Abstract
The phase behavior of a new psedoternary system of clove oil/Tween 20 has been studied. Several compositions from the single-phase region were selected and their stability toward time, temperature, and electrolytes has been examined. A particular composition(clove oil/Tween 20/water as 5/30/65) was chosen as the drug delivery system from the clear oil-in-water zone of the pseudoternary system. The droplet dimension and the polydispersity state of the particular composition was determined by dynamic light scattering. A bioactive compound quarcetin was encapsulated in the vehicle. The efficacy of the drug in the vehicle was examined against leishmaniasis in hamster models. The hepatotoxicity of the vehicle (o/w microemulsion) with and without the drug quarcetin was examined by estimating serum alkaline phosphatase, glutamate pyruvate transaminase, urea, and creatinine.
An administered drug is absorbed and distributed within the body. But only a small portion of the administered dose reaches the pharmacological site of action. The remaining fraction can act on nonpharmacological sites to cause undesirable side effects. Colloidal drug delivery systems can improve the therapeutic index of drugs by enhancing their efficacy and reducing their unwanted side effects (Sarkar et al. Citation2002). Microemulsions are colloidal systems that have been either examined or exploited in this respect. (Attwood Citation1994; Baroli et al. Citation2000; Lianli, Nandi, and Kim Citation2002; Malcomson and Lawerence Citation1993, Citation1998; Moreno et al. Citation2000; Trotta Citation1999). They have the unique ability to solubilize nonpolar compounds in polar media and vice-versa, thus having good potential in pharmacy and medicine (Acharya et al. Citation2001a; Moreno, Frutos, and Ballesteros Citation2001). Oil-in–water microemulsions have been proposed as aqueous-based vehicles for delivery of a range of drugs (Attwood Citation1994; Das, Bhattacharya and Moulik Citation1991; Hazra et al. Citation1998; Khalweit, Busse, and Faulhaber Citation1995; Lawrence Citation1996; Paul and MoulikCitation1997; Tenjarala Citation1999).
For the last decade, there have been reports of microemulsion formulations suitable for oral and topical delivery but reports of microemulsion systems for subcutaneous and parenteral use are relatively scarse (Corswant, Thorom, and Engstrom Citation1998). The oil-in-water microemulsions are attractive vehicles for parenteral administrations because of their high solubilizing capacity of organic compounds and their stability (Hazra et al. Citation1998; Majhi and Moulik Citation1999; Park and Kim Citation1999). However, because of toxicity, only a limited number of surfactants and cosurfactants can be used to prepare pharmaceutical microemulsions. For in vivo applications, the delivery vehicle must be prepared from biocompatible and biotolerable components. Vegetable oils, triglycerides, and esters of fatty acids have been used as nonaqueous components for preparation of pharmaceutical microemulsions (Hazra et al. Citation1998; Corswant et al. Citation1998; Malcomson et al. Citation1998). The surfactants that are commonly used are Aerosol OT, polysorbates and alkyl polyethers, and sorbitan monoesters (Attwood Citation1994; Acharya et al. Citation2001a, Citation2001b; Changez and Varshney Citation2000). The presence of cosurfactants (short chain alcohols) is generally considered undesirable because of toxicity (Corswant et al. Citation1998).
Colloidal drug delivery systems are accumulated and taken up nonspecifically by the reticuloendothelial systems (RES) (Basu Citation1994; Sarkar et al. Citation2002). So the drugs to be targeted to the macrophages of the RES are expected to yield good results when they are encapsulated in microemulsions and administered intravenously. In the present work, experimental leishmaniasis were used as a model disease where the amastigotes of the causative parasite Leishmania donovani resides and proliferates within the macrophages of the RES. Thus, antileishmanial drugs after their incorporation in appropriate delivery systems could be targeted to the RES and examined for their efficacy.
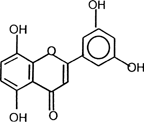
Quercetin (), the bioactive compound used in the present study, is a flavonoid known to be a potent antioxidant. It promotes topoisomerase mediated site-specific DNA cleavage in mammalian cells in vitro (Austin et al. Citation1992;Yamashita, Kowada, and Nakano 1990). Recently, quercetin has been reported to have antileishmanial properties and found to be apoptic to Leishmania donovani (Mittra et al. Citation2000; Sarkar et al. Citation2002). For the present study, the antileishmanial property of quercetin has been re-examined after its incorporation in the oil-in-water microemulsion of clove oil/Tween 20/water, a newly designed colloidal delivery system.
MATERIALS AND METHODS
The surfactant Tween 20 (polyoxyethylene sorbitan monolaurate) was obtained from Sigma Chemicals (St. Louis, MO, USA). Clove oil was from SD Fine Chemicals India (acid value 5.12 mgKOH/gm, saponification value 5.023 mgKOH/gm, iodine value 88.34%, eugenol content 80%. Sodium dihydrogen phosphate and disodium hydrogen phosphate were from SD Fine Chemicals. Sodium chloride and potassium chloride were purchased from Merck, India, and BDH, India, respectively. Tris(hydroxymethyl)aminomethane hydrochloride was a product of SISCO Research Laboratory Mumbai, India. Dextrose was a product of Sarabhai Merck, India. P-nitrophenyl phosphate(PNPP), p-nitrophenol, DL-alanine, and pyruvic acid were purchased from SRL, Mumbai, India, 2,4 dinitrophenyl hydrazine came from Aldrich, U.S.A; α -oxoglutaric acid, aniline, and citric acid were from Merck, Germany. The bioactive compound quarcetin was extracted from the Indian plant Fagopyrum esculentum (buckwheat) by following the reported method (Sarkar et al. Citation2002) in the Medicinal Chemistry Division of Indian Institute of Chemical Biology, Kolkata. Doubly distilled water was used in all preparations.
Ternary Phase Diagram of the Microemulsion Forming System
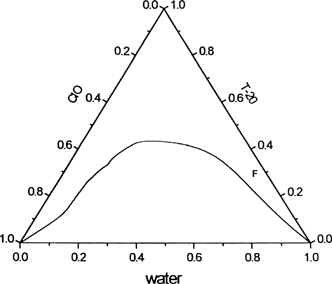
To construct the phase diagram of the ternary system of clove oil, Tween 20, and water, Tween 20 was dissolved in clove oil in different weight ratios in several stoppered test tubes placed in a constant temperature water bath. To each of these samples, water was added from a micropipette under constant stirring condition until the solution became just turbid from phase separation. The measurements were checked for reproducibility, and the compositions were expressed in weight (wt%) to construct the phase diagram on triangular coordinates.
Effect of Additives
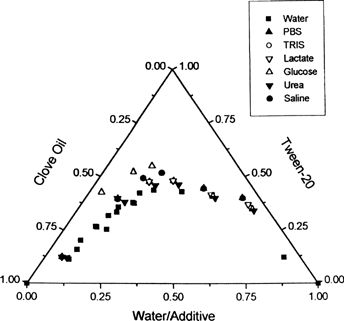
Six compositions—82/12/7(A), 48/38/15(B), 33/46/21(C), 25/46/29(D), 15/40/45(E), 5/30/65(F)—were chosen from the clear zone of the ternary phase diagram of clove oil/Tween 20/water and the effect of additives on the stability of these compositions were studied. To each of these samples, phosphate buffered solution (PBS) (pH 7.2), Tris HCl buffer(pH 7.4), Ringer lactate solution (compound sodium lactate injection ip), urea solution (30 mg/ml), glucose solution (100 mg/ml), and 0.9% saline were added respectively, in place of water and their effect on the stability state of compositions (A–E) was observed. The effect of temperature on the samples with the additives also was studied in the range of 288–313 K by subjecting them to heating and cooling cyles. No turbidity was observed with urea and NaCl for all the 6 compositions in the studied temperature range. However, below 303 K, PBS and glucose imparted turbidity in A. Tris-HCl imparted turbidity in D, and PBS, glucose, and Tris-HCl imparted turbidity in E. But the solutions became transparent on raising the temperature above 303 K.
Dynamic Light Scattering Measurements
The dimensions of the nanodispersions were measured in a dynamic light scattering spectrophotometer (DLS 700, Otsuka Electronics, Japan) using a He-Ne laser of wavelength 632.8 nm (Moulik et al. Citation1999). The sample cell was placed in the thermostated chamber of the goniometer, and the scattered intensity was monitored at 90°. The autocorrelation function of the scattered intensity was obtained from a 1024-channel photon correlator. The experimental solutions were repeatedly (three to four times) filtered through Millipore filters of pore size 0.45 μ m to remove extraneous particles. The measurements for each sample were processed using the instrument's software to obtain the hydrodynamic diameter (dh), the polydispersity index (the ratio of the standard deviation (SD) in dh and the average dh), and the diffusion coefficient of the dispersed droplets.
Selection of Vehicle Composition
For the encapsulation of drug, a wt % composition of clove oil/Tween 20/PBS (pH 7.2) designated as 5/30/65 F was selected (marked as F in the oil-in-water region of the ternary phase diagram). The system has low and moderate percentages of oil and surfactant respectively, and was expected to impart low toxicity.
Quercetin Incorporation
1 mg of quercetin was dissolved in 0.24 ml of clove oil and kept overnight to ensure complete dissolution of the drug. Next 1.4 ml of Tween 20 was added in the preparation followed by 3.25 ml of PBS (pH7.2). They were throughly mixed in a vortex mixer. The final concentration of the drug in the preparation was 0.20 mg/ml (0.7 mmoldm− 3). The microemulsion containing the drug is designated as FD.
Stability Studies
The ternary drug delivery vehicle of compositions of dispersed clove oil droplets stabilized by Tween 20 in PBS without drug (E) and with drug encapsulation (FD) was found to be very stable. The stability was observed for 1 year in the range of 278–313 K. Biological studies were undertaken with these samples.
In Vivo Studies in Hamsters
A colony of golden hamsters (Mesocristatus auratus) originally from Haffkline Research Institute (Bombay, India) was used to maintain Leishmania donovani strain AG83 from an Indian Kala-azar patient by intracardial passage every 6 weeks. Amastigotes were isolated from hamster spleens according to the method of Looker, Bereus, and Marr (Citation1983) with some modifications followed by Das et al. (Citation1990). Each animal was injected intracardially with 2 × 106 amastigotes. The mean therapeutic dose was taken to be 3 mg/Kg of body weight as recommended (Sarkar et al. Citation2002).
For chemotherapy, a multiple dose treatment was followed. A group of 16 hamsters of same age, sex, and average body weight of 100 g were taken for treatment, 30 days postinfection. The animals were divided into 4 groups having 4 animals in each: infected untreated control, free-drug treated, microemulsion-incorporated drug treated, and placebo microemulsion-treated. For free-drug treatment 300 μ g of quercetin, dissolved initially in minimum volume of DMSO and diluted to 0.5 ml with PBS was injected subcutaneously into each hamster every 3 days for a total of 6 doses. For the drug containing microemulsion, 300 μ g of the drug, incorporated in the requisite volume of the vehicle, was injected into each hamster subcutaneously each time. An equal volume of the empty microemulsion (without drug) was injected into each of the placebo group. The animals were killed by an overdose of chloroform after 7 days from the last injection. The splenic impression smears were taken on slides, fixed in absolute methanol, and stained with Giemsa strain (1:4 v/v) for microscopic examinations. The parasitic load in the spleen was calculated using the Stauber's formula (Stauber, Franchino, and Grun Citation1958).
Investigations on Drug Toxicity
For the determination of drug toxicity, parameters such as specific enzyme levels related to normal liver function and biochemistry related to kidney function were analyzed. Fresh blood was collected from the hamsters by cardiac puncture just before sacrifice and sera were collected by centrifugation. Serum alkaline phosphatase (SAP) and serum glutamate pyruvate transaminase (SGPT) were assayed according to published protocols (Bessey, Lowry, and Brock Citation1946; Reitman and Frankel Citation1957).
For the assay of SAP the serum was incubated with p-nitrophenyl phosphate in alkaline buffer (pH 10.4) for 30 min at 37°C. The reaction was stopped with 0.02 (N) NaOH. The absorbance was measured at 410 nm and the amount of p-nitrophenol released was determined from a standard curve of known concentrations of p-nitrophenol versus absorbance.
For SGPT, the serum was incubated with phosphate buffer (pH 7.5) containing DL-alanine and α -oxoglutaric acid for 30 min at 37°C. The mixture was further treated with aniline citrate and 2,4-dinitrophenyl hydrazine hydrochloride reagents. The reaction was stopped by adding 0.4(N) NaOH and the absorbance was measured at 520 nm. The amount of sodium pyruvate released was determined from a standard curve of known concentrations of sodium pyruvate versus absorbance.
For assessing nephrotoxicity, urea and creatinine were assayed using standard kits (Preccugent®, Pinnacle Marketing, Mumbai, India).
RESULTS AND DISCUSSION
Physicochemical Properties
The phase behavior of the ternary system of clove oil/ Tween 20/water is depicted in . In this diagram, a large clear monophasic zone of 48% of the total area was obtained without using a cosurfactant, making the composition simple and striking. Such a large monophasic microemulsion zone is seldom attainable, especially without a cosurfactant. Microemulsion systems for drug delivery, reported in the literature, suffer complexity in composition due to use of a large number of components (Richardson et al. Citation1997; Trotta, Ugazio, and Gasco Citation1995). The prepared microemulsion samples (A–F) were both water-in-oil (w/o) and oil-in-water (o/w) types. The samples A, B, and C were distinctly w/o and E and F were o/w types. D was bicontinuous, i.e., having simultaneous dispersions of both w/o and o/w.
The effect of additives on the 6 different compositions in the different regions of the clear zone of the ternary phase diagram is depicted in . Additives do not cause pronounced shifts in the phase boundary of the ternary system, except for glucose where the boundary moderately shifted more toward the oil rich region.
FIG. 3. Effect of additives on the phase diagrams of clove oil/ Tween 20/water system at 6 different compositions 82/12/7 (A), 47/38/15 (B), 33/46/21 (C), 25/46/29 (D), 15/40/45 (E), and 5/30/65(F), as designated in text.
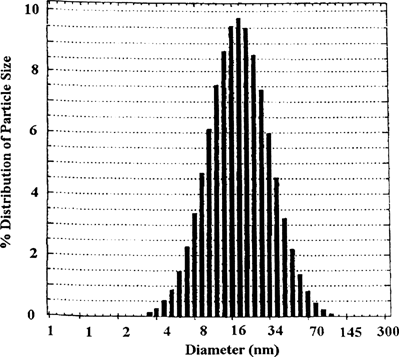
The size distribution of the composition (sample F mentioned above) used for drug delivery as determined by DLS is depicted in . The average size was found to be 17.0 nm. The system was polydispers with broad distribution of size and PDI value 0.369 ≫ 0.01(meant for monodispersity). A small size distribution of the vehicle should be advantageous for higher circulation time (t1/2) as well as for parenteral delivery since chances for capillary blockage by the nano-sized droplets would be minimum. The studied o/w dispersion vehicle had dimensions much lower than the diameter (400 nm) of the smallest blood capillary (Kreuter Citation1994). Thus, it is unlikely to be involved in capillary blockage.
FIG. 4. Percentage of particle size distribution versus diametera (nm) obtained from DLS measurement for the microemulsion composition 5/30/65 (F).
In the present work, clove oil was used as the internal phase in which the drug (natural product quercetin) is soluble (solubility 1 mg/ml). It is an essential oil with eugenol as the major component (80%) and furfurol, eugenyl acetate, and pinene as the other components. It is free of fatty acids and triglycerides. Eugenol causes irritation to skin but according to an FDA report, its acceptable limit for human intake is 2.5 mg/kg (Selvi and Niranjali Citation1998), which is much more than the amount present in the present delivery system (0.04 mg/ml of delivery system). The biological acceptability of the nonionic surfactants used in this study was already established (Florence and Attwood Citation1983)(Citation1998).
Amphiphiles with high HLB (hydrophilic-lipophilic balance) values are known to form good o/w microemulsions. The HLB value of Tween 20 herein used is 16.7 (Myers Citation1988). The stability of nonionic surfactant-derived microemulsions is known to be less vulnerable to electrolyte addition (Florence and Attwood Citation1998; Robb Citation1982). This is an important prerequisite for drug delivery systems as these systems are likely to be mixed with electrolytes present in the biological fluid after being incorporated in vivo The stability of the present system with time and temperature was found to be excellent (as observed for 1 year in the temperature range of 280–313 K).
Biological Activities
The effects of chemotherapy on hamsters infected for 30 days with L. donovani are shown in . For chemotherapy, the same equivalent drug concentration viz. 3 mg Kg− 1 body weight for each hamster (Sarkar et al. Citation2002) was used in the free form as well as in microemulsion encapsulated form and injected subcutaneously (SC) every 3 days for a total of 6 doses. The placebo microemulsion also was administered simultaneously in equivalent amounts. The SC injection of the free drug reduced the spleen parasite load by 30%, whereas the microemulsion-incorporated drug reduced it by 50%. The placebo microemulsion, probably due to the adjuvant effect, reduced the spleen parasite burden by 11%. Thus, microemulsion-incorporated quercetin has shown significantly greater efficacy than the free drug against experimental leishmaniasis in hamsters.
TABLE 1 Effect of quercetin on 30-day infected hamster models undergoing experimental leishmaniasis
The SAP, SGPT, urea, and creatinine levels of the placebo, microemulsion-treated, free-drug treated and drug-loaded microemulsion-treated hamsters undergoing experimental leihsmaniasis were more or less comparable. The findings reported here at least suggest that the incorporation of the drug into the microemulsion did not incresase the hepato- and nephrotoxicity of the formulation, although a decrease would have been advantageous. But our observation of the efficacy of quercetin in the lowering of spleen parasite load considerably on encapsulation in microemulsion over that of the free drug has made the study worth while. To develop formulations containing a lower percentage clove oil or its mixture with other oils to show reduced toxicity, further work is warranted. This is in progress.
CONCLUSION
The clove oil/Tween 20/water can form a very stable (temperature and salt resistant) o/w microemulsion system with reasonably low droplet dimension. Although the vehicle is hepatotoxic and nephrotoxic, the drug-incorporated vehicle seems to function better against experimental leishmaniasis in hamster models than the free drug.
S. Gupta, S. K. Sanyal, and S. Datta thank the Department of Science and Technology, government of India, for financial support to the work. M. K. Basu and S. Lala thank the Council of Scientific and Industrial Research for financial support and Emeritus Scientist position respectively. S. P. Moulik acknowledges with appreciation the Senior Scientist position offered to him by the Indian National Science Academy. Quarcetin was a gift sample obtained by the courtesy of S. Mukhopadhyay of the Medicinal Chemistry Division, Indian Institute of Chemical Biology, Kolkata.
REFERENCES
- Attwood D. Microemulsion. Colloidal Drug Delivery System, J. Kreuter. Marcel Dekker, New York 1994; 31–65
- Acharya A., Sanyal S. K., Moulik S. P. Formation and characterization of a pharmaceutically useful microemulsion derived from isopropyl yristate, polyoxyethylene(4) lauryl ether (Brij 30) isopropyl alcohol and water. Current. Sci. 2001a; 81: 362–370, [CSA]
- Acharya A., Sanyal S. K., Moulik S. P. Physicochemical investigations on microemulsification of eucalyptol and water in presence of polyoxyethylene(4) lauryl ether (Brij 30) and ethanol. Int J. Pharm. 2001b; 229: 213–226, [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Acharya A., Sanyal S. K., Moulik S. P. Formation and characterization of an useful biological system using mixed oil (ricebran and isopropyl myristate) polyoxyethylene (2) oleyl ether (Brij 92), isopropyl alcohol and water. J. Disp. Sci. Tech. 2001c; 22: 551–561, [CROSSREF], [CSA]
- Austin C. A., Patel S., Ono K., Nakane H., Fisher L. M. Site specific DNA cleavage by mammalian DNA topoisomerase II induced by novel flavone and catechin derivatives. Biochem. J. 1992; 282: 883–889, [PUBMED], [INFOTRIEVE], [CSA]
- Baroli B., Lopez-quintela M. A., Delagdo-Charro M., Fades A. M., Lanc-Mendez J. B. Microemulsion for topical delivery of 8- methoxalen. J. Contr. Rel. 2000; 69: 209–218, [CROSSREF], [CSA]
- Basu M. K. Liposomes in drug targeting. Biotech. & Genet. Engg. Rev. 1994; 12: 383–408, [CSA]
- Methods of Enzymatic Analysis, 2nd ed., K. Bergmeyer, Gawehn. Academic Press, New York. vol. 1: 856–864
- Bessey O. A., Lowry O. H., Brock M. A method for rapid determination of alkaline phosphatase with five cubic millimetres of scrum. J. Biol. Chem. 1946; 164: 321–329, [CSA]
- Changez M., Varshney M. Aerosol OT microemulsion as transdermal carriers of tetracaine hydrochloride. Drug Dev. Ind. Pharm. 2000; 26: 507–512, [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- von Corswant C., Thorem P., Engstrom S. Triglyceride based microemulsion for intraveneous administration of sparingly soluble substances. J. Pharm. 1998; 87: 200–209, [CSA]
- Das M. L., Bhattacharya P. K., Moulik S. P. Model biological microemulsion2. Water (cholesteryl benzoate+heptane)-Tx 100-butanol microemulsion containing dextran, bovine serum albumin and NaCl. Langmuir 1991; 7: 636–642, [CROSSREF], [CSA]
- Das N., Mahato S. B., Naskar K., Ghosh B. K., Basu M. K. Targeting of urea stibamine encapsulated in liposomes to reticuloendothelial system for the treatment of experimental leishmaniasis. Biochem. Med. Metab. Biol 1990; 43: 13–133, [CROSSREF], [CSA]
- Florence A. T., Attwood D. Surfactant Systems: Their Chemistry, Pharmacy and Biology. Chapman and Hall, London and New York 1983
- Physicochemical Principles of Pharmacy, A. T. Florence, D. Attwood. Macmillan, London 1998
- Hazra B., Gupta S., Sarkar R., Moulik S. P. Microemulsion encapsulation of diopyrin, a plant derived bisnaphthoquinonoid of potential chemotherapeutic activity. J. Pharm. Pharmacol. 1998; 50: 191, (suppl)[CSA]
- Kahlweit M., Busse G., Faulhaber B. Preparing nontoxic microemulsions. Langmuir 1995; 11: 4185–4187, [CROSSREF], [CSA]
- Nanoparticles. Colloidal Drug Delivery System, J. Kreuter. Marcel Dekker, New York 1994
- Lawrence M. J. Microemulsion as drug delivery vehicles. Current Opin. Colloid Inter. Sci. 1996; 1: 826–832, [CSA]
- Lianli I. L., Nandi I., Kim K. H. Development of an ethyl laurate based microemulsion for rapid- onset of intranasal delivery of diazepam. Int J. Pharm. 2002; 237: 77–85, [CROSSREF], [CSA]
- Looker D. L., Bereus R. L., Marr J. J. Purine metabolism in Leishmania donovani amastigotes and promastigotes. Mol. Biochem. Parasitol. 1983; 19: 15–28, [CROSSREF], [CSA]
- Majhi P. R., Moulik S. P. Physicochemical studies on biological macro and microemulsion VI: Mixing behaviour of eucalyptus oil, water and polyoxyethylene sorbitan monolaurate (Tween 20) assisted by n-butanol and cinnamic alcohol. J. Disp. Sci. Tech. 1999; 20: 1407–1427, [CSA]
- Malcomson C., Lawrence M. J. Comparison of model steroids into nonionic micellar and microemulsion systems. J Pharm. Pharmacol. 1993; 45: 141–143, [CSA]
- Malcomson C., Satra C., Kantaria S., Sidhu A., Lawrence M. J. Effect of oil on the level of solubilisation of testosterone propionate into nonionic oil-in-water microemulsion. J. Pharm. Sci. 1998; 87: 109–116, [CROSSREF], [CSA]
- Mitra B., Saha A., Roy Chowdhury A., Pal C., Mandal S., Mukhopadhyay S., Majumder H. K. Luteolin—an abundant dietary component is a potent antileishmanial agent that acts by inducing topoisomerase-II mediated kinetoplast DNA cleavage leading to apoptosis. Mol. Med. 2000; 6: 527–541, [CROSSREF], [CSA]
- Moulik S. P., Dey G. C., Bhowmik B. B., Panda A. K. Physicochemical studies in microemulsion VI: dynamics and thermodynamics of percolation of water/AOT/n-heptane in presence of sodium cholate and salicylate. J. Phys. Chem. B 1999; 103: 7122–7129, [CROSSREF], [CSA]
- Moreno M. A., Frutos P., Ballesteros M. P. Lyophilised lecithin based oil-water microemulsions as a new and low toxic delivery system for Amphotericin B. Pharm. Res. 2001; 18: 344–351, [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Moreno M., Frutos P., Ballesteros M. P., Lastres J. l. Release of Nortriptylene hydrochloride from oil-in-water microemulsion. Chem. Pharm. Bull. 2000; 48: 1623–1627, [PUBMED], [INFOTRIEVE], [CSA]
- Mukhopadyay L., Mitra N., Bhattacharya P. K., Moulik S. P. Thermodynamics of formation of biological microemulsion (with cinnamic alcohol, Aerosol OT, Tween 20 and water)Êand kinetics of alkaline fading of crystal violet in it. J. Coll. Int. Sci. 1997; 186: 1–8, [CROSSREF], [CSA]
- Myers D. Surfactant Science and Technology. VCH Publishers, New York 1988; 235–238
- Park K. M., Kim C. K. Preparation and characterization of flurbiprofen-loaded microemulsion for parenteral delivery. Int. J. Pharm. 1999; 181: 173–179, [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Paul B. K., Moulik S. P. Microemulsions: an overview. J. Disp. Sci. 1997; 18: 301–367, [CSA]
- Reitman S., Frankel S. A colorimetric method for the determination of serum oxaloacetic and glutamic pyruvic transaminase. Am. J. Clin. Pathol. 1957; 28: 56–63, Cited In Handbook of Enzymatic Methods of Analysis, ed. G. G. Giulbault, pp 121–123. New York and Basel: Marcel Dekker. 121–123[PUBMED], [INFOTRIEVE], [CSA]
- Richardson C. J., Mbanefo A., Aboofazeli R., Lawrence M. J., Barlow D. J. Prediction of phase behavior in microemulsion systems using artificial neural network. J. Coll. Int. Sci. 1997; 187: 296–303, [CROSSREF], [CSA]
- Microemulsions, I. D. Robb. Plenum Press, New York 1982
- Sarkar S., Mandal S., Sinha J., Mukhopadhyay S., Das N., Basu M. K. Quercetin: critical evaluation as an antileishmanial agent in vivo in hamsters using different vesicular delivery modes. J. Drug Target 2002; 10: 573–578, [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Selvi R. T., Niranjali S. Inhibition by euginol of diethylnitrosamine- induced microsomal degranulation. Fitotherapia 1998; 69: 115–117, [CSA]
- Stauber L. A., Franchino E., Grun J. An eight day method for screening compounds against Leishmania donovani in golden hamster. J. Protozool. 1958; 5: 263–273, [CSA]
- Tenjarala S. Microemulsions: an overview and pharmaceutical applications. Crit. Rev. Therap. Drug Carrier Syst. 1999; 16: 461–521, [CSA]
- Trotta M., Ugazio E., Gasco M. R. Pseudo-ternary phase diagram of lecithin-based microemulsions: Influence of monoalkyl phosphates. J. Pharm. Pharmacol. 1995; 47: 451–454, [PUBMED], [INFOTRIEVE], [CSA]
- Trotta M. Influence of phase transformation of indomethacin release from microemulsion. J. Cont. Rel. 1999; 60: 399–405, [CROSSREF], [CSA]
- Yamashita Y., Kowada S. Z., Nakano H. Induction of mammalian topoisomerase II dependent DNA cleavage by non-interactive flavonoids, genistein and orobol. Biochem. Pharmacol. 1990; 39: 737–744, [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]