Abstract
We have previously demonstrated that low-density lipoprotein (LDL) can be oxidized by iron in the lysosomes of macrophages. Some of the iron content of lysosomes might be delivered through autophagy of ferritin (the main iron-storage protein in the body). We have now investigated the effects of ferritin-mediated LDL oxidation on macrophage function. The addition of ferritin to human THP-1 cells and human monocyte-derived macrophages increased lysosomal lipid peroxidation, as shown by LPO-Foam, a fluorescent probe targeted to lysosomes. Incubating THP-1 cells with ferritin and native LDL or LDL aggregated by sphingomyelinase, to allow their endocytosis and delivery to lysosomes, led to the formation of lysosomal ceroid (an advanced lipid oxidation product), indicative of lysosomal LDL oxidation. Incubating THP-1 cells with ferritin and LDL caused metabolic activation of the cells, as shown by increased extracellular acidification and oxygen consumption measured by a Seahorse analyzer. LDL oxidized by ferritin in lysosomes might be released from macrophages when the cells die and lyse and affect neighboring cells in atherosclerotic lesions. Adding LDL oxidized by ferritin at lysosomal pH (pH 4.5) to macrophages increased their intracellular reactive oxygen species formation, shown using dihydroethidium, and increased apoptosis. Ferritin might therefore contribute to LDL oxidation in the lysosomes of macrophages and have atherogenic effects.
Graphical abstract
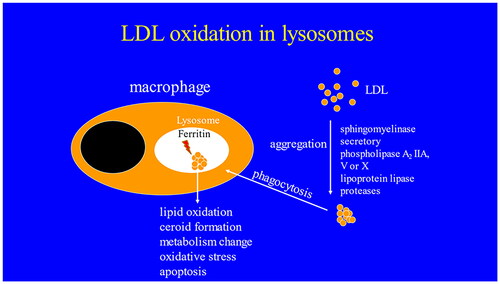
Introduction
Cardiovascular diseases are the main cause of death worldwide and atherosclerosis is the underlying cause of most cases of cardiovascular disease. Macrophages play a major role in the pathogenesis of atherosclerosis. They accumulate lipid from low-density lipoprotein (LDL) and become foam cells, secrete pro-inflammatory cytokines and proteases, which destabilize atherosclerotic lesions leading to fissure of the fibrous cap causing thrombosis and myocardial infarctions and thrombotic strokes [Citation1].
We have proposed that LDL modified and aggregated by various processes in the extracellular space of atherosclerotic lesions is phagocytosed by macrophages and then oxidized by iron inside lysosomes [Citation2]. Lysosomal oxidation of LDL increases the pH of lysosomes and increases the secretion of pro-inflammatory cytokines, all of which can be decreased by cysteamine, an antioxidant which accumulates in lysosomes [Citation3]. Cysteamine decreases the formation of atherosclerotic lesions [Citation4] and causes the regression of existing atherosclerotic lesions in LDL receptor-deficient mice [Citation5].
Ferritin is the main store of iron in the body and its iron is made available to the cell after ferritin is autophagocytosed from the cytosol into lysosomes [Citation6]. We have shown that ferritin can oxidize LDL at lysosomal pH (about pH 4.5) [Citation7] and this process might therefore contribute to LDL oxidation within macrophage lysosomes in atherosclerotic lesions.
Macrophage death by apoptosis, necrosis, pyroptosis, or ferroptosis is probably important in atherosclerosis [Citation8] and can be caused by oxidized LDL in cultured macrophages [Citation9].
Atherosclerotic lesions contain both classically activated inflammatory (M1) macrophages and alternatively activated anti-inflammatory (M2) macrophages [Citation10]. The metabolism of these macrophages differs, with M1 macrophages taking up large amounts of glucose and relying on glycolysis for energy production with impaired oxidative phosphorylation, whereas M2 macrophages exhibit increased oxidative phosphorylation and fatty acid oxidation [Citation10]. Oxidized LDL has been shown to greatly increase glycolysis in macrophages [Citation11].
In the study reported here, we show that LDL oxidation by ferritin can affect macrophage function in terms of lipid peroxidation, metabolism, reactive oxygen species generation, and apoptosis.
Materials and methods
Materials
The apoptosis kit was purchased from BioLegend, California, USA. All other laboratory chemicals and reagents were purchased from Sigma-Aldrich Ltd., Dorset, UK or Fisher Scientific Ltd., Loughborough, UK, unless otherwise indicated. Equine spleen ferritin with a protein molecular weight of 440,000 Da was obtained from Sigma.
Isolation of LDL
Native LDL (density 1.019 − 1.063 g/ml) was isolated from pooled plasma of blood taken from one to three volunteers after an overnight fast, who gave their informed consent. LDL was isolated using sequential ultracentrifugation of the pooled plasma as previously described [Citation12]. The LDL was stored at 4 °C in the presence of 100 μM EDTA for up to one month. Ethical permission to take human blood for the isolation of LDL and monocytes was obtained from the University of Reading Research Ethics Committee (Project No. 12/07).
Preparation of sphingomyelinase-aggregated LDL
LDL was aggregated by sphingomyelinase [Citation13], as described previously [Citation4].
Cell culture
THP-1 monocytes were cultured in RPMI 1640 medium containing l-glutamine, phenol red, HEPES, sodium pyruvate with low sodium bicarbonate, and high glucose. The medium was supplemented with 10% (v/v) heat-inactivated fetal calf serum (FCS), penicillin (50 UI/ml), streptomycin (50 µg/ml), and amphotericin (0.95 µg/ml). Cells were subcultured every 3–4 days. THP-1 monocytes were differentiated to adherent macrophages by incubating 300,000 cells/ml with phorbol 12-myristate 12-acetate (PMA; 25 ng/ml) for 72 h in tissue culture plastic. THP1-derived macrophages were washed with phosphate-buffered saline (PBS), re-suspended in fresh media and rested for 48 h before experiments were carried out.
Human monocytes were isolated from blood donated by healthy volunteers [Citation14]. Briefly, after isolation, the cell concentration was adjusted to 5 × 106 cells/ml in RPMI 1640 containing 0.05% (v/v) human serum and antibiotics, incubated in 6-well nonadherent plates for 40 h and then plated in adherent tissue culture plates (6-well) using RPMI 1640 containing 10% (v/v) heat-inactivated human serum and antibiotics. The media was changed every 3 days. The differentiated human monocyte-derived macrophages (HMDM) were used 10 days after taking blood.
Measurement of lysosomal lipid peroxidation
Lysosomal lipid peroxidation was measured using the lipid peroxidation probe LPO-Foam (a kind gift from Dr. Xiao, Dalian University of Technology, PR China). LPO-Foam accumulates in lysosomes and its conjugated diene moiety degrades in response to lipid peroxidation causing a spectral shift which can be detected by flow cytometry [Citation15]. THP-1 macrophages or HMDM seeded at 500,000 cells per well were treated with pre-warmed RPMI 1640 with 10% human serum (2 ml per well) alone or with ferritin (100 µg protein/ml; 0.227 µM) for 24 h. The cells were then incubated for another 24 h without ferritin but with native LDL or sphingomyelinase-modified LDL or with ferritin alone or ferritin together with native LDL or sphingomyelinase-modified LDL (all LDL concentrations were 200 µg protein/ml). The cells were washed three times with PBS (pre-warmed to 37 °C), culture media added to them, scraped into a clear 96-well round bottom microplate (Greiner CellStar) and centrifuged at 500 g at room temperature for 5 min. The cells were re-suspended in 200 µl of media (without serum) containing 2 µM Foam-LPO and incubated for 15 min at room temperature in the dark. They were then centrifuged at 500 g for 5 min at 4 °C, the supernatant was discarded and the cells re-suspended in PBS and washed three times. The cells were re-suspended in flow cytometer buffer and analyzed by flow cytometry (BD Biosciences C6 flow cytometer). FlowJo software was used to determine the mean fluorescence intensity of the two channels and the ratio of green channel (FL1) to red channel (FL2) was used as a measure of lipid peroxidation in lysosomes.
Measurement of ceroid levels in macrophages
THP-1 cells (45,000) were differentiated with PMA (25 ng/ml) on sterilized coverslips in 6-well tissue culture plates. Some cells were pretreated with ferritin (100 µg protein/ml; 0.227 µM) for 24 h. Cells were washed with PBS and incubated with RPMI 1640 containing serum and antibiotics (2 ml) with or without native LDL (200 µg protein/ml) or sphingomyelinase-modified LDL (200 µg protein/ml) for 24 h. Some of the cells also had ferritin (100 µg protein/ml) as appropriate. The cells were then washed with PBS and incubated with 2 ml of RPMI 1640 containing 10% (v/v) lipoprotein-deficient serum and antibiotics with or without ferritin (100 µg protein/ml) as appropriate. [Lipoprotein-deficient heat-inactivated FCS was prepared by ultracentrifugation at a density of 1.25 g/ml (using KBr) for 48 h at 4 °C and gav of 117,500, followed by dialysis against PBS and passage through a 0.22 µm filter.] The medium was replaced every two days for seven days. The cells were then washed with PBS and fixed with paraformaldehyde (4%, w/v) for 15 min. They were gently washed with purified water and then PBS and incubated with xylene for 5 min, rinsed with 60% (v/v) isopropanol and incubated with ethanol for 5 min to remove “soluble lipids” before being stained for 15 min with Oil Red O (0.5%, w/v, which had been passed through a 0.22 µm filter). The cells were gently rinsed with water several times and ceroid was measured by light microscopy (Axioscope 2, Carl Zeiss) with images being captured using AxioVision software and analyzed using Image J. The mean intensity of at least 50 cells was calculated.
Measurement of macrophage respiration and metabolism
THP-1 macrophages were detached using Accutase and seeded at 60,000 cells per well in an Agilent Seahorse XF 8-well cell culture miniplate in 200 µl of Seahorse XF base medium. Two wells received medium only for background correction. The cells in three wells were pretreated for 24 h with ferritin (88 µg protein/ml; 0.2 µM) in RPMI 1640 medium containing serum and antibiotics. One well continued this treatment for 24 h, whereas the other two wells received native LDL (100 µg protein/ml) for 24 h in addition to the ferritin. Two wells without ferritin pre-incubation received RPMI 1640 medium containing serum and antibiotics alone and one well received this medium and native LDL (100 µg protein/ml).
For analysis, the culture medium was replaced, washed, and incubated with XF base media containing glucose (10 mM), pyruvate (1 mM), and glutamine (2 mM) at pH 7.4 and the plate placed in a non-CO2 incubator at 37 °C for 1 h. A mitochondrial stressor mix was immediately prepared in accordance with the Agilent Seahorse XFp cell energy phenotype kit using oligomycin (2 µM) and carbonyl cyanide-4 (trifluoromethoxy) phenylhydrazone (FCCP) (2 µM). The oxygen consumption rate (OCR) and extracellular acidification rate (ECAR) were measured using a Seahorse XFp analyzer.
Determination of intracellular reactive oxygen species formation
LDL (500 µg protein/ml) was incubated with ferritin (440 µg/ml; 1 µM) at 37 °C for 24 h in 0.15 M NaCl/10 mM sodium acetate buffer, pH 4.5. LDL (500 µg protein/ml) or ferritin (440 µg/ml) in buffer of pH 4.5 were also incubated alone at 37 °C for 24 h. THP-1 macrophages (45,000 cells) on coverslips in 6-well plates were incubated for 24 h with pre-warmed RPMI 1640 media containing serum and antibiotics (2 ml per well) with or without LDL (50 µg protein/ml), ferritin (44 µg protein/ml; 0.1 µM), ferritin-oxidized LDL (50 µg protein/ml), or NaCl/sodium acetate buffer, pH 4.5. The cells were then washed twice with PBS and incubated for 30 min at 37 °C with 10 µM dihydroethidium (DHE) in PBS in an incubator without CO2 in the dark. The cells were washed twice with PBS and mounted with fluorescence mounting media containing DAPI. Images were captured using an Axio Imager (Zeiss) and the intensity of the fluorescence determined using ImageJ software.
Determination of apoptosis of macrophages
Oxidized LDL was prepared by incubating LDL (1 mg LDL protein/ml) with ferritin (880 µg protein/ml; 2 µM) at 37 °C for 24 h in NaCl/sodium acetate buffer (pH 4.5). LDL (1 mg protein/ml) and ferritin (2 µM) were incubated separately in this buffer at 37 °C for 24 h. THP-1 macrophages were incubated for 48 h with pre-warmed RPMI 1640 containing serum and antibiotics alone (2 ml per well) or containing NaCl/sodium acetate buffer or LDL (100 µg protein/ml) or ferritin (88 µg protein/ml; 0.2 µM) or ferritin-oxidized LDL (100 µg protein/ml). The medium was removed from each well and retained. The cells were detached by incubation with Accutase (750 µl) for 10 min at 37 °C. The removed medium was added to the appropriate cells and centrifuged in an Eppendorf tube at 100 g for 5 min. The pellet was washed twice with cell staining buffer. The cells were re-suspended in 800 µl annexin V binding buffer and 250 µl was transferred to a brown Eppendorf tube for protection from light. Propidium iodide (PI) (2.5 µg) and fluorescein isothiocyanate (FITC)-annexin V (0.5 µg) was added and incubated for 15 min at room temperature in the dark. The volume was made up to 500 µl and analyzed immediately using an Accuri C6 flow cytometer (BD Biosciences).
Statistics
The data are shown as the mean ± SEM of the number of independent experiments stated. Data were statistically analyzed using one-way analysis of variance (ANOVA) followed by Tukey’s post hoc test but when comparing multiple parameters, two-way ANOVA and a Bonferroni post hoc test were used. GraphPad Prism 5 software was used for all statistical analyses. The results were considered significant if p was <0.05.
Results
Ferritin promotes lipid peroxidation within lysosomes of macrophages
We tested if ferritin could increase lysosomal lipid oxidation in macrophages using the probe Foam-LPO, which can detect lipid oxidation in lysosomes [Citation15]. Ferritin was added to cultured macrophages so that it could be endocytosed by them and delivered to lysosomes (to mimic what would happen in vivo when ferritin is delivered to lysosomes by autophagy). Lysosomal lipid oxidation was increased substantially by ferritin in both human THP-1 macrophages and HMDM ().
Figure 1. Flow cytometry analysis of lipid peroxidation in macrophages. THP-1 macrophages were incubated (A) for 24 h with medium alone and then for 24 h with LDL (200 µg protein/ml) or (B) for 24 h with ferritin (100 µg protein/ml; 0.227 µM) and then for 24 h with ferritin plus LDL (200 µg protein/ml) or (C) for 24 h with ferritin and for 24 h with ferritin alone. The cells were then incubated with Foam-LPO (2 µM) for 15 min at room temperature and analyzed by flow cytometry. The fluorescence in the red channel is shown. (D and E) THP-1 macrophages or (F) human monocyte-derived macrophages were incubated alone or with ferritin (100 µg protein/ml; 0.227 µM) for 24 h. The cells were then incubated for another 24 h without ferritin but with native LDL or sphingomyelinase-modified LDL or with ferritin alone or ferritin together with native LDL or sphingomyelinase-modified LDL (all LDL concentrations were 200 µg protein/ml). The cells were then incubated with Foam-LPO (2 µM) for 15 min at room temperature and analyzed by flow cytometry. Lysosomal lipid peroxidation was quantified by the ratio of mean fluorescence intensity in the green and red channels (FL1/FL2). The control cells were not exposed to ferritin or LDL. *p < 0.05, **p < 0.01, and ***p < 0.001 compared with control cells, ≠≠p < 0.01 and ≠≠≠p < 0.001 for the shown comparison for three independent experiments.
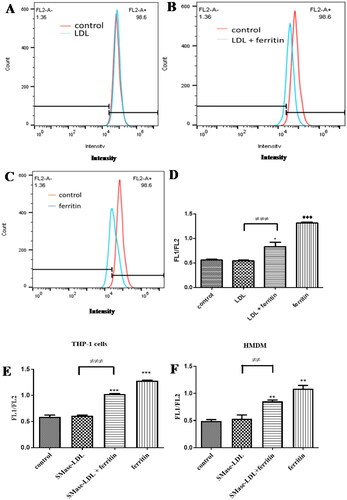
We incubated macrophages with native LDL and sphingomyelinase-modified (aggregated) LDL because aggregated LDL generated in the extracellular space of the arterial intima might well be responsible for converting macrophages into foam cells in atherosclerotic lesions [Citation16,Citation17]. Sphingomyelinase-aggregated LDL is taken up and oxidized in the lysosomes of macrophages [Citation4]. In these experiments, lysosomal lipid oxidation measured by the oxidation of Foam-LPO was not increased by native LDL or sphingomyelinase-modified (aggregated) LDL (). Native LDL and sphingomyelinase-modified LDL decreased the oxidation of Foam-LPO by ferritin possibly because the LDL competed with the Foam-LPO for oxidation by ferritin ().
Effects of ferritin on intracellular ceroid
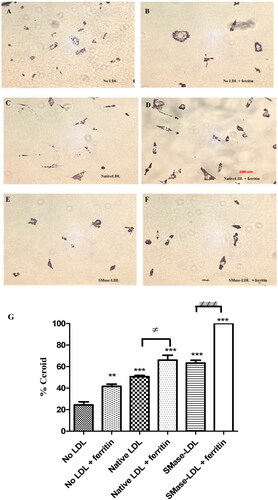
The lysosomal oxidation of LDL leads to the formation of ceroid, an advanced oxidation product of polymerized lipid and protein [Citation2]. The levels of ceroid in THP-1 macrophages were significantly increased by incubation with native LDL and more so by incubation with sphingomyelinase-aggregated LDL (). Ceroid was significantly increased in all cases when the cells were pre-incubated with ferritin to allow time for the ferritin to be endocytosed into lysosomes (). The endocytosis of ferritin and its delivery to lysosomes therefore increases the oxidation of lipids in these organelles.
Figure 2. Detection of ceroid in THP-1 macrophages. Some cells were pretreated with ferritin (100 µg protein/ml; 0.227 µM) for 24 h. The cells were then incubated for 24 h with or without native LDL (200 µg protein/ml), sphingomyelinase-modified LDL (200 µg protein/ml) or ferritin (100 µg protein/ml). They were then incubated for 7 days with medium containing 10% (v/v) lipoprotein-deficient serum with or without ferritin (100 µg protein/ml). The “soluble” lipids were removed with ethanol and xylene and the cells stained for ceroid with Oil Red O (A–F). These images are representative of three independent experiments. The levels of ceroid were quantified by ImageJ and presented as percentage ceroid compared with cells incubated with ferritin and SMase-LDL (G). The control cells (No LDL) were not exposed to ferritin or LDL. **p < 0.01 and ***p < 0.001 compared with the cells without LDL. ≠p < 0.05 and ≠≠≠p < 0.001 for the indicated comparison.
Effect of ferritin on macrophage metabolism
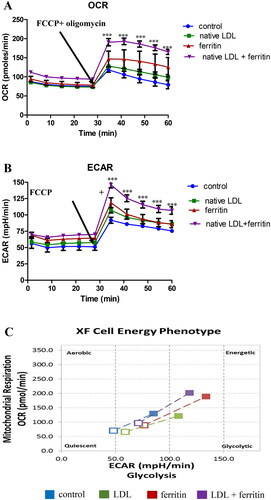
The metabolic reprogramming of energy production in macrophages might contribute to inflammation in atherosclerotic lesions [Citation10]. We therefore investigated the effect of ferritin and LDL on the metabolic energy phenotype of macrophages using Agilent Seahorse technology and the mitochondrial stress test using oligomycin to inhibit ATP synthase and FCCP as a proton uncoupler. The OCR and ECAR in cells treated with ferritin plus LDL showed considerable increases, but no significant increase was observed with LDL alone (). The results show that cells pre-incubated with ferritin, to allow its delivery to lysosomes, and then incubated with LDL were more metabolically energetic and had the capacity to consume more oxygen and produce more extracellular acids ().
Figure 3. Effect of ferritin and LDL on macrophage metabolism. THP-1 macrophages were incubated for 24 h with or without ferritin (88 µg/ml; 0.2 µM). They were then incubated for 24 h with or without native LDL (100 µg protein/ml) or ferritin (88 µg/ml). The cells were then washed and incubated with medium containing glucose, glutamine and pyruvate as fuels and analyzed using Agilent Seahorse technology to determine their metabolic phenotype before (unfilled squares) and after (filled in squares) being stressed by oligomycin and FCCP. (A) Oxygen consumption rate (OCR), (B) extracellular acidification rate (ECAR), and (C) metabolic profile are shown. The control cells were not exposed to ferritin or LDL. ***p < 0.001 compared with the control cells for four independent experiments.
Effects of ferritin-oxidized LDL on intracellular reactive oxygen species formation
Macrophage death by apoptosis or some other means occurs in atherosclerotic lesions [Citation8]. If the dead cells or cell fragments are not phagocytosed by other cells (efferocytosis), they would lyse and expose the remaining live cells to their contents, including oxidized LDL, some of which might have been oxidized by ferritin in lysosomes. We therefore incubated macrophages with LDL oxidized by ferritin at lysosomal pH to see if it would affect the function of the cells.
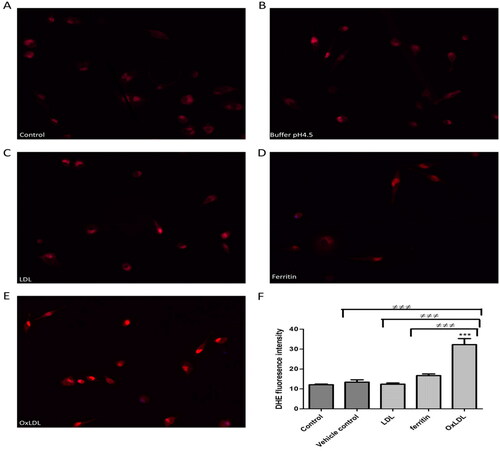
Reactive oxygen species might play an important role in atherogenesis [Citation18] and so we investigated the effect of LDL oxidized by ferritin on reactive oxygen species generation by macrophages. The intracellular level of reactive oxygen species was increased considerably by oxidized LDL, whereas native LDL and ferritin had little, if any, effect ().
Figure 4. Effect of ferritin-oxidized LDL on intracellular reactive oxygen species formation by THP-1 macrophages. Cells (45,000) were plated on cover slips and incubated for 24 h in (A) RPMI 1640 medium, serum, and antibiotics alone or containing (B) sodium acetate buffer, pH 4.5 (10% (v/v) as a vehicle control, (C) native LDL (50 µg protein/ml), (D) ferritin (44 µg/ml; 0.1 µM), or (E) ferritin-oxidized LDL (50 µg protein/ml). The cells were then washed with PBS and incubated with 10 µM DHE for 30 min in the dark at 37 °C in an incubator without CO2, washed with PBS and mounted using fluorescence mounting medium containing DAPI. (F) The fluorescence intensity was measured with an epifluorescence microscope. The control cells were not exposed to acidic buffer, LDL or ferritin. ***p < 0.001 compared with the control cells. ≠≠≠p < 0.001 for the indicated comparison for three independent experiments.
Effect of ferritin-oxidized LDL on macrophage apoptosis
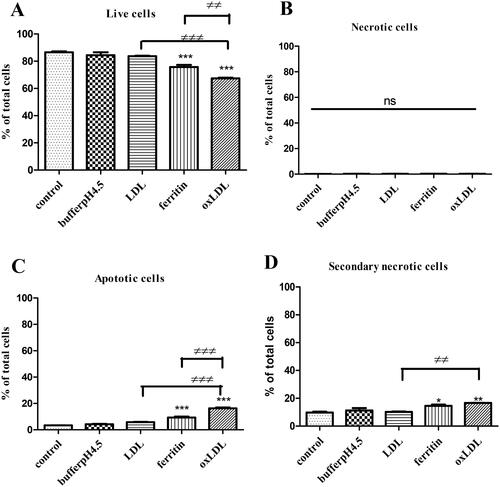
As apoptosis is important in atherosclerosis [Citation8], we investigated the effect of LDL oxidized by ferritin on cell death in macrophages because, as mentioned above, macrophages might be exposed to LDL oxidized at least partially due to ferritin when cells die and lyse in atherosclerotic lesions. Apoptosis was measured by the externalization of phosphatidylserine and necrosis by the permeabilization of the plasma membrane allowing PI to enter the cell and bind to DNA. Ferritin-oxidized LDL decreased the proportion of live cells, as did ferritin to a lesser extent (). Native LDL had no effect. Ferritin-oxidized LDL and to a lesser extent ferritin increased apoptosis (). Secondary necrosis (apoptosis followed by the permeabilization of the plasma membrane) was increased by ferritin-oxidized LDL and by ferritin (). Primary necrosis (the permeabilization of the plasma membrane in the absence of apoptosis) was very low in all conditions ().
Figure 5. Effects of ferritin-oxidized LDL on apoptosis in THP-1 macrophages. Ferritin-oxidized LDL was prepared by incubating LDL (1 mg protein/ml) with ferritin 880 µg/ml; 2 µM) at 37 °C for 24 h in NaCl/sodium acetate buffer, pH 4.5. THP-1 cells (500,000 cells per well) were cultured in RPMI 1640 containing serum and antibiotics alone or with NaCl/sodium acetate buffer, pH 4.5 (10%), native LDL (100 µg protein/ml), ferritin (88 µg/ml; 0.2 µM) alone or ferritin-oxidized LDL (100 µg protein/ml). After incubation for 48 h, the cells were harvested and assayed by flow cytometry. The percentage of (A) live cells, (B) necrotic cells, (C) apoptotic cells, and (D) secondary necrotic cells are shown. The control cells were not exposed to acidic buffer, LDL, or ferritin. *p < 0.05, **p < 0.01, and ***p < 0.001 compared with the control cells for three independent experiments. ≠≠p < 0.01 and ≠≠≠p < 0.001 for the indicated comparison.
Discussion
Macrophages are well recognized to play an important role in all stages of atherosclerosis, as they constitute major cellular components of early and advanced atherosclerotic lesions [Citation1]. Lysosomal dysfunction in macrophages is induced by oxidized LDL and cholesterol crystals [Citation19,Citation20]. Our previous findings suggest that ferritin might be involved in oxidizing LDL in the lysosomes of macrophages [Citation7]. In the study reported here, we have investigated the possible effects that LDL oxidation by ferritin might have on the function of macrophages.
We obtained evidence that ferritin could cause oxidative stress in lysosomes by incubating THP-1 macrophages and HMDM with ferritin and then adding a probe (Foam-LPO) for lysosomal lipid peroxidation. There was a considerable increase in the signal from the probe in both types of cell, suggesting that the ferritin had been endocytosed and delivered to lysosomes and was oxidizing the probe in these organelles (). This suggests that ferritin has the potential to oxidize LDL inside lysosomes.
Our laboratory has previously shown that THP-1 macrophages [Citation21], J774 mouse macrophages and HMDM [Citation2] treated with aggregated LDL formed ceroid in their lysosomes due to iron-catalyzed oxidation. In the present study, we found that ceroid in THP-1 macrophages was increased by pre-incubation with ferritin, to allow it to be delivered to lysosomes, especially when the cells were then incubated with sphingomyelinase-modified LDL (). We interpret this finding as endocytosed ferritin releasing catalytically active iron that can catalyze LDL oxidation in the lysosomes of macrophages. It should be noted that most of the ferritin in lysosomes in vivo is probably derived from the autophagy of cytosolic ferritin.
Induction of a pro-inflammatory response in macrophages is characterized by a shift in metabolic pathways to aerobic glycolysis, production of lactic acid and decreased oxidative phosphorylation and uptake of oxygen [Citation10,Citation22]. Oxidized LDL has been shown to increase the ECAR by macrophages [Citation23,Citation24]. We found that pre-incubating macrophages with ferritin to allow its delivery to lysosomes and then incubating with LDL increased their release of acids, presumably mainly lactic acid. We interpret this, in the light of other findings from this study, as the cells endocytosing ferritin and LDL and oxidizing the LDL in lysosomes leading to oxidative stress and metabolic alterations. The acidification of the extracellular space of atherosclerotic lesions would increase LDL binding to glycosaminoglycans in the arterial intima [Citation25], increase LDL modification [Citation26,Citation27] and affect the function of the cells in the artery [Citation27–29].
Reactive oxygen species might be involved in atherogenesis in a number of ways [Citation18]. The superoxide radical and its more reactive protonated form, the hydroperoxyl radical, might be involved in lysososomal LDL oxidation by iron [Citation21] and ferritin [Citation7]. Oxidized LDL stimulates the intracellular production of reactive oxygen species in cells [Citation30–32]. Macrophages in atherosclerotic lesions might be exposed to oxidized LDL, some of which might have been oxidized by ferritin in lysosomes, when other macrophages die and lyse and release their contents into the extracellular space. We therefore added ferritin-oxidized LDL to THP-1 macrophages and found that this increased their formation of reactive oxygen species several-fold ().
Cell death by apoptosis or some other means is undoubtedly involved in atherosclerosis and it is well established that oxidized LDL is cytotoxic to cells [Citation8]. We show here that ferritin-oxidized LDL, which might be released from dead and lysed macrophages, can induce apoptosis, but not primary necrosis, in THP-1 macrophages (). The toxicity of ferritin-oxidized LDL was not unexpected. Ferritin oxidation of LDL causes the formation of 7-ketocholesterol and cholesteryl ester hydroperoxides [Citation7] and these oxidized lipids have known to be cytotoxic. As discussed above, ferritin-oxidized LDL also increased the formation of reactive oxygen species by the macrophages and this might possibly be involved in the induction of apoptosis in the cells [Citation33].
Conclusion
Our findings support our proposal that ferritin contributes to the oxidation of LDL in the lysosomes of macrophages and causes oxidative stress, metabolic changes and apoptosis in the cells, which have atherogenic effects.
Author contributions
O.O.O. carried out the experiments and O.O.O. and D.S.L. wrote the paper.
Acknowledgements
The authors are grateful to Tertiary Education Trust Fund (TETFund) of Nigeria for a PhD studentship for O.O.O. We thank our colleagues, who volunteered to donate blood and Dr. Kim G. Jackson and Ms. Rada G. Mihaylova for skillfully taking blood for LDL isolation. The authors are grateful to Professor Yi Xiao, Dalian University of Technology, PR China for kindly giving us Foam-LPO.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Bjorkegren JLM, Lusis AJ. Atherosclerosis: recent developments. Cell. 2022;185(10):1630–1645.
- Wen Y, Leake DS. Low density lipoprotein undergoes oxidation within lysosomes in cells. Circ Res. 2007;100(9):1337–1343.
- Ahmad F, Leake DS. Lysosomal oxidation of LDL alters lysosomal pH, induces senescence, and increases secretion of pro-inflammatory cytokines in human macrophages. J Lipid Res. 2019;60(1):98–110.
- Wen Y, Ahmad F, Mohri Z, et al. Cysteamine inhibits lysosomal oxidation of low density lipoprotein in human macrophages and reduces atherosclerosis in mice. Atherosclerosis. 2019;291:9–18.
- Ahmad F, Mitchell RD, Houben T, et al. Cysteamine decreases low-density lipoprotein oxidation, causes regression of atherosclerosis, and improves liver and muscle function in low-density lipoprotein receptor-deficient mice. J Am Heart Assoc. 2021;10(18):e017524.
- Lv HH, Shang P. The significance, trafficking and determination of labile iron in cytosol, mitochondria and lysosomes. Metallomics. 2018;10(7):899–916.
- Ojo OO, Leake DS. Low density lipoprotein oxidation by ferritin at lysosomal pH. Chem Phys Lipids. 2018;217:51–57.
- Martinet W, Coornaert I, Puylaert P, et al. Macrophage death as a pharmacological target in atherosclerosis. Front Pharmacol. 2019;10. article 306.
- Hardwick SJ, Hegyi L, Clare K, et al. Apoptosis in human monocyte-macrophages exposed to oxidized low density lipoprotein. J Pathol. 1996;179(3):294–302.
- Koelwyn GJ, Corr EM, Erbay E, et al. Regulation of macrophage immunometabolism in atherosclerosis. Nat Immunol. 2018;19(6):526–537.
- Lee SJ, Quach CHT, Jung KH, et al. Oxidized low-density lipoprotein stimulates macrophage F-18-FDG uptake via hypoxia-inducible factor-1α activation through Nox2-dependent reactive oxygen species generation. J Nucl Med. 2014;55(10):1699–1705.
- Wilkins GM, Leake DS. The effect of inhibitors of free radical generating-enzymes on low-density lipoprotein oxidation by macrophages. Biochim Biophys Acta. 1994;1211(1):69–78.
- Walters MJ, Wrenn SP. Size-selective uptake of colloidal low density lipoprotein aggregates by cultured white blood cells. J Colloid Interface Sci. 2010;350(2):494–501.
- Firth CA, Yang YT, Gieseg SP. Lipid oxidation predominates over protein hydroperoxide formation in human monocyte-derived macrophages exposed to aqueous peroxyl radicals. Free Radic Res. 2007;41(7):839–848.
- Zhang XF, Wang BL, Wang C, et al. Monitoring lipid peroxidation within foam cells by lysosome-targetable and ratiometric probe. Anal Chem. 2015;87(16):8292–8300.
- Lorey MB, Oorni K, Kovanen PT. Modified lipoproteins induce arterial wall inflammation during atherogenesis. Front Cardiovasc Med. 2022;9:841545.
- Devlin CM, Leventhal AR, Kuriakose G, et al. Acid sphingomyelinase promotes lipoprotein retention within early atheromata and accelerates lesion progression. ATVB. 2008;28(10):1723–1730.
- Nowak WN, Deng JC, Ruan XZ, et al. Reactive oxygen species generation and atherosclerosis. Arterioscler Thromb Vasc Biol. 2017;37(5):E41–E52.
- Emanuel R, Sergin I, Bhattacharya S, et al. Induction of lysosomal biogenesis in atherosclerotic macrophages can rescue lipid-induced lysosomal dysfunction and downstream sequelae. Arterioscler Thromb Vasc Biol. 2014;34(9):1942–1952.
- Cox BE, Griffin EE, Ullery JC, et al. Effects of cellular cholesterol loading on macrophage foam cell lysosome acidification. J Lipid Res. 2007;48(5):1012–1021.
- Ahmad F, Leake DS. Antioxidants inhibit low density lipoprotein oxidation less at lysosomal pH: a possible explanation as to why the clinical trials of antioxidants might have failed. Chem Phys Lipids. 2018;213:13–24.
- Freemerman AJ, Johnson AR, Sacks GN, et al. Metabolic reprogramming of macrophages glucose transporter 1 (GLUT1)-mediated glucose metabolism drives a proinflammatory phenotype. J Biol Chem. 2014;289(11):7884–7896.
- Chen YL, Yang M, Huang WX, others, et al. Mitochondrial metabolic reprogramming by CD36 signaling drives macrophage inflammatory responses. Circ Res. 2019;125(12):1087–1102.
- Kumar A, Gupta P, Rana M, et al. Role of pyruvate kinase M2 in oxidized LDL-induced macrophage foam cell formation and inflammation. J Lipid Res. 2020;61(3):351–364.
- Glise L, Rutberg M, Haversen L, et al. pH-dependent protonation of histidine residues is critical for electrostatic binding of low-density lipoproteins to human coronary arteries. Arterioscler Thromb Vasc Biol. 2022;42(8):1037–1047.
- Leake DS. Does an acidic pH explain why low density lipoprotein is oxidised in atherosclerotic lesions? Atherosclerosis. 1997;129(2):149–157.
- Oorni K, Rajamaki K, Nguyen SD, et al. Acidification of the intimal fluid: the perfect storm for atherogenesis. J Lipid Res. 2015;56(2):203–214.
- Gerry AB, Leake DS. A moderate reduction in extracellular pH protects macrophages against apoptosis induced by oxidized low density lipoprotein. J. Lipid Res. 2008;49(4):782–789.
- Gerry AB, Leake DS. Effect of low extracellular pH on NF-κB activation in macrophages. Atherosclerosis. 2014;233(2):537–544.
- Hsieh CC, Yen MH, Yen CH, et al. Oxidized low density lipoprotein induces apoptosis via generation of reactive oxygen species in vascular smooth muscle cells. Cardiovasc Res. 2001;49(1):135–145.
- van Aalst JA, Zhang DM, Miyazaki K, et al. Role of reactive oxygen species in inhibition of endothelial cell migration by oxidized low-density lipoprotein. J Vasc Surg. 2004;40(6):1208–1215.
- Zmijewski JW, Moellering DR, Le Goffe C, et al. Oxidized LDL induces mitochondrially associated reactive oxygen/nitrogen species formation in endothelial cells. Am J Physiol Heart Circ Physiol. 2005;289(2):H852–H861.
- Fujii J, Homma T, Osaki T. Superoxide radicals in the execution of cell death. Antioxidants. 2022;11(3):501.
