E., and Froines, J.R. (2004). Determination of four quinones in diesel exhaust particles, SRM 1649a, an atmospheric PM2.5. Aerosol Sci. Technol., 38, 68–81.
- Choosong, T., Chomanee, J., Tekasakul, P., Tekasakul, S., Otani, Y., Hata, M., and Furuuchi, M. (2010). Workplace environment and personal exposure of PM and PAHs to workers in natural rubber sheet factories contaminated by wood burning smoke. Aerosol Air Qual. Res., 10, 8–21.
- Chow, J.C., Watson, J.G., Crow, D., Lowenthal, D.H., and Merrifield, T. (2001). Comparison of IMPROVE and NIOSH carbon measurements. Aerosol Sci. Technol., 34, 23–34.
- CONCAWE. (2005). Fuel Effects on Emissions from Advanced Diesel Engines and Vehicles. . Report No. 2/05: Conservation of Clean Air and Water in Europe. Fuel Quality and Emissions Management Group. Brussels, Belgium.
- Correa, S.M., and Arbilla, G. (2006). Aromatic hydrocarbons emissions in diesel and biodiesel exhaust. Atmos. Environ., 40, 6821–6826.
- Correa, S.M., and Arbilla, G. (2008a). Carbonyl emissions in diesel and biodiesel exhaust. Atmos. Environ., 42, 769–775.
- Correa, S.M., and Arbilla, G. (2008b). Mercaptans emissions in diesel and biodiesel exhaust. Atmos. Environ., 42, 6721–6725.
- Corro, G. (2002). Sulfur impact on diesel emission control – a review. React. Kin. Catal. Lett., 75, 89–106.
- Crimmins, B.S., and Baker, J.E. (2006). Improved GC/MS methods for measuring hourly PAH and nitro-PAH concentrations in urban particulate matter. Atmos. Environ., 40, 6764–6779.
- Dabek-Zlotorzynska, E., and Piechowski, M. (2007). Application of CE with novel dynamic coatings and field-amplified sample injection to the sensitive determination of isomeric benzoic acids in atmospheric aerosols and vehicular emission. Electrophoresis, 28, 3526–3534.
- Dallmann, T.R., and Harley, R.A. (2010). Evaluation of mobile source emission trends in the United States. J. Geophys. Res. Atmos., 115, D14305, doi: 10.1029/2010JD013862
- Dane, J., and Voorhees, K.J. (2010). Investigation of nitro-organic compounds in diesel exhaust. . Report No. NREL/SR-540–45597: United States Department of Energy, National Renewable Energy Laboratory. Golden, CO.
- Delhomme, O., Herckes, P., and Millet, M. (2007). Determination of nitro-polycyclic aromatic hydrocarbons in atmospheric aerosols using HPLC fluorescence with a post-column derivatisation technique. Anal. Bioanal. Chem., 389, 1953–1959.
- Di, Y.G., Cheung, C.S., and Huang, Z.H. (2009). Comparison of the effect of biodiesel-diesel and ethanol-diesel on the gaseous emission of a direct-injection diesel engine. Atmos. Environ., 43, 2721–2730.
- Durbin, T.D., Cocker, D.R., Sawant, A.A., Johnson, K., Miller, J.W., Holden, B.B., Helgeson, N.L., and Jack, J.A. (2007). Regulated emissions from biodiesel fuels from on/off-road applications. Atmos. Environ., 41, 5647–5658.
- ECETOC. (2005). Guidance for the Interpretation of Biomonitoring Data. . 44: European Centre for Ecotoxicology and Toxicology of Chemicals.
- Eiguren-Fernandez, A., Miguel, A.H., Lu, R., Purvis, K., Grant, B., Mayo, P., Di Stefano, E., Cho, A.K., and Froines, J. (2008). Atmospheric formation of 9,10-phenanthraquinone in the Los Angeles air basin. Atmos. Environ., 42, 2312–2319.
- Elihn, K., Ulvestad, B., Hetland, S., Wallen, A., and Randem, B.G. (2008). Exposure to ultrafine particles in asphalt work. J. Occup. Environ. Health, 5, 771–779.
- Enya, T., Suzuki, H., Watanabe, T., Hirayama, T., and Hisamatsu, Y. (1997). 3-nitrobenzanthrone, a powerful bacterial mutagen and suspected human carcinogen found in diesel exhaust and airborne particulates. Environ. Sci. Technol., 31, 2772–2776.
- Ferreira, S.L., dos Santos, A.M., de Souza, G.R., and Polito, W.L. (2008). Analysis of the emissions of volatile organic compounds from the compression ignition engine fueled by diesel-biodiesel blend and diesel oil using gas chromatography. Energy, 33, 1801–1806.
- Forster, K., Preuss, R., Rossbach, B., Bruning, T., Angerer, J., and Simon, P. (2008). 3-Hydroxybenzo[a]pyrene in the urine of workers with occupational exposure to polycyclic aromatic hydrocarbons in different industries. Occup. Environ. Med., 65, 224–229.
- Fraser, M.P., Cass, G.R., and Simoneit, B.R. T. (2003). Air quality model evaluation data for organics. 6. C-3-C-24 organic acids. Environ. Sci. Technol., 37, 446–453.
- Fraser, M. P., Cass, G. R., and Simoneit, B. R. T. (1999). Particulate organic compounds emitted from motor vehicle exhaust and in the urban atmosphere. Atmos. Environ., 33, 2715–2724.
- Gamble, J. (2010). Lung cancer and diesel exhaust: A critical review of the occupational epidemiology literature. Crit. Rev. Toxicol., 40, 189–244.
- Gendre, C., Lafontaine, M., Delsaut, P., and Simon, P. (2004). Exposure to polycyclic aromatic hydrocarbons and excretion of urinary 3-hydroxybenzo[A]pyrene: assessment of an appropriate sampling time. Polycyclic Aromat. Compd., 24, 433–439.
- Gertler, A.W., Gilles, J.A., Pierson, W.R., Rogers, C.F., Sagebiel, J.C., Abu-Allaban, M., Coulombe, W., Tarnay, L., and Cahill, T.A. (2002). Real-world particulate matter and gaseous emissions from motor vehicles in a highway tunnel. In Emissions from diesel and gasoline engines measured in highway tunnels (pp. 5–56). Boston, MA: Health Effects Institute.
- Giannouli, M., Kalognomou, E.A., Mellios, G., Moussiopoulos, N., Samaras, Z., and Fiala, J. (2011). Impact of European emission control strategies on urban and local air quality. Atmos. Environ., 45, 4753–4762.
- Giechaskiel, B., Ntziachristos, L., Samaras, Z., Scheer, V., Casati, R., and Vogt, R. (2005). Formation potential of vehicle exhaust nucleation mode particles on-road and in the laboratory. Atmos. Environ., 39, 3191–3198.
- Gil, F., and Pla, A. (2001). Biomarkers as biological indicators of xenobiotic exposure. J. Appl. Toxicol., 21, 245–255.
- Godschalk, R.W. L., Van Schooten, F.J., and Bartsch, H. (2003). A critical evaluation of DNA adducts as biological markers for human exposure to polycyclic aromatic compounds. J. Biochem. Molec. Biol., 36, 1–11.
- Gould, T., Larson, T., Stewart, J., Kaufman, J.D., Slater, D., and McEwen, N. (2008). A controlled inhalation diesel exhaust exposure facility with dynamic feedback control of PM concentration. Inhal. Toxicol., 20, 49–52.
- Graboski, M.S., McCormick, R.L., Alleman, T.L., and Herring, A.M. (2003). The effect of biodiesel composition on engine emissions from a DDC Series 60 Diesel Engine. . Report NREL/SR-510–31461: United States Department of Energy, National Renewable Energy Lab.
- Grahame, T.J. (2009). Does improved exposure information for PM2.5 constituents explain differing results among epidemiological studies? Inhal. Toxicol., 21, 381–393.
- Granath, F., Ehrenberg, L., and Tornqvist, M. (1992). Degree of alkylation of macromolecules in vivo from variable exposure. Mutat. Res., 284, 297–306.
- Grosjean, D., Grosjean, E., and Gertler, A.W. (2001). On-road emissions of carbonyls from light-duty and heavy-duty vehicles. Environ. Sci. Technol., 35, 45–53.
- Guarieiro, L.L. N., de Souza, A.F., Torres, E.A., and de Andrade, J.B. (2009). Emission profile of 18 carbonyl compounds, CO, CO2, and NOx emitted by a diesel engine fuelled with diesel and ternary blends containing diesel, ethanol and biodiesel or vegetable oils. Atmos. Environ., 43, 2754–2761.
- Gundel, J., Mannschreck, C., Buttner, K., Ewers, U., and Angerer, J. (1996). Urinary levels of 1-hydroxypyrene, 1-, 2-, 3-, and 4-hydroxyphenanthrene in females living in an industrial area of Germany. Arch. Environ. Contam. Toxicol., 31, 585–590.
- Haas, M.J., Scott, K.M., Alleman, T.L., and McCormick, R.L. (2001). Engine performance of biodiesel fuel prepared from soybean soapstock: A high quality renewable fuel produced from a waste feedstock. Energy Fuels, 15, 1207–1212.
- Hallquist, M., Wenger, J.C., Baltensperger, U., Rudich, Y., Simpson, D., Claeys, M., Dommen, J., Donahue, N.M., George, C., Goldstein, A.H., Hamilton, J.F., Herrmann, H., Hoffmann, T., Iinuma, Y., Jang, M., Jenkin, M.E., Jimenez, J.L., Kiendler-Scharr, A., Maenhaut, W., McFiggans, G., Mentel, T.F., Monod, A., Prevot, A.S. H., Seinfeld, J.H., Surratt, J.D., Szmigielski, R., and Wildt, J. (2009). The formation, properties and impact of secondary organic aerosol: current and emerging issues. Atmos. Chem. Phys., 9, 5155–5236.
- Hansen, A.M., Mathiesen, L., Pedersen, M., and Knudsen, L.E. (2008). Urinary 1-hydroxypyrene (1-HP) in environmental and occupational studies – a review. Int. J. Hyg. Environ. Health, 211, 471–503.
- Happonen, M., Lahde, T., Messing, M.E., Sarjovaara, T., Larmi, M., Wallenberg, L.R., Virtanen, A., and Keskinen, J. (2010). The comparison of particle oxidation and surface structure of diesel soot particles between fossil fuel and novel renewable diesel fuel. Fuel, 89, 4008–4013.
- Hara, K., Hanaoka, T., Yamano, Y., and Itani, T. (1997). Urinary 1-hydroxypyrene levels of garbage collectors with low-level exposure to polycyclic aromatic hydrocarbons. Sci. Total Environ., 199, 159–164.
- Havey, C.D., Dane, A.J., Abbas-Hawks, C., and Voorhees, K.J. (2009). Detection of nitro-polycyclic aromatic hydrocarbons in mainstream and sidestream tobacco smoke using electron monochromator-mass spectrometry. Environ. Chem. Lett., 7, 331–336.
- Hays, S.M., Becker, R.A., Leung, H.W., Aylward, L.L., and Pyatt, D.W. (2007). Biomonitoring equivalents: A screening approach for interpreting biomonitoring results from a public health risk perspective. Regul. Toxicol. Pharmacol., 47, 96–109.
- He, C., Ge, Y.S., Tan, J.W., You, K.W., Han, X.K., and Wang, J.F. (2010). Characteristics of polycyclic aromatic hydrocarbons emissions of diesel engine fueled with biodiesel and diesel. Fuel, 89, 2040–2046.
- He, C., Ge, Y.S., Tan, J.W., You, K.W., Han, X.K., Wang, J.F., You, Q.W., and Shah, A.N. (2009). Comparison of carbonyl compounds emissions from diesel engine fueled with biodiesel and diesel. Atmos. Environ., 43, 3657–3661.
- Heeb, N.V., Schmid, P., Kohler, M., Gujer, E., Zennegg, M., Wenger, D., Wichser, A., Ulrich, A., Gfeller, U., Honegger, P., Zeyer, K., Emmenegger, L., Petermann, J.L., Czerwinski, J., Mosimann, T., Kasper, M., and Mayer, A. (2008). Secondary effects of catalytic diesel particulate filters: Conversion of PAHs versus formation of Nitro-PAHs. Environ. Sci. Technol., 42, 3773–3779.
- Hemminki, K., Zhang, L.F., Kruger, J., Autrup, H., Tornqvist, M., and Norbeck, H.E. (1994). Exposure of us and taxi drivers to urban air pollutants as measured by DNA and protein adducts. Toxicol. Lett., 72, 171–174.
- Henderson, R.F., Bechtold, W.E., Bond, J.A., and Sun, J.D. (1989). The use of biological markers in toxicology. Crit. Rev. Toxicol., 20, 65–82.
- Herner, J.D., Hu, S.H., Robertson, W.H., Huai, T., Collins, J.F., Dwyer, H., and Ayala, A. (2009). Effect of advanced aftertreatment for PM and NOx control on heavy-duty diesel truck emissions. Environ. Sci. Technol., 43, 5928–5933.
- Hesterberg, T.W., Long, C.M., Sax, S.N., Lapin, C.A., McClellan, R.O., Bunn, W.B., and Valberg, P.A. (2011). Particulate matter in new technology diesel exhaust (NTDE) is quantitatively and qualitatively very different from that found in traditional diesel exhaust (TDE). J. Air Waste Manage. Assoc., 61, 894–913.
- Himmelstein, M.W., Boogaard, P.J., Cadet, J., Farmer, P.B., Kim, J.H., Martin, E.A., Persaud, R., and Shuker, D.E. G. (2009). Creating context for the use of DNA adduct data in cancer risk assessment: II. Overview of methods of identification and quantitation of DNA damage. Crit. Rev. Toxicol., 39, 679–694.
- Ho, K.F., Lee, S.C., Ho, S.S. H., Kawamura, K., Tachibana, E., Cheng, Y., and Zhu, T. (2010). Dicarboxylic acids, ketocarboxylic acids, alpha-dicarbonyls, fatty acids, and benzoic acid in urban aerosols collected during the 2006 Campaign of Air Quality Research in Beijing (CAREBeijing-2006). J. Geophys. Res. Atmos., 115, D19312, doi:1029–2009JD013304.
- Holmen, B.A., and Ayala, A. (2002). Ultrafine PM emissions from natural gas, oxidation- catalyst diesel, and particle-trap diesel heavy-duty transit buses. Environ. Sci. Technol., 36, 5041–5050.
- Hsu, Y., and Mullen, M. (2007). Compilation of Diesel Speciation Data. . CRC Report No. E-75: Coordinating Research Council. Alpharetta, GA.
- Huang, W., Smith, T.J., Ngo, L., Wang, T., Chen, H.Q., Wu, F.G., Herrick, R.F., Christiani, D.C., and Ding, H. (2007). Characterizing and biological monitoring of polycyclic aromatic hydrocarbons in exposures to diesel exhaust. Environ. Sci. Technol., 41, 2711–2716.
- Huang, W.L., Grainger, J., Patterson, D.G., Turner, W.E., Caudill, S.P., Needham, L.L., Pirkle, J.L., and Sampson, E.J. (2004). Comparison of 1-hydroxypyrene exposure in the US population with that in occupational exposure studies. Int. Arch. Occup. Environ. Health, 77, 491–498.
- Huyck, S., Ohman-Strickland, P., Zhang, L., Tong, J., Xu, X.U., and Zhang, J.J. (2010). Determining times to maximum urine excretion of 1-aminopyrene after diesel exhaust exposure. J. Expo. Sci. Environ. Epidemiol., 20, 650–655.
- Inazu, K., Nam, V.D., Asato, T., Okochi, H., Hisamatsu, Y., Kobayashi, T., and Baba, T. (2008). Atmospheric occurrence of 2-nitrobenzanthrone associated with airborne particles in central Tokyo. Polycyclic Aromat. Compd., 28, 562–577.
- Jakober, C.A., Charles, M.J., Kleeman, M.J., and Green, P.G. (2006). LC-MS analysis of carbonyl compounds and their occurrence in diesel emissions. Anal. Chem., 78, 5086–5093.
- Jakober, C.A., Riddle, S.G., Robert, M.A., Destaillats, H., Charles, M.J., Green, P.G., and Kleeman, M.J. (2007). Quinone emissions from gasoline and diesel motor vehicles. Environ. Sci. Technol., 41, 4548–4554.
- Jakober, C.A., Robert, M.A., Riddle, S.G., Destaillats, H., Charles, M.J., Green, P.G., and Kleeman, M.J. (2008). Carbonyl emissions from gasoline and diesel motor vehicles. Environ. Sci. Technol., 42, 4697–4703.
- Jakubowski, M., and Trzcinka-Ochocka, M. (2005). Biological monitoring of exposure: Trends and key developments. J. Occup. Health, 47, 22–48.
- Janssen, N.A. H., van Vliet, P.H. N., Aarts, F., Harssema, H., and Brunekreef, B. (2001). Assessment of exposure to traffic related air pollution of children attending schools near motorways. Atmos. Environ., 35, 3875–3884.
- Jarvholm, B., and Reuterwall, C. (2012). A comparison of occupational and non-occupational exposure to diesel exhausts and its consequences for studying health effects. Occup. Environ. Med., 69, 851–852.
- Jerrett, M., Arain, A., Kanaroglou, P., Beckerman, B., Potoglou, D., Sahsuvaroglu, T., Morrison, J., and Giovis, C. (2005). A review and evaluation of intraurban air pollution exposure models. J. Expo. Anal. Environ. Epidemiol., 15, 185–204.
- Johnson, T.V. (2006). Diesel emission control in review. . Society of Automotive Engineers World Congress, SAE International. Detroit, MI.
- Jones, K., Garfitt, S., Emms, V., Warren, N., Cocker, J., and Farmer, P. (2006). Correlation of haemoglobin-acrylamide adducts with airborne exposure: An occupational survey. Toxicol. Lett., 162, 174–180.
- Jongeneelen, F.J. (2001). Benchmark guideline for urinary 1-hydroxypyrene as biomarker of occupational exposure to polycyclic aromatic hydrocarbons. Ann. Occup. Hyg., 45, 3–13.
- Jung, J.S., Tsatsral, B., Kim, Y.J., and Kawamura, K. (2010). Organic and inorganic aerosol compositions in Ulaanbaatar, Mongolia, during the cold winter of 2007 to 2008: Dicarboxylic acids, ketocarboxylic acids, and alpha-dicarbonyls. J. Geophys. Res. Atmos., 115, D22203. doi:10.1029/2010JD014339.
- Karahalil, B., Burgaz, S., Fisek, G., and Karakaya, A.E. (1998). Biological monitoring of young workers exposed to polycyclic aromatic hydrocarbons in engine repair workshops. Mutat. Res. Genet. Toxicol., 412, 261–269.
- Karavalakis, G., Deves, G., Fontaras, G., Stournas, S., Samaras, Z., and Bakeas, E. (2010). The impact of soy-based biodiesel on PAH, nitro-PAH and oxy-PAH emissions from a passenger car operated over regulated and nonregulated driving cycles. Fuel, 89, 3876–3883.
- Karavalakis, G., Stournas, S., and Bakeas, E. (2009). Light vehicle regulated and unregulated emissions from different biodiesels. Sci. Total Environ., 407, 3338–3346.
- Kawamura, K., and Kaplan, I.R. (1987). Motor exhaust emissions as a primary source for dicarboxylic acids in Los Angeles ambient air. Environ. Sci. Technol., 21, 105–110.
- Kawanaka, Y., Sakamoto, K., Wang, N., and Yun, S.J. (2007). Simple and sensitive method for determination of nitrated polycyclic aromatic hydrocarbons in diesel exhaust particles by gas chromatography-negative ion chemical ionisation tandem mass spectrometry. J. Chromatogr. A, 1163, 312–317.
- Khalek, I.A., Bougher, T.L., Merritt, P.M., and Zielinska, B. (2011). Regulated and unregulated emissions from highway heavy-duty diesel engines complying with U.S. Environmental Protection Agency 2007 emissions standards. J. Air Waste Manag. Assoc., 61, 427–442.
- Kim, D., Kumfer, B.M., Anastasio, C., Kennedy, I.M., and Young, T.M. (2009). Environmental aging of polycyclic aromatic hydrocarbons on soot and its effect on source identification. Chemosphere, 76, 1075–1081.
- Kim, H., Cho, S.H., Kang, J.W., Kim, Y.D., Nan, H.M., Lee, C.H., Lee, H., and Kawamoto, T. (2001). Urinary 1-hydroxypyrene and 2-naphthol concentrations in male Koreans. Int. Arch. Occup. Environ. Health, 74, 59–62.
- Kittelson, D.B. (1998). Engines and nanoparticles: A review. J. Aerosol Sci., 29, 575–588.
- Kittelson, D.B., Watts, W.F., Johnson, J.P., Rowntree, C., Payne, M., Goodier, S., Warrens, C., Preston, H., Zink, U., Ortiz, M., Goersmann, C., Twigg, M.V., Walker, A.P., and Caldow, R. (2006). On-road evaluation of two diesel exhaust aftertreatment devices. J. Aerosol Sci., 37, 1140–1151.
- Knudsen, L.E., Gaskell, M., Martin, E.A., Poole, J., Scheepers, P.T. J., Jensen, A., Autrup, H., and Farmer, P.B. (2005). Genotoxic damage in mine workers exposed to diesel exhaust, and the effects of glutathione transferase genotypes. Mutat. Res. Genet. Tox., 583, 120–132.
- Kojima, Y., Inazu, K., Hisamatsu, Y., Okochi, H., Baba, T., and Nagoya, T. (2010). Influence of secondary formation on atmospheric occurrences of oxygenated polycyclic aromatic hydrocarbons in airborne particles. Atmos. Environ., 44, 2873–2880.
- Krahl, J., Munack, A., Schroder, O., Stein, H., and Bunger, J. (2003). Influence biodiesel and different designed diesel fuels on the exhaust gas emissions and health effects. . Report SAE 2003–01–3199: Society of Automotive Engineers. Warrendale, PA.
- Kuusimaki, L., Peltonen, Y., Mutanen, P., Peltonen, K., and Savela, K. (2004). Urinary hydroxy-metabolites of naphthalene, phenanthrene and pyrene as markers of exposure to diesel exhaust. Int. Arch. Occup. Environ. Health, 77, 23–30.
- Lafontaine, M., Champmartin, C., Simon, P., Delsaut, P., and Funck-Brentano, C. (2006). 3-Hydroxybenzo[a]pyrene in the urine of smokers and non-smokers. Toxicol. Lett., 162, 181–185.
- Lafontaine, M., Gendre, C., Delsaut, P., and Simon, P. (2004). Urinary 3-hydroxybenzo[A]pyrene as a biomarker of exposure to polycyclic aromatic hydrocarbons: An approach for determining a biological limit value. Polycyclic Aromat. Compd., 24, 441–450.
- Lambe, A.T., Miracolo, M.A., Hennigan, C.J., Robinson, A.L., and Donahue, N.M. (2009). Effective rate constants and uptake coefficients for the reactions of organic molecular markers (n-alkanes, hopanes, and steranes) in motor oil and diesel primary organic aerosols with hydroxyl radicals. Environ. Sci. Technol., 43, 8794–8800.
- Lanni, T., Chatterjee, S., Conway, R., Windawi, H., Rosenblatt, D., Bush, C., Lowell, D., Evans, J., and McLean, R. (2001). Performance and durability evaluation of continuously regenerating particulate filters on diesel powered urban buses at NY city transit. . Report SAE 2001–01–0511: Society of Automotive Engineers. Warrendale, PA.
- Lapuerta, M., Armas, O., and Rodriguez-Fernandez, J. (2008). Effect of biodiesel fuels on diesel engine emissions. Prog. Energy Combust. Sci., 34, 198–223.
- Laumann, S., Micic, V., Kruge, M.A., Achten, C., Sachsenhofer, R.F., Schwarzbauer, J., and Hofmann, T. (2011). Variations in concentrations and compositions of polycyclic aromatic hydrocarbons (PAHs) in coals related to the coal rank and origin. Environ. Pollut., 159, 2690–2697.
- Laumbach, R., Tong, J., Zhang, L., Ohman-Stickland, P., Stern, A., Fiedler, N., Kipen, H., Kelly-NcNeil, K., Lioy, P., and Zhang, J. (2009). Quantification of 1-aminopyrene in human urine after a controlled exposure to diesel exhaust. J. Environ. Monitor., 11, 153–159.
- Layshock, J.A., Wilson, G., and Anderson, K.A. (2010). Ketone and quinone-substituted polycyclic aromatic hydrocarbons in mussel tissue, sediment, urban dust, and diesel particulate matrices. Environ. Toxicol. Chem., 29, 2450–2460.
- Lee, C.Y., Lee, J.Y., Kang, J.W., and Kim, H. (2001). Effects of genetic polymorphisms of CYP1A1, CYP2E1, GSTM1, and GSTT1 on the urinary levels of 1-hydroxypyrene and 2-naphthol in aircraft maintenance workers. Toxicol. Lett., 123, 115–124.
- Legreid, G., Reimann, S., Steinbacher, M., Staehelin, J., Young, D., and Stemmler, K. (2007). Measurements of OVOCs and NMHCs in a swiss highway tunnel for estimation of road transport emissions. Environ. Sci. Technol., 41, 7060–7066.
- Leotz-Gartziandia, E., Tatry, V., and Carlier, P. (2000). Sampling and analysis of polycyclic aromatic hydrocarbons (PAH) and oxygenated PAH in diesel exhaust and ambient air. Polycyclic Aromat. Compd., 20, 245–258.
- Leung, D.Y. C., Luo, Y., and Chan, T.L. (2006). Optimization of exhaust emissions of a diesel engine fuelled with biodiesel. Energy Fuels, 20, 1015–1023.
- Lewtas, J. (2007). Air pollution combustion emissions: Characterization of causative agents and mechanisms associated with cancer, reproductive, and cardiovascular effects. Mutat. Res. Rev. Mutat., 636, 95–133.
- Li, Z., Romanoff, L.C., Trinidad, D.A., Hussain, N., Jones, R.S., Porter, E.N., Patterson, D.G., and Sjodin, A. (2006). Measurement of urinary monohydroxy polycyclic aromatic hydrocarbons using automated liquid-liquid extraction and gas chromatography/isotope dilution high-resolution mass spectrometry. Anal. Chem., 78, 5744–5751.
- Liang, F.Y., Lu, M.M., Birch, M.E., Keener, T.C., and Liu, Z.F. (2006). Determination of polycyclic aromatic sulfur heterocycles in diesel particulate matter and diesel fuel by gas chromatography with atomic emission detection. J. Chromatogr. A, 1114, 145–153.
- Lim, M.C. H., Ayoko, G.A., Morawska, L., Ristovski, Z.D., and Jayaratne, E.R. (2005). Effect of fuel composition and engine operating conditions on polycyclic aromatic hydrocarbon emissions from a fleet of heavy-duty diesel buses. Atmos. Environ., 39, 7836–7848.
- Lin, P.H., Chen, D.R., Wang, T.W., Lin, C.H., and Chuang, M.C. (2009). Investigation of the cumulative tissue doses of naphthoquinones in human serum using protein adducts as biomarker of exposure. Chem. Biol. Interact., 181, 107–114.
- Lipsky, E.M., and Robinson, A.L. (2006). Effects of dilution on fine particle mass and partitioning of semivolatile organics in diesel exhaust and wood smoke. Environ. Sci. Technol., 40, 155–162.
- Liu, L.J. S., Phuleria, H.C., Webber, W., Davey, M., Lawson, D.R., Ireson, R.G., Zielinska, B., Ondov, J.M., Weaver, C.S., Lapin, C.A., Easter, M., Hesterberg, T.W., and Larson, T. (2010). Quantification of self pollution from two diesel school buses using three independent methods. Atmos. Environ., 44, 3422–3431.
- Lloyd, A.C., and Cackette, T.A. (2001). Diesel engines: Environmental impact and control. J. Air Waste Manage. Assoc., 51, 809–847.
- Maina, G., Manzari, M., Palmas, A., Passini, V., and Filon, F.L. (2007). Risk assessment of occupational exposure to polycyclic aromatic hydrocarbons by means of urinary 1-hydroxypyrene. Toxicol. Ind. Health, 23, 55–59.
- Manabe, S., Izumikawa, S., Asakuni, K., Wada, O., and Kanai, Y. (1991). Detection of carcinogenic amino-alpha-carbolines and amono-gamma-carbolines in diesel-exhaust particles. Environ. Pollut., 70, 255–265.
- Manini, P., De Palma, G., and Mutti, A. (2007). Exposure assessment at the workplace: Implications of biological variability. Toxicol. Lett., 168, 210–218.
- Marchant, C.A., Briggs, K.A., and Long, A. (2008). In silico tools for sharing data and knowledge on toxicity and metabolism: Derek for windows, meteor, and vitic. Toxicol. Mech. Methods, 18, 177–187.
- Mauderly, J.L., and Chow, J.C. (2008). Health effects of organic aerosols. Inhal. Toxicol., 20, 257–288.
- McClellan, R.O., Hesterberg, T.W., and Wall, J.C. (2012). Evaluation of carcinogenic hazard of diesel engine exhaust needs to consider revolutionary changes in diesel technology. Regul. Toxicol. Pharmacol., 63, 225–258.
- McCreanor, J., Cullinan, P., Nieuwenhuijsen, M.J., Stewart-Evans, J., Malliarou, E., Jarup, L., Harrington, R., Svartengren, M., Han, I., Ohman-Strickland, P., Chung, K.F., and Zhang, J.F. (2007). Respiratory effects of exposure to diesel traffic in persons with asthma. N. Engl. J. Med., 357, 2348–2358.
- Migliaccio, C.T., Bergauff, M.A., Palmer, C.P., Jessop, F., Noonan, C.W., and Ward, T.J. (2009). Urinary levoglucosan as a biomarker of wood smoke exposure: Observations in a mouse model and in children. Environ. Health Perspect., 117, 74–79.
- Miller-Schulze, J.P., Paulsen, M., Kameda, T., Toriba, A., Tang, N., Tamura, K., Dong, L., Zhang, X., Hayakawa, K., Yost, M.G., and Simpson, C.D. (2013). Evaluation of urinary metabolites of 1-nitropyrene as biomarkers for exposure to diesel exhaust in taxi drivers of Shenyang, China. J. Expo. Anal. Environ. Epidemiol., 23, 170–175.
- Miller-Schulze, J.P., Paulsen, M., Toriba, A., Hayakawa, K., and Simpson, C.D. (2007). Analysis of 1-nitropyrene in air particulate matter standard reference materials by using two-dimensional high performance liquid chromatography with online reduction and tandem mass spectrometry detection. J. Chromatogr. A, 1167, 154–160.
- Miller-Schulze, J.P., Paulsen, M., Toriba, A., Tang, N., Hayakawa, K., Tamura, K., Dong, L.J., Zhang, X.M., and Simpson, C.D. (2010). Exposures to particulate air pollution and nitro-polycyclic aromatic hydrocarbons among taxi drivers in Shenyang, China. Environ. Sci. Technol., 44, 216–221.
- Millstein, D.E., and Harley, R.A. (2010). Effects of retrofitting emission control systems on in-use heavy diesel vehicles. Environ. Sci. Technol., 44, 5042–5048.
- Miracolo, M.A., Presto, A.A., Lambe, A.T., Hennigan, C.J., Donahue, N.M., Kroll, J.H., Worsnop, D.R., and Robinson, A.L. (2010). Photo-oxidation of low-volatility organics found in motor vehicle emissions: Production and chemical evolution of organic aerosol mass. Environ. Sci. Technol., 44, 1638–1643.
- Mirivel, G., Riffault, V., and Galloo, J.C. (2010). Simultaneous determination by ultra-performance liquid chromatography-atmospheric pressure chemical ionization time-of-flight mass spectrometry of nitrated and oxygenated PAHs found in air and soot particles. Anal. Bioanal. Chem., 397, 243–256.
- MSHA. (2001). Diesel Particulate Matter Exposure of Coal Miners; Proposed Rule. Mine Safety and Health Administration. 30 CFR Part 72. Fed. Regist., 30, 5526.
- MSHA. (2005). Diesel Particulate Matter Exposure of Underground Metal and Nonmetal Miners; Final Rule. Mine Safety and Health Administration. 30 CFR Part 57. Fed. Regist., 70, 32868.
- Mullen, M. (2010). Diesel Unregulated Emissions Characterization. . CRC Report No. E-75–2: Coordinating Research Council. Alpharetta, GA.
- Murakami, M., Yamada, J., Kumata, H., and Takada, H. (2008). Sorptive behavior of nitro-PAHs in street runoff and their potential as indicators of diesel vehicle exhaust particles. Environ. Sci. Technol., 42, 1144–1150.
- Murashov, V., Engel, S., Savolainen, K., Fullam, B., Lee, M., and Kearns, P. (2009). Occupational safety and health in nanotechnology and Organisation for Economic Cooperation and Development. J. Nanopart. Res., 11, 1587–1591.
- Murillo-Tovar, M.A., Amador-Munoz, O., Villalobos-Pietrini, R., and Marriott, P.J. (2010). Selective separation of oxy-PAH from n-alkanes and PAH in complex organic mixtures extracted from airborne PM2.5. Chromatographia, 72, 913–921.
- Murugesan, A., Umarani, C., Subramanian, R., and Nedunchezhian, N. (2009). Bio-diesel as an alternative fuel for diesel engines-A review. Renew. Sus. Energy Rev., 13, 653–662.
- Nagy, E., Adachi, S., Takamura-Enya, T., Zeisig, M., and Moller, L. (2007). DNA adduct formation and oxidative stress from the carcinogenic urban air pollutant 3-nitrobenzanthrone and its isomer 2-nitrobenzanthrone, in vitro and in vivo. Mutagenesis, 22, 135–145.
- Needham, L.L., Calafat, A.M., and Barr, D.B. (2007). Uses and issues of biomonitoring. Int. J. Hyg. Environ. Health, 210, 229–238.
- Nelson, P.F., Tibbett, A.R., and Day, S.J. (2008). Effects of vehicle type and fuel quality on real world toxic emissions from diesel vehicles. Atmos. Environ., 42, 5291–5303.
- Nielsen, P.S., Andreassen, A., Farmer, P.B., Ovrebo, S., and Autrup, H. (1996). Biomonitoring of diesel exhaust-exposed workers. DNA and hemoglobin adducts and urinary 1-hydroxypyrene as markers of exposure. Toxicol. Lett., 86, 27–37.
- Ning, Z., Cheung, C.S., and Liu, S.X. (2004). Experimental investigation of the effect of exhaust gas cooling on diesel particulate. J. Aerosol Sci., 35, 333–345.
- Ning, Z., and Sioutas, C. (2010). Atmospheric processes influencing aerosols generated by combustion and the inference of their impact on public exposure: A review. Aerosol Air Qual. Res., 10, 43–58.
- Nishino, N., Atkinson, R., and Arey, J. (2008). Formation of nitro products from the gas-phase OH radical-initiated reactions of toluene, naphthalene, and biphenyl: Effect of NO2 concentration. Environ. Sci. Technol., 42, 9203–9209.
- NIST. (2006). Certificate of Analysis. Standard Reference Material® 1650b. Diesel Particulate Matter. United States Department of Commerce, National Institute of Standards and Technology. . Gaithersburg, MD.
- Noll, J.D., Timko, R.J., McWilliams, L., Hall, P., and Haney, R. (2005). Sampling results of the improved SKC diesel particulate matter cassette. J. Occup. Environ. Health, 2, 29–37.
- Oda, J., Maeda, I., Mori, T., Yasuhara, A., and Saito, Y. (1998). The relative proportions of polycyclic aromatic hydrocarbons and oxygenated derivatives in accumulated organic particulates as affected by air pollution sources. Environ. Technol., 19, 961–976.
- OECD. (2009). Preliminary Analysis of Exposure Measurement and Exposure Mitigation in Occupational Settings: Manufactured Nanomaterials. Series on the Safety of Manufactured Nanomaterials. . Report No. 8 : Organisation for Economic Co-operation and Development, Environment Directorate. Paris, France.
- Ogawa, M., Oyama, T., Isse, T., Yamaguchi, T., Murakami, T., Endo, Y., and Kawamoto, T. (2006). Hemoglobin adducts as a marker of exposure to chemical substances, especially PRTR class I designated chemical substances. J. Occup. Health, 48, 314–328.
- Ono-Ogasawara, M., and Smith, T.J. (2004). Diesel exhaust particles in the work environment and their analysis. Ind. Health, 42, 389–399.
- OSHA. (2005). Chemical Sampling Information (CSI) [online]. Diesel Exhaust United States Department of Labor. Occupational Safety & Health Administration. Salt Lake Technical Center. Sandy, UT. . http://www.osha.gov/dts/chemicalsampling/data/CH_234650.html. . (accessed November 2010).
- Oukebdane, K., Portet-Koltalo, F., Machour, N., Dionnet, F., and Desbene, P.L. (2010). Comparison of hot Soxhlet and accelerated solvent extractions with microwave and supercritical fluid extractions for the determination of polycyclic aromatic hydrocarbons and nitrated derivatives strongly adsorbed on soot collected inside a diesel particulate filter. Talanta, 82, 227–236.
- Ozaki, N., Takemoto, N., and Kindaichi, T. (2010). Nitro-PAHs and PAHs in atmospheric particulate matters and sea sediments in Hiroshima Bay area, Japan. Water Air Soil Pollut., 207, 263–271.
- Palli, D., Russo, A., Masala, G., Saieva, C., Guarrera, S., Carturan, S., Munnia, A., Matullo, G., and Peluso, M. (2001). DNA adduct levels and DNA repair polymorphisms in traffic-exposed workers and a general population sample. Int. J. Cancer, 94, 121–127.
- Park, S., Kim, H., and Choi, B. (2009). Emission characteristics of exhaust gases and nanoparticles from a diesel engine with biodiesel-diesel blended fuel (BD20). J. Mech. Sci. Technol., 23, 2555–2564.
- Payri, F., Bermudez, V.R., Tormos, B., and Linares, W.G. (2009). Hydrocarbon emissions speciation in diesel and biodiesel exhausts. Atmos. Environ., 43, 1273–1279.
- Phousongphouang, P.T., and Arey, J. (2003). Sources of the atmospheric contaminants, 2-nitrobenzanthrone and 3-nitrobenzanthrone. Atmos. Environ., 37, 3189–3199.
- Pohjola, S.K., Savela, K., Kuusimaki, L., Kanno, T., Kawanishi, M., and Weyand, E. (2004). Polycyclic aromatic hydrocarbons of diesel and gasoline exhaust and DNA adduct detection in calf thymus DNA and lymphocyte DNA of workers exposed to diesel exhaust. Polycyclic Aromat. Compd., 24, 451–465.
- Prasad, R., and Bella, V.R. (2010). A review on diesel soot emission, its effect and control. Bull. Chem. React. Eng. Catal., 5, 69–86.
- Qu, S.X., Leigh, J., Koelmeyer, H., and Stacey, N.H. (1997). DNA adducts in coal miners: Association with exposures to diesel engine emissions. Biomarkers, 2, 95–102.
- Raheman, H., and Ghadge, S.V. (2008). Performance of diesel engine with biodiesel at varying compression ratio and ignition timing. Fuel, 87, 2659–2666.
- Rakopoulos, C.D., Rakopoulos, D.C., Hountalas, D.T., Giakoumis, E.G., and Andritsakis, E.C. (2008). Performance and emissions of bus engine using blends of diesel fuel with bio-diesel of sunflower or cottonseed oils derived from Greek feedstock. Fuel, 87, 147–157.
- Rappaport, S.M., Waidyanatha, S., Qu, Q.S., Shore, R., Jin, X.M., Cohen, B., Chen, L.C., Melikian, A.A., Li, G.L., Yin, S.N., Yan, H.F., Xu, B.H., Mu, R.D., Li, Y.Y., Zhang, X.L., and Li, K.Q. (2002). Albumin adducts of benzene oxide and 1,4-benzoquinone as measures of human benzene metabolism. Cancer Res., 62, 1330–1337.
- Rappaport, S.M., Waidyanatha, S., and Serdar, B. (2004). Naphthalene and its biomarkers as measures of occupational exposure to polycyclic aromatic hydrocarbons. J. Environ. Monitor., 6, 413–416.
- Ratcliff, M.A., Dane, A.J., Williams, A., Ireland, J., Luecke, J., McCormick, R.L., and Voorhees, K.J. (2010). Diesel particle filter and fuel effects on heavy-duty diesel engine emissions. Environ. Sci. Technol., 44, 8343–8349.
- Reisen, F., and Arey, J. (2005). Atmospheric reactions influence seasonal PAH and nitro-PAH concentrations in the Los Angeles basin. Environ. Sci. Technol., 39, 64–73.
- Ris, C. (2007). US EPA health assessment for diesel engine exhaust: A review. Inhal. Toxicol., 19, 229–239.
- Ryan, P.H., LeMasters, G.K., Levin, L., Burkle, J., Biswas, P., Hu, S.H., Grinshpun, S., and Reponen, T. (2008). A land-use regression model for estimating microenvironmental diesel exposure given multiple addresses from birth through childhood. Sci. Total Environ., 404, 139–147.
- Samy, S., and Zielinska, B. (2010). Secondary organic aerosol production from modern diesel engine emissions. Atmos. Chem. Phys., 10, 609–625.
- Sarigiannis, D.A., and Saisana, M. (2008). Multi-objective optimization of air quality monitoring. Environ. Monit. Assess., 136, 87–99.
- Sauvain, J.J., Duc, T.V., and Guillemin, M. (2003). Exposure to carcinogenic polycyclic aromatic compounds and health risk assessment for diesel-exhaust exposed workers. Int. Arch. Occup. Environ. Health, 76, 443–455.
- Sawant, A.A., Shah, S.D., Zhu, X.N., Miller, J.W., and Cocker, D.R. (2007). Real-world emissions of carbonyl compounds from in-use heavy-duty diesel trucks and diesel Back-Up Generators (BUGS). Atmos. Environ., 41, 4535–4547.
- Schauer, C., Niessner, R., and Poschl, U. (2004). Analysis of nitrated polycyclic aromatic hydrocarbons by liquid chromatography with fluorescence and mass spectrometry detection: air particulate matter, soot, and reaction product studies. Anal. Bioanal. Chem., 378, 725–736.
- Schauer, J.J., Kleeman, M.J., Cass, G.R., and Simoneit, B.R. T. (1999). Measurement of emissions from air pollution sources. 2. C-1 through C-30 organic compounds from medium duty diesel trucks. Environ. Sci. Technol., 33, 1578–1587.
- Schauer, J.J., Kleeman, M.J., Cass, G.R., and Simoneit, B.R. T. (2002). Measurement of emissions from air pollution sources. 4. C-1-C-27 organic compounds from cooking with seed oils. Environ. Sci. Technol., 36, 567–575.
- Scheepers, P.T. J. (2008). The use of biomarkers for improved retrospective exposure assessment in epidemiological studies: Summary of an ECETOC workshop. Biomarkers, 13, 734–748.
- Scheepers, P.T. J., Anzion, R., Micka, V., Poole, J., Coggon, D., and Bos, R.R. (2004). Evaluation of the use of 1-nitropyrene, pyrene, and 1-hydroxypyrene as markers of diesel exhaust exposure in underground mining: Results from the biomodem study. Polycyclic Aromat. Compd., 24, 405–417.
- Scheepers, P.T. J., Coggon, D., Knudsen, L.E., Anzion, R., Autrup, H., Bogovski, S., Bos, R.P., Dahmann, D., Farmer, P., Martin, E.A., Micka, V., Muzyka, V., Neumann, H.G., Poole, J., Schmidt-Ott, A., Seiler, F., Volf, J., and Zwirner-Baier, I. (2002). Biomarkers for occupational diesel exhaust exposure monitoring (BIOMODEM) – a study in underground mining. Toxicol. Lett., 134, 305–317.
- Scheepers, P.T. J., Micka, V., Muzyka, V., Anzion, R., Dahmann, D., Poole, J., and Bos, R.P. (2003). Exposure to dust and particle-associated 1-nitropyrene of drivers of diesel-powered equipment in underground mining. Ann. Occup. Hyg., 47, 379–388.
- Scheepers, P.T. J., and Vermeulen, R.C. H. (2012). Diesel engine exhaust classified as a human lung carcinogen. How will this affect occupational exposures? Occup. Environ. Med., 69, 691–693.
- Scherer, G. (2005). Biomonitoring of inhaled complex mixtures – ambient air, diesel exhaust and cigarette smoke. Exp. Toxicol. Pathol., 57, 75–110.
- Schoket, B. (1999). DNA damage in humans exposed to environmental and dietary polycyclic aromatic hydrocarbons. Mutat. Res. Fund. Mol., 424, 143–153.
- Schulz, C., Angerer, J., Ewers, U., and Kolossa-Gehring, M. (2007). The German Human Biomonitoring Commission. Int. J. Hyg. Environ. Health, 210, 373–382.
- Seaton, A., Tran, L., Aitken, R., and Donaldson, K. (2010). Nanoparticles, human health hazard and regulation. J. R. Soc. Interface, 7:S119–S129.
- Seidel, A., Dahmann, D., Krekeler, H., and Jacob, J. (2002). Biomonitoring of polycyclic aromatic compounds in the urine of mining workers occupationally exposed to diesel exhaust. Int. J. Hyg. Environ. Health, 204, 333–338.
- Serdar, B., Waidyanatha, S., Zheng, Y.X., and Rappaport, S.M. (2003). Simultaneous determination of urinary 1-and 2-naphthols, 3-and 9-phenanthrols, and 1-pyrenol in coke oven workers. Biomarkers, 8, 93–109.
- Shah, A.N., Yun-shan, G., and Jian-wei, T. (2009). Carbonyls emission comparison of a turbocharged diesel engine fuelled with diesel, biodiesel, and biodiesel-diesel blend. Jordan J. Mech. Ind. Eng., 3, 111–118.
- Shakya, K.M., and Griffin, R.J. (2010). Secondary organic aerosol from photooxidation of polycyclic aromatic hydrocarbons. Environ. Sci. Technol., 44, 8134–8139.
- Shibata, K., Yanagisawa, N., Tashiro, Y., Mukunashi, T., Onodera, T., and Sakamoto, K. (2010). Reduction in the emissions and toxicity of polycyclic aromatic hydrocarbons from a heavy-duty diesel engine with the latest aftertreatment devices. J. Health Sci., 56, 31–40.
- Sidhu, S., Gullett, B., Striebich, R., Klosterman, J., Contreras, J., and DeVito, M. (2005). Endocrine disrupting chemical emissions from combustion sources: diesel particulate emissions and domestic waste open burn emissions. Atmos. Environ., 39, 801–811.
- Simcox, A. (2009). Update on Ozone and PM2.5 Programs. Westborough, MA: Air and Waste Management Association-NES Fall 2009 Conference.
- Sobus, J.R., Pleil, J.D., Madden, M.C., Funk, W.E., Hubbard, H.F., and Rappaport, S.M. (2008). Identification of surrogate measures of diesel exhaust exposure in a controlled chamber study. Environ. Sci. Technol., 42, 8822–8828.
- Sobus, J.R., Waidyanatha, S., McClean, M.D., Herrick, R.F., Smith, T.J., Garshick, E., Laden, F., Hart, J.E., Zheng, Y., and Rappaport, S.M. (2009). Urinary naphthalene and phenanthrene as biomarkers of occupational exposure to polycyclic aromatic hydrocarbons. Occup. Environ. Med., 66, 99–104.
- Soderstrom, H., Hajslova, J., Kocourek, V., Siegmund, B., Kocan, A., Obiedzinski, W., Tysklind, M., and Bergqvist, P.A. (2005). PAHs and nitrated PAHs in air of five European countries determined using SPMDs as passive samplers. Atmos. Environ., 39, 1627–1640.
- Stiborova, M., Martinek, V., Svobodova, M., Sistkova, J., Dvorak, Z., Ulrichova, J., Simanek, V., Frei, E., Schmeiser, H.H., Phillips, D.H., and Arlt, V.M. (2010). Mechanisms of the different DNA adduct forming potentials of the urban air pollutants 2-nitrobenzanthrone and carcinogenic 3-nitrobenzanthrone. Chem. Res. Toxicol., 23, 1192–1201.
- Su, D.S., Muller, J.O., Jentoft, R.E., Rothe, D., Jacob, E., and Schlogl, R. (2004). Fullerene-like soot from EuroIV diesel engine: Consequences for catalytic automotive pollution control. Top. Catal., 30–1, 241–245.
- Tan, P.Q., Hu, Z.Y., and Lou, D.M. (2009). Regulated and unregulated emissions from a light-duty diesel engine with different sulfur content fuels. Fuel, 88, 1086–1091.
- Toriba, A., Kitaoka, H., Dills, R.L., Mizukami, S., Tanabe, K., Takeuchi, N., Ueno, M., Kameda, T., Tang, N., Hayakawa, K., and Simpson, C.D. (2007). Identification and quantification of 1-nitropyrene metabolites in human urine as a proposed biomarker for exposure to diesel exhaust. Chem. Res. Toxicol., 20, 999–1007.
- Tornqvist, M., and Kautiainen, A. (1993). Adducted proteins for identification of endogeneous electrophiles. Environ. Health Perspect., 99, 39–44.
- Traviss, N., Thelen, B.A., Ingalls, J.K., and Treadwell, M.D. (2010). Biodiesel versus diesel: A pilot study comparing exhaust exposures for employees at a rural municipal facility. J. Air Waste Manage. Assoc., 60, 1026–1033.
- Tsai, P.J., Shih, T.S., Chen, H.L., Lee, W.J., Lai, C.H., and Liou, S.H. (2004). Urinary 1-hydroxypyrene as an indicator for assessing the exposures of booth attendants of a highway toll station to polycyclic aromatic hydrocarbons. Environ. Sci. Technol., 38, 56–61.
- Tschoeke, H., Graf, A., Stein, J., Kruger, M., Schaller, J., Breuer, N., Engeljehringer, K., and Schindler, W. (2010). Diesel Engine Exhaust Emissions. In K. Mollenhauer, and H. Tschoeke (Eds.), Handbook of diesel engines (pp. 417–485). Berlin: Springer-Verlag.
- Turrio-Baldassarri, L., Battistelli, C.L., Conti, L., Crebelli, R., De Berardis, B., Iamiceli, A.L., Gambino, M., and Iannaccone, S. (2004). Emission comparison of urban bus engine fueled with diesel oil and ‘biodiesel’ blend. Sci. Total Environ., 327, 147–162.
- Turrio-Baldassarri, L., Battistelli, C.L., and Iamiceli, A.L. (2003). Evaluation of the efficiency of extraction of PAHs from diesel particulate matter with pressurized solvents. Anal. Bioanal. Chem., 375, 589–595.
- Tzamkiozis, T., Ntziachristos, L., and Samaras, Z. (2010). Diesel passenger car PM emissions: From Euro 1 to Euro 4 with particle filter. Atmos. Environ., 44, 909–916.
- USEPA. (2011).Estimation Program Interface (EPI) Suite [online]. . United States Environmental Protection Agency, Office of Prevention, Pesticides and Toxic Substances.http://www.epa.gov/opptintr/exposure/pubs/episuite.htm (accessed March 2011).
- Vaaraslahti, K., Virtanen, A., Ristimaki, J., and Keskinen, J. (2004). Nucleation mode formation in heavy-duty diesel exhaust with and without a particulate filter. Environ. Sci. Technol., 38, 4884–4890.
- Valavanidis, A., Fiotakis, K., Vlahogianni, T., Papadimitriou, V., and Pantikaki, V. (2006). Determination of selective quinones and quinoid radicals in airborne particulate matter and vehicular exhaust particles. Environ. Chem., 3, 118–123.
- van Bekkum, Y.M., Scheepers, P.T. J., vandenBroek, P.H. H., Velders, D.D., Noordhoek, J., and Bos, R.P. (1997). Determination of hemoglobin adducts following oral administration of 1-nitropyrene to rats using gas chromatography tandem mass spectrometry. J. Chromatogr. B, 701, 19–28.
- van Bekkum, Y.M., van den Broek, P.H. H., Scheepers, P.T. J., Noordhoek, J., and Bos, R.P. (1999). Biological fate of [C-14]-l-nitropyrene in rats following intragastric administration. Chem. Biol. Interact., 117, 15–33.
- Van Roosbroeck, S., Li, R.F., Hoek, G., Lebret, E., Brunekreef, B., and Spiegelman, D. (2008). Traffic-related outdoor air pollution and respiratory symptoms in children – the impact of adjustment for exposure measurement error. Epidemiology, 19, 409–416.
- van Veen, M.P. (2001). CONSEXPO 3.0 Consumer Exposure and Uptake Models. . RIVM report 612810011: National Institute of Public Health and the Environment.
- Vasconcellos, P.D., Sanchez-Ccoyllo, O., Balducci, C., Mabilia, R., and Cecinato, A. (2008). Occurrence and concentration levels of Nitro-PAH in the air of three Brazilian cities experiencing different emission impacts. Water Air and Soil Pollution, 190, 87–94.
- Waidyanatha, S., Zheng, Y.X., and Rappaport, S.M. (2003). Determination of polypyclic aromatic hydrocarbons in urine of coke oven workers by headspace solid phase microextraction and gas chromatography-mass spectrometry. Chem. Biol. Interact., 145, 165–174.
- Waidyanatha, S., Zheng, Y.X., Serdar, B., and Rappaport, S.M. (2004). Albumin adducts of naphthalene metabolites as biomarkers of exposure to polycyclic aromatic hydrocarbons. Cancer Epidemiol. Biomarkers Prevent., 13, 117–124.
- Wal, R.L. V., Bryg, V.M., and Hays, M.D. (2010). Fingerprinting soot (towards source identification): Physical structure and chemical composition. J. Aerosol Sci., 41, 108–117.
- Walgraeve, C., Demeestere, K., Dewulf, J., Zimmermann, R., and Van Langenhove, H. (2010). Oxygenated polycyclic aromatic hydrocarbons in atmospheric particulate matter: Molecular characterization and occurrence. Atmos. Environ., 44, 1831–1846.
- Wang, H.B., Kawamura, K., Ho, K.F., and Lee, S.C. (2006). Low molecular weight dicarboxylic acids, ketoacids, and dicarbonyls in the fine particles from a roadway tunnel: Possible secondary production from the precursors. Environ. Sci. Technol., 40, 6255–6260.
- Wang, L., Atkinson, R., and Arey, J. (2010). Comparison of alkylnitronaphthalenes formed in NO3 and OH radical-initiated chamber reactions with those observed in ambient air. Environ. Sci. Technol., 44, 2981–2987.
- Watson, W.P., and Mutti, A. (2004). Role of biomarkers in monitoring exposures to chemicals: present position, future prospects. Biomarkers, 9, 211–242.
- Williams, D.T., and Blanchfield, B.J. (1974). Retention, excretion and metabolism of phthalic acid administered orally to rat. Bull. Environ. Contam. Toxicol., 12, 109–112.
- Yang, H.H., Chien, S.M., Lo, M.Y., Lan, J.C. W., Lu, W.C., and Ku, Y.Y. (2007). Effects of biodiesel on emissions of regulated air pollutants and polycyclic aromatic hydrocarbons under engine durability testing. Atmos. Environ., 41, 7232–7240.
- Yanowitz, J., and McCormick, R.L. (2009). Effect of biodiesel blends on North American heavy-duty diesel engine emissions. Eur. J. Lipid Dci. Technol., 111, 763–772.
- Yassine, M.M., and Dabek-Zlotorzynska, E. (2010). Determination of benzene polycarboxylic acids in atmospheric aerosols and vehicular emissions by liquid chromatography-mass spectrometry. Anal. Method, 2, 129–134.
- Zhang, J., McCreanor, J.E., Cullinan, P., Chung, K.F., Ohlman-Strickland, P., Han, I.K., Järup, L., and Nieuwenhuijsen, M.J. (2009). Health effects of real-world exposures to diesel exhaust in persons with asthma. . Research Report No. 138. Boston, MA: Health Effects Institute.
- Zhang, K.M., and Wexler, A.S. (2004). Evolution of particle number distribution near roadways – part I: Analysis of aerosol dynamics and its implications for engine emission measurement. Atmos. Environ., 38, 6643–6653.
- Zhang, Z.H., Cheung, C.S., Chan, T.L., and Yao, C.D. (2010). Experimental investigation of regulated and unregulated emissions from a diesel engine fueled with Euro V diesel fuel and fumigation methanol. Atmos. Environ., 44, 1054–1061.
- Zielinska, B. (2005). Atmospheric transformation of diesel emissions. Exp. Toxicol. Pathol., 57, 31–42.
- Zielinska, B., Campbell, D., Lawson, D.R., Ireson, R.G., Weaver, C.S., Hesterberg, T.W., Larson, T., Davey, M., and Liu, L.J. S. (2008). Detailed characterization and profiles of crankcase and diesel particulate matter exhaust emissions using speciated organics. Environ. Sci. Technol., 42, 5661–5666.
- Zielinska, B., McDonald, J.D., and Seagrave, J. (2010). Atmospheric transformation of diesel emissions. . Research Report No. 147: Health Effects Institute. Boston, MA.
- Zielinska, B., Sagebiel, J., Arnott, W.P., Rogers, C.F., Kelly, K.E., Wagner, D.A., Lighty, J.S., Sarofim, A.F., and Palmer, G. (2004). Phase and size distribution of polycyclic aromatic hydrocarbons in diesel and gasoline vehicle emissions. Environ. Sci. Technol., 38, 2557–2567.
- Zielinska, B., and Samy, S. (2006). Analysis of nitrated polycyclic aromatic hydrocarbons. Anal. Bioanal. Chem., 386, 883–890.
- Zou, B., Wilson, J.G., Zhan, F.B., and Zeng, Y.N. (2009). Air pollution exposure assessment methods utilized in epidemiological studies. J. Environ. Monitor., 11, 475–490.
- Zwirner-Baier, I., and Neumann, H.G. (1999). Polycyclic nitroarenes (nitro-PAHs) as biomarkers of exposure to diesel exhaust. Mutat. Res. Genet. Tox., 441, 135–144.
