Abstract
Drug treatment of pulmonary hypertension may be limited by systemic hypotension. Selective action of a vasodilator drug in pulmonary arteries could be achieved by administering a vasodilator gas into systemic venous blood so that it dilates pulmonary arteries before immediate first-pass elimination via exhalation. This article presents in vivo data to show that a pharmacologically active gas can be delivered safely into systemic venous blood where it has a distribution pattern and physiologic effects similar to those observed when the gas is inhaled into pulmonary venous (systemic arterial) blood. This is a first step toward development of first-pass pulmonary clearance as a mechanism to concentrate drugs in pulmonary arteries.
Pharmacotherapy commonly relies on drug dynamics to achieve a desired pharmacologic effect at an intended site of action and with an acceptable minimum of side effects (Ross and Kenakin Citation2001). Targeted drug action is less frequently achieved pharmacokinetically by taking advantage of variable absorptive rates or of internal barriers such as the blood-brain barrier, or, in the case of nitric oxide, of very rapid inactivation of the drug (Wilkinson Citation2001). Most drugs, however, are administered with the expectation that they will reach the central circulation and undergo redistribution throughout the entire body.
Like other drugs, anesthetic gases enter the central circulation where, due to high blood flows to the central nervous system, they quickly reach their primary site of action in the brain while simultaneously undergoing distribution throughout the body. Unlike almost all other drugs, anesthetic gases enter the central circulation via inhalation. Inhaled anesthetic agents rapidly diffuse through the pulmonary capillary membrane into the pulmonary venous and hence systemic arterial blood. The arterial blood concentration of an anesthetic gas then depends primarily on three factors:
Continued agent delivery to the alveolus.
Agent solubility in blood and tissues.
Cardiac output and distribution (Eger Citation1974).
The mechanism by which modern anesthetic gases are eliminated from the body during recovery from anesthesia also is unique because almost all of the administered drug is exhaled, unchanged, making alveolar ventilation the primary mechanism for both termination of drug action and elimination of drug from the body. Elimination by exhalation of any gas that is dissolved in the systemic venous blood also depends on alveolar ventilation, blood:gas solubility of the gas, and cardiac output (Farhi Citation1967; Eger Citation1974). During emergence from inhalational anesthesia (anesthetic gas exhalation), a concentration gradient exists between the pulmonary arterial and pulmonary venous blood that is directly proportional to the amount of drug that is cleared by the lungs. During anesthesia emergence, therefore, the amount of anesthetic gas that is in the pulmonary arteries is greater than that in the systemic arteries by the amount that is exhaled.
In clinical settings characterized by pulmonary hypertension, such as right heart failure, acute lung failure, or congenital heart disease with right-to-left shunting of blood, a selective vasodilator effect on the pulmonary arteries may improve overall cardiopulmonary performance and gas exchange. To date, nitric oxide has been the most extensively studied and effective therapy of pulmonary hypertension because it selectively lowers pulmonary vascular resistance without causing systemic hypotension. Nitric oxide, however, requires highly specialized equipment and has not yet shown proven clinical benefit (Taylor et al. Citation2004). Treatment of pulmonary hypertension with a direct-acting vasodilator also could be accomplished by creating a concentration gradient of a dilator drug in which higher concentrations of the drug are present in pulmonary arterial blood than are present in systemic arterial blood. Such a concentration gradient theoretically could be achieved by administering a gaseous vasodilator with low blood solubility directly into the systemic venous blood. The gas would then enter the pulmonary arteries followed by immediate first-pass pulmonary clearance (exhalation) leaving a less effective or ineffective concentration of the gas in the systemic arteries.
In this report, we provide preliminary in vivo verification that such a system is possible. We report that a gaseous drug, entering the body via the systemic veins, leads to a drug uptake and distribution pattern and to physiologic effects that are similar to those that are observed when the drug is inhaled and enters the body via the pulmonary veins. This is a first step toward developing the concept of first-pass pulmonary clearance as a practical method to selectively deliver drugs to the pulmonary arteries.
MATERIALS AND METHODS
This study was approved by the local Institutional Animal Care and Use Committee and followed the Guide for the Care and Use of Laboratory Animals established at our institution.
Preparation of a Gas-Containing Intravenous Solution
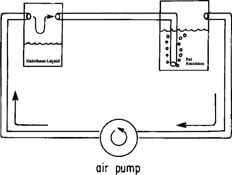
A closed, bubble-through system with an in-circuit gas pump was used to prepare a solution of halothane gas-dissolved in liquid. Air, used as a carrier gas, was first passed over liquid halothane (vapor pressure = 243 mmHg @ 20°C) (Halocarbon Laboratories, NJ, USA) in a glass vessel. The effluent, halothane-containing gas was then gently bubbled through a gas scintillation device immersed in a commercially available intravenous fat emulsion (IntralipidR, Cutter, CA, USA) placed in a second glass vessel. The gas was then passed back through the pump into the first vessel (). A fat emulsion was chosen as the liquid carrier for halothane gas because of the very high solubility of halothane in fat (Nunn Citation1960) allowing for large quantities of halothane gas to be dissolved in a relatively small volume of liquid fat emulsion. Preparation of the halothane-containing lipid solution was terminated when we estimated that ∼ 40 ml of halothane vapor was present in 1 ml of the final intravenous fat solution, as determined by the disappearance (vaporization) of liquid halothane from the first vessel. Final determination of halothane concentration in the fat emulsion was made by weight as follows:
where:
H = total dissolved halothane vapor (ml),
FEw = total weight (grams) of original intravenous fat emulsion,
Hw = total weight (grams) of dissolved halothane vapor, and
D = density halothane vapor @ 20°C = 8.21 mg/ml (0.00821 g/ml).
[H] = concentration of halothane vapor in final solution (volumes %),
H = total dissolved halothane vapor (ml), and
(FE + H)v = volume of final solution of intravenous fat emulsion and halothane vapor (ml).
Halothane Administration Protocol
Mongrel dogs were anesthetized with intravenous pentobarbital (30 mg/kg) and their tracheas were intubated after which they breathed 100% oxygen spontaneously through a closed circle breathing system with a carbon dioxide absorber and to which was added metabolic quantities of oxygen. The circuit included a 2 L reservoir bag to accommodate peak inspiratory and expiratory flows. A flow-directed pulmonary artery catheter and an intra-arterial catheter were inserted via the femoral vein and artery, respectively. Then 1 hr after the intravenous injection of pentobarbital, halothane was administered via either the inhaled (control group) or intravenous (experimental group) route. Inhaled halothane was administered by injection of calculated amounts of liquid halothane into the exhalation limb of the closed breathing system. The liquid halothane immediately vaporized into predetermined quantities of halothane gas. An equal amount of intravenous halothane gas was administered to the experimental group via a peripheral vein using a calibrated glass syringe infusion pump (Auto Syringe, Travenol, NH, USA). In both groups, halothane was administered incrementally according to weight based on a previously published closed breathing circuit drug delivery schedule (Lowe and Ernst Citation1981) (see Schedule at end of article). The schedule was formatted to achieve and maintain an end-tidal halothane concentration of ∼ 0.7%. End-tidal halothane concentrations were measured (Engstrom Emma, Engstrom Medical, Sweden) at baseline and at 9, 16, 25, 36, 49, 64, and 81 min after the start of halothane administration, according to the square root of time formula (Lowe and Ernst Citation1981) (Schedule 1).
Hemodynamic variables including cardiac output, systemic and pulmonary arterial pressures and resistances, heart rate, and central venous and pulmonary artery occlusion pressures were determined at baseline (after pentobarbital) and at 30 and 60 min after the initiation of halothane administration. Arterial blood gas analysis was performed at the same times. Dogs receiving intravenous halothane were again anesthetized 48 hr after the initial experiment and the peripheral vein that was used for halothane injection was excised for gross morphological examination. Continuous physiological variables were compared within the between groups using ANOVA techniques or a paired student's t-test, as appropriate, with a p value of less than 0.05 taken to represent statistical significance.
RESULTS
A total of 8 dogs, weighing between 25 and 35 kg, were studied; 4 received inhaled halothane and 4 received intravenous halothane. The average volume of gas-containing intravenous lipid solution administered to each dog was 0.75 ml/kg/hour (∼25 to 40 ml total over 90 min). End-tidal halothane concentrations were constant in both groups and were not significantly different between groups at any time (). Hemodynamic variables compared between groups were not significantly different at any time for any of the values measured (). As would be expected with halothane, systolic arterial pressure decreased significantly in both groups compared with their respective baseline values (). Arterial blood gas analyses also were not significantly different between groups, although, again as expected, both groups showed an increase in arterial carbon dioxide tension following the administration of halothane via either route (). Examination of the peripheral veins in the intravenous halothane group revealed thrombosis in all veins that used for the halothane intravenous infusion.
FIG. 2. Measured end-tidal halothane concentrations when halothane vapor is administered quantitatively via either the inhalational (inhaled) or the intravenous (injected) route.
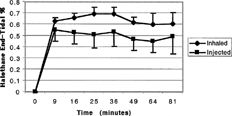
TABLe 1 Mean hemodynamic and blood gas measurements (S.D.) during inhalation of halothane gas (inhaled) or intravenous infusion of halothane gas-containing solution (injected)
DISCUSSION
This study tested, first, the feasibility of dissolving known quantities of a pharmacologically active gas in a medium that is suitable for intravenous administration. Second, we showed that the resulting solution can be administered quantitatively to create a stable and predictable drug concentration in vivo. Third, we showed that relevant clinical concentrations of an anesthetic gas, delivered in intravenous carrier fluid, can be achieved without intravenous fluid overload. Finally, the cardiopulmonary effects of intravenous gas delivery were found to be identical to those of inhaled gas delivery.
Henry's Law governs the extent to which a gas in equilibrium with a solvent will dissolve in the solvent. Although Henry's Law is frequently assumed to describe a linear relationship, it is, in fact, nonlinear for practically all known solvent-solute systems. (Gerrard Citation1980) Taking advantage of this nonlinearity, we found that concentrations of halothane gas ∼ 40 ml of gaseous halothane per ml of lipid emulsion were relatively easy to produce. With these concentrations of vaporized halothane gas in a lipid emulsion, we were able to administer pharmacologically relevent concentrations of halothane gas (0.5 to 0.7 vols % end-tidal) into the systemic venous system without causing blood volume overload. We also were able, for the first time, to deliver the intravenous halothane gas quantitatively to produce predictable in vivo concentrations in contrast to earlier studies that used an uncontrolled mixing of liquid anesthetic with a carrier vehicle (Biber, Martner, and Wenner Citation1982; Biber et al. Citation1984; Krantz, Cascorbi, and Rudo Citation1962). Thus, predictable and quantitative delivery of a gas is feasible via intravenous delivery.
Quantitative, closed circuit inhalation anesthesia is a well-defined technique for administering precise, predefined quantities of an anesthetic gas to a closed, body-plus-breathing circuit system during surgery (Lowe and Ernst Citation1981). During this or any inhalational anesthetic technique, alveolar (end-tidal) gas tension of the anesthetic agent commonly is used to measure the effective concentration of anesthetic gas that is present in vivo because alveolar gas tension closely parallels arterial gas tension. During anesthesia, anesthetic gas enters the alveolus by one of two routes: in the inspired gas mixture or through the capillary membrane from mixed venous blood. While alveolar gas tension is reflective of anesthetic depth (Eger Citation1974), there is no particular reason, beyond convenience, why a given alveolar gas tension has to be achieved by administering the anesthetic gas through the inhalation route. According to accepted models of uptake and distribution, gas concentrations in the alveolus also could be maintained by delivery of the gas into systemic venous blood. Our results provide in vivo confirmation of this concept because end-expiratory concentrations of halothane were maintained at constant and equivalent levels by either the inhalational or the intravenous route of administration. The slightly lower end-tidal concentration of halothane in the intravenous group was probably caused by some loss of the agent during handling of the halothane containing solution or possibly to an increase in the solubility of the blood as a consequence of adding lipid or altered peripheral uptake of the agent (Sakaeda et al, Citation1994).
The measured parameters of cardiopulmonary function also were found to be similar in the two groups. Intravenous lipid solutions have been shown to be without cardiopulmonary effects in most clinical settings (Jarnberg, Lindholm, and Eklund Citation1981), whereas the cardiopulmonary effects of halothane vapor, primarily depressant, are well known (Eger Citation1974). In this study, the measures of cardiopulmonary function were not different between the two groups and were similar to what has been previously reported for halothane including mild depression of cardiac output and pulmonary ventilation ().
Finally, while thrombosis at peripheral intravenous injection sites may limit the usefulness of this particular technique for some agents, it is important to note that other mechanisms for the quantifiable delivery of pharmacologically active gases into the systemic venous blood are already available. Recent advances in microfiber technology, for example, have led to the development of intravenous membrane gas exchangers that can be placed in the inferior vena cava where they deliver oxygen to venous blood before the blood reaches the pulmonary circulation thereby leading to improvements in systemic oxygenation. (Zwischenberger, Tao, and Bidani Citation1999; Zwischenberger and Alpard Citation2002). Theoretically, a pharmacologically active gas also could be administered using this same technique.
In summary, we found that a pharmacologically active gas can be reliably and predictably administered into the systemic venous blood to achieve a drug concentration in the body that is the same as when the gas is administered by inhalation. The results of this study are a first step in the development of a pharmacokinetically-based method that uses first-pass pulmonary clearance to achieve selective delivery of a drug to the pulmonary arteries.
SCHEDULE
At a constant arterial concentration, the rate of anesthetic uptake of each organ (Qant) can be calculated using the exponential saturation equation:
where: e = the base of natural logarithms, 2.71828, Ca = blood concentration of the anesthetic (= alveolar concentration x blood: gas partition coefficient), Q = total cardiac output, and Qt = distribution of cardiac output to each organ, Vt = organ volume, and λt = anesthetic solubility in each organ.
The rate of whole body anesthetic uptake (Qan) at any time (t) is the sum of (Σ) all the individual organ rates of uptake:
Using the tissue-gas partition coefficients for halothane and the accepted physiological values for organ volumes and blood flow, the whole body halothane requirement can be calculated based on body weight. An important observation from these data is the constant relationship of the rate of whole body uptake to the reciprocal of the square root of time so the amount of an anesthetic vapor that is absorbed by the body during time periods 0–1, 1–4, 4–9, 9–16 min is the same (from Lowe and Ernst Citation1981).
REFERENCES
- Biber B., Martner J., Werner O. Halothane by the I.V. route in experimental animals. Acta Anaesthesiol. Scand. 1982; 26(6)658–659, [PUBMED], [INFOTRIEVE], [CSA]
- Biber B., Johannesson G., Lennander O., Martner J., Sonander H., Werner O. Intravenous infusion of halothane dissolved in fat haemodynamic effects in dogs. Acta Anaesth. Scand. 1984; 28: 385–389, [PUBMED], [INFOTRIEVE], [CSA]
- Eger E. I. Anesthetic Uptake and Action. Williams and Wilkins, Baltimore 1974; 1–26
- Farhi L. E. Elimination of inert gas by the lung. Resp. Physiol. 1967; 3: 1–11, [CROSSREF], [CSA]
- Gerrard W. Gas Solubilities, Widespread Applications. Pergamon Press, New York 1980; 361–388
- Jarnberg P. O., Lindholm M., Eklund J. Lipid infusion in critically ill patients. Cri. Care. Med. 1981; 9(1)27–31, [CSA]
- Krantz J. C., Jr., Cascorbi H. F., Rudo F. G. Intravenous administration of methoxyflurane (penthrane) emulsions in animals and man. Anesth. Analg. 1962; 41: 257–262, [PUBMED], [INFOTRIEVE], [CSA]
- Lowe H. J., Ernst E. A. Quantitative Practice of Anesthesia Use of Closed Circuit. William and Wilkins, Baltimore 1981
- Nunn J. F. The solubility of volatile anesthetics in oil. Br. J. Anaesth. 1960; 32: 346, [PUBMED], [INFOTRIEVE], [CSA]
- Ross E. M., Kenakin T. P. Mechanisms of drug action and the relationship between drug concentration and effect. The Pharmacological Basis of Therapeutics 10th ed., J. Hardman, L. Limbird. McGraw-Hill, New York 2001; 31–43
- Sakaeda T., Takahashi K., Nishihara Y., Hirano K. O/W lipid emulsions for parenteral drug delivery. I. Pharmacokinetics of the oil particles and incorporated sudan II. Biol. Pharm. Bull. 1994; 17(11)1490–1495, [PUBMED], [INFOTRIEVE], [CSA]
- Taylor R. W., Zimmerman J. L., Dellinger R. P., Starube R. C., Criner G. J., Davis K. D., Jr., Kelly K. M., Smith T. C., Small R. J. Low-dose inhaled nitric oxide in patients with acute lung injury. A randomized controlled trial. JAMA 2004; 291(13)1603–1609, [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Wilkinson G. R. Pharmacokinetics: The dynamics of drug absorption, distribution, and elimination. The Pharmacological Basis of Therapeutics, 10th ed., J. Hardman, L. Limbird. McGraw-Hill, New York 2001; 3–29
- Zwischenberger J. B., Tao W., Bidani A. Intravascular membrane oxygenator and carbon dioxide removal devices: a review of performance and improvements. ASAIO. J. 1999; 45(1)41–46, [PUBMED], [INFOTRIEVE], [CSA]
- Zwischenberger J. B., Alpard S. K. Artificial lungs: a new inspiration. Perfusion 2002; 17(4)253–268, [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]