Abstract
The objective of this study was to establish a drug transport study using human nasal epithelial (HNE) cell monolayers cultured by the air-liquid interface (ALI) method using serum-free medium (BEGM:DME/F12, 50:50). The cells were developed and characterized in comparison to those that have been previously cultured by the liquid-covered culture (LCC) method. The epithelial cell monolayer cultured by the ALI method resulted in a significantly higher transepithelial electrical resistance value (3,453 ± 302 ohm × cm2) that was maintained (>1,000 ohm × cm2) for up to 20 days compared with that cultured by the LCC method. Observation by scanning electron microscopy revealed mature cilia after 2 weeks in the ALI culture, while flatten unhealthy ciliated cells were observed in the LCC method. After 21 days, higher level of MUC5AC and 8 mRNA were expressed in ALI culture which confirmed the secretory differentiation of HNE monolayers in vitro. No significant difference in the permeability coefficients of a model hydrophilic marker (14C-mannitol) and a lipophilic drug (budesonide) was observed between the two conditions on day 7. The passage 2–3 of the HNE monolayer using ALI condition retained the morphology and differentiated features of normal epithelium. Thus it would be a suitable model for in vitro nasal drug delivery studies.
During the past decades, nasal drug delivery has emerged as a noninvasive alternative for drugs ranging from small metal ions to large macromolecular proteins. Attractive features of nasal drug delivery include the large surface area of the nasal cavity, avoidance of the hepatic first-pass effect, and the relatively high blood flow, which promotes rapid absorption (Hussain, Hirai, and Bawarshi Citation1981; Faraj et al. Citation1990; Merkkus, Schipper, and Verhoef Citation1996). Various in vivo and in vitro models on nasal drug delivery have been described in literature (Krishnamoorthy and Mitra Citation1998; Cornaz and Burri Citation1994), where in vivo animal models or in vitro excised animal tissue models have been used due to the methodological and ethical limitations associated with use of human specimens. On the other hand, research is being actively conducted in developing and validating human nasal cell culture systems to serve as alternatives to animal models (Werner and Kissel Citation1995; Kissel and Werner Citation1998; Agu et al. Citation2001, Citation2002, Citation2003).
Recent studies on human nasal epithelial (HNE) cell culture have provided valuable in vitro models for the study of nasal physiology in healthy and disease states (Dormer et al. Citation2005; Harris et al. Citation2004). Although these recent advances appear promising, problems including the limited amount of available cells and epithelial cell differentiation still remain. To address this problem, a passaged human nasal cell culture system has been developed by our research group focusing on the development of tight junctions for drug transport studies (Roh et al. Citation1999; Yoo et al. Citation2003). Each passage culture up to passage 4 formed a tight monolayer with high TEER, although the differentiation of cilia and mucin secreting cells were not complete.
In nasal epithelium, the important properties of cultured epithelial cells include the formation of confluent cell layers interconnected by tight junction, the expression of apical cilia, and the production and secretion of mucin. Among them, ciliated cells in nasal epithelium culture play an important role in the physiological function, such as mucocilliary clearance, which also may be relevant to nasal drug delivery (Schipper, Verhoef, and Merkus Citation1991). Additionally, differentiation of transporters and the presence of enzymatic systems increase the predictive potential of the in vitro cell culture models. However, compared with the in vivo nasal epithelium, relatively undifferentiated epithelium with few ciliated cells existed in our previous report on passaged nasal culture model (Yoo et al. Citation2003). The expression of differentiated phenotype as well as the morphological and functional features of cells may be affected by the culture condition, such as selecting the substratum and the right mix of media additives including differentiation inducer (e.g., retinoic acid) and creating an air-liquid interface (ALI) condition. Moreover, the development of serum-free hormone-supplemented culture medium has made it possible to induce the differentiation of airway epithelial cells with similar morphologic epithelium in vivo (Wu et al. Citation1985; Gruenert, Basbanm, and Widdicombe Citation1990). In recent studies, ALI culture condition with serum-free medium is commonly used for airway epithelial cell culture monolayers (Mathias et al. Citation1995; Mattinger et al. Citation2002; Agu et al. Citation2001) to produce more ciliated cells and/or to enhance mucin secretion.
The present work is an attempt to systematically improve the differentiation of the serially passage cultured human nasal cell monolayers by applying the ALI condition to produce more ciliated cells for drug transport studies. The properties of the nasal epithelium using ALI as well as LCC culture conditions were investigated using bioelectric and morphological studies. To determine the secretory differentiation of nasal epithelium, RT-PCR for the mucin gene expression was analyzed. The drug transport of 14C-mannitol and budesonide was also studied as the paracellular and the transcellular route markers, respectively.
MATERIALS AND METHODS
Culture Conditions
Nasal specimens were obtained during surgery from inferior turbinate mucosa of patients suffering from septal deviation or chronic sinusitis. The isolation and expansion of human nasal cells used in this study has been previously described in detail (Yoo et al. Citation2003). Briefly, the nasal specimens were dissociated enzymatically overnight at 4°C using 1.0% Pronase (type XIV protease, Sigma, St. Louis, MO, USA). Dissociated epithelial cells were washed and suspended in DMEM/F12 (Dulbecco's Modified Eagle Medium: nutrient mixture F12 [Ham] 1:1) containing 10% fetal bovine serum (FBS). Cell suspension was preplated on a plastic dish at 37°C for 1 hr to eliminate fibroblasts, endothelial cells, and myoblasts. Suspended epithelial cells were frozen using DMSO and stored in a liquid nitrogen tank. Frozen passage-1 stocks were thawed and seeded at 500 cells/cm2 in plastic tissue culture dishes. Culture incubator was maintained at 37°C in an atmosphere of 5% CO2 and 95% relative humidity. The medium of serum-free BEGM (Bronchial Epithelial Growth Medium) was changed every 2 days.
When cultures reached 70–80% confluency, the cells were detached with 0.1% trypsin-EDTA (Gibco BRL, Gaithersburg, MD, USA) and were seeded on Transwell® at a density of 5 × 105 cells/cm2 (Costar®, 12 mm, polyester 0.4 μm, Cambridge, MA, USA). The volumes of apical and basolateral culture media were 0.5 and 1.5 mL, respectively. Both sides of the Transwell® were filled with BEGM:DMEM/F12 (50:50) supplmented with hydrocortisone (0.5 μ g/mL), insulin (5 μ g/mL), transferrin (10 μ g/mL), epinephrine (0.5 μ g/mL), triiodothyronine (6.5 μ g/mL), gentamycin (50 μ g/mL), amphotericin-B (50 μ g/mL), retinoic acid (0.1 ng/mL), and epidermal growth factor (0.5 ng/mL human recombinant) (all supplied by Clonetics Corp., San Diego, CA, USA), and then was incubated at 37°C in an atmosphere of 5% CO2 and 95% relative humidity. For LCC culture condition, the media in both sides were changed after 24 hr of seeding and then every 2 days. For ALI culture condition, the media in both sides were changed after 24 hr and 72 hr of seeding, and then the apical surface of the monolayer was exposed to air after the nasal cells reached confluence on day 3, after which only the media in the basolateral side was changed every 2 days. The remaining cells after seeding were kept in a liquid nitrogen tank until serially subcultured on a plastic culture dish, as mentioned above.
Transepithelial Electrical Resistance Measurement
After seeding, the transepithelial electrical resistance (TEER) was measured daily using an EVOM voltohmmeter device (WPI, Sarasota, FL, USA), and corrected by subtracting the background due to the blank transwell insets and medium. For ALI monolayer, 0.5 mL and 1.5 mL of pre-equilibrated medium were added to the apical and basolateral reservoirs, respectively, the monolayers were allowed to attain a steady potential for 5 min prior to making bioelectric measurements. The integrity of the monolayer of nasal cells also was determined by measuring TEER values before and after the transport studies.
Scanning Electron Microscopy
Scanning electron microscopy (SEM) was performed to characterize the morphology of passage-2 nasal epithelial cells using LCC and ALI condition after 7, 14, and 21 days of seeding. The cells were rinsed with 0.1 M PBS and were fixed in 2.5% glutaraldehyde in 0.1 M PBS (pH 7.4) for 2 hr at 4°C. After rinsing with PBS, the specimens were stained with 1% osmium tetroxide for 1 hr at 4°C. Subsequently, the specimens were dehydrated through serial ethanol solutions and dipped in hexamethyldisilazane for 15 sec. The specimens were coated with gold in a sputter coater (E-1030, Hitachi, Tokyo, Japan) and examined by the scanning electron microscope (S-4300, Hitachi, Tokyo, Japan).
Reverse Transcription-Polymerase Chain Reaction
Reverse transcription-polymerase chain reaction (RT-PCR) was performed for the detection of mucin gene mRNAs. The methods for detection of mucin gene mRNAs previously have been described in detail (Yoon et al. Citation2000). Oligonucleotide primers were designed according to the published sequences for human mucin gene MUC5AC (680bp, 5′ primer: TCC GGC TCA TCT TCT TCC, 3′ primer: ACT TGG GCA CTG GTG CTG), MUC5B (338bp, 5′ primer: ACT CCA GAG ACT GTC CAC AC, 3′ primer: TAC CAC TGG TCT GTG TGC TA), and MUC8 (239bp, 5′ primer: ACA GGG TTT CTC CTC ATT G, 3′primer: CGT TTA TTC CAG CAC TGT TC).
Total RNAs of LCC and ALI human nasal monolayer on 7 and 21 days were extracted with TRIzol® reagent according to the manufacturer's recommendations. Total RNA (2 μ g) was reverse-transcribed into complementary DNA (cDNA) with random primers (Promega) and Superscript II reverse transcriptase (Gibco BRL). RT-PCRs were performed with a Perkin Elmer Cetus DNA Thermal Cycler. Denaturation was carried out at 94°C for 30 sec. The annealing temperature was at 52°C for 1 min. Extension was performed at 72°C for 1 min.
The PCR products were separated by electrophoresis on a 1.2% agarose gel containing 50 ng/mL ethidium bromide and photographed with Polaroid type 55 films. To verify that the amplified products were from mRNA and not genomic DNA contamination, negative controls were performed by omitting reverse transcriptase from RT-PCT reaction. In the absence of reverse transcriptase, no PCR products were observed (data not shown).
Transport Studies
The transport studies were performed by initially incubating the monolayers in transport medium (Hank's Balanced Salt Solution supplemented with 15 mM glucose and 15 mM HEPES buffer, pH 7.4) for 15 min at 37°C. After measuring the TEER value, each transport experiment was performed by adding 0.4 mL of transport medium containing budesonide (20 μ g/mL, Sigma) or 4 μ M 14C-mannitol (specific activity of 56.0 Ci/mmol, Amersham, Arlington, IL, USA) in apical side, and 1.0 mL of blank transport medium in basolateral side. At predetermined time intervals, 1.0 mL samples were withdrawn from the basolateral side and quickly replaced with an equal volume of fresh transport medium. Permeation studies were conducted with the monolayer on day 7 (passage 2 or 3). After 1 hr transport experiments, the TEER value was determined to check the integrity of the cell monolayers.
For analysis of 14C-mannitol samples, 0.3 mL of each sample was mixed with 2.0 mL scintillation cocktail (Ultima Gold, Packard, Netherlands) and was analyzed by the Tri-Carb 2200CA liquid scintillation counter (Packard Instrument, Meriden, CT, USA). The concentration of budesonide in samples was determined using HPLC system equipped with a binary pump system (Gilson Model 305 and 306) and an automatic injector (Gilson Model 234). Merck C18 LiChroCART® 125-4 column (5 μm, 125 × 4 mm, Merck, Darmstadt, Germany) was used with a mobile phase of 70% methanol and 30% water. With a flow of 1.0 mL/min and a detection wavelength of 242 nm, retention time was 4.2 min.
The apparent permeability coefficient (Papp, cm/s) of 14C-mannitol and budesonide was estimated using the following equation:
where, dQ/dt is the solute flux obtained from linear regression, A is the surface area of a permeable support (1.0 cm2), and C0 is the initial drug concentration.
RESULTS AND DISCUSSION
Change of TEER Value
shows the change of TEER values of epithelial cell monolayers (passage 2) at the seeding density of 5 × 105 cells/cm2 for 20 days. In ALI condition, when air-liquid interface condition was initiated from day 3 after seeding, a maximum TEER (3,453 ± 302 ohm × cm2) was observed on day 5. The high TEER value (> 1,000 ohm × cm2) was maintained for at least 10 days. In LCC condition, however, the maximum TEER value (1,963 ± 413 ohm × cm2) appeared on day 2 and decreased quickly. Thus, it was possible to culture human nasal epithelial cells at an air-liquid interface that exhibited better electrophysiological characteristics than those cultured by the conventional liquid-covered culture, which developed high TEER value in relatively short term and decreased slowly.
FIG. 1. Change of transepithelial electrical resistance (TEER) of HNE cell monolayers grown under the LCC (•) or ALI (▴) condition. Each value is the mean ± SD of 3 determinations (passage 2).
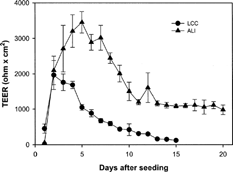
The TEER values that we have obtained in both culture conditions were higher than those reported from conventional primary cultures. In human nasal primary culture (Remigius et al. Citation2001) and rabbit tracheal primary culture systems (Mathias et al. Citation1995), maximum TEER were reported to be 1,349 ± 508 ohm × cm2 in 8 days and about 1,500 ohm × cm2 in 7 days, respectively. The air-liquid interface (i.e., exposing fluid-free on the apical surface of the nasal cell layers) condition and/or selection of culture media most probably played a critical role in developing the tight junction, thereby increasing the TEER value.
ALI cultures have been found to grow epithelial cells in a configuration resembling in vivo conditions. It has shown to not only mimic the morphologic features of the epithelium, but also to perform many of its physiologic and biochemical activities (Gray et al. Citation1996). Moreover, the selection of culture medium used in ALI also may have played an important role. BEGM, which is normally the medium used for ALI, is reported to be suitable for obtaining more homogeneous cell morphology than DMEM (Mattinger et al. Citation2002) which was used in the LCC method. DMEM with 10% FBS seems to be critical to stimulate cell proliferation in the early stage of the culture (Yoo et al. Citation2003), while retinoic acid in BEGM seems to be an important factor for the growth and differentiation of airway epithelial cells (Karrtinen et al. Citation1993; Yoon et al. Citation2000). Therefore, in this study, the mixture of BEGM and DMEM/F12 (50:50) with all supplements was used for the ALI cultures, which may have caused the increased and prolonged TEER values ().
Scanning Electron Microscopy
The morphological characteristics of the nasal epithelial monolayers, specially the development of cilia, were observed by SEM study. The most distinguished morphological difference between the two methods was the development of ciliated cells. The presence of cilia on the apical surface of ALI culture was shown on day 7 (). The number of ciliated cells increased on the 14th day (). After 3 weeks of ALI culture, markedly increased number of ciliated cells matured with long and healthy cilia were observed ( and ). In LCC conditions, however, denuded and flattened ciliated cells and numerable microvillus were observed during the 3 weeks (, , , and ).
FIG. 2. Scanning electron microscopy of the human nasal epithelial cell monolayer (passage 2). (A) ALI condition after 7 days, (B) LCC condition after 7 days, (C) ALI condition after 14 days, (D) LCC condition after 14 days, (E) and (G) ALI condition after 21 days, (F) and (H) LCC condition after 21 days. (A)–(F) are ×3000 magnification; (G) and (H) are of × 10,000 magnification.
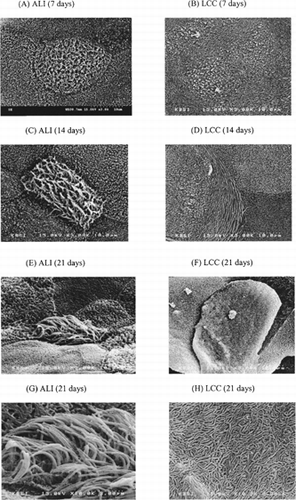
In ALI culture, the number of ciliated cells was more than 10% after 14 days of culture, which is consistent with the previous reports of airway epithelium cultures (Kaartinen et al. Citation1993; Mathias et al. Citation1995). They reported that submerged cultures resulted in a lower epithelial thickness (flattened cells) with a loss of cilia and a decrease in the number of ciliated cells, while ALI could acquire a cubical or cobblestone appearance. The morphological appearance of nasal epithelium of ALI culture was known to be much closer to the nasal tissue in vivo than that of LCC condition. The morphological and functional characteristics in the nasal epithelial cell layers grown under ALI condition can improve delivery of oxygen across the thin film of liquid to the cells that may change the cellular respiration to a more aerobic nature (Mathias et al. Citation1995). Thus, limited numbers of reports are found on LCC condition for the development of differentiated nasal epithelium (Werner and Kissel Citation1995; Kissel and Werner Citation1998; Yoo et al. Citation2003).
RT-PCR
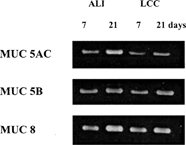
Mucin was selected as the major mucous secretion in this study to observe the secretory cell differentiation, since drug transport might be affected by mucin on the surface of the epithelial cells (Marttin et al. Citation1998). Mucin is the major airway secretion produced by mucous cells, whereas nonmucin secretions, such as lysozyme, lactoferrin, secretory IgA, and secretory leukocyte protease inhibitor, are secreted by serous cells (Yoon et al. Citation2000). Among 13 human mucin genes, MUC5AC, 5B, and 8 mRNAs were chosen in this study because the first two are known to be the major mucin gene in the goblet cell of the respiratory mucosa and MUC 8 was found to be the major mucin gene in the polyp epithelium (Buisine et al. Citation1999; Hoverberg, Davies, and Carlstedt Citation1996).
Levels of mRNA expressions of three mucin genes (MUC5AC, 5B, and 8 mRNAs) were measured by RT-PCR analysis after 7 and 21 days of LCC and ALI cultures. As shown in , MUC5AC, 5B, and 8 mRNAs were expressed in both ALI and LCC culture of passaged HNE cells, which suggests that culture of HNE cells induced mucous cell differentiation. This is also consistent with our previous report that observed the mucin granules in TEM study (Yoo et al. Citation2003) and with the recent reports on the differentiation process of nasal epithelial cells (Yoon et al. Citation2003; Hanamaure, Deguchi, and Ohyama Citation1994; Usui et al. Citation2000; Gray et al. Citation1996). Moreover, mucin gene expression was more significant in ALI culture in 21 days than that of LCC in 21 days, which again confirmed the importance of retinoic acid in BEGM and ALI condition on the growth and differentiation of airway epithelial cells (Yoon et al. Citation2000; Karrtinen et al. Citation1993). ALI culture probably can deliver oxygen across the thin film of liquid to the cells that may subsequently change cellular respiration to a more aerobic nature, thereby improving the differentiation of nasal epithelial cells than LCC condition and maintain higher TEER value for up to 21 days.
Transport Studies
The comparison of in vitro transport studies between ALI and LCC nasal epithelium was performed using 14C-mannitol and budesonide as a paracellular and a transcellular route marker, respectively. Based on the previous study (Yoo et al. Citation2003), nasal cell monolayers with TEER value of higher than 500 ohm × cm2 were used for drug transport studies of hydrophilic and lipophilic markers. Although high TEER value of ALI culture maintained up to 21 days, the transport studies were conducted on day 7 when TEER value of LCC monolayer was above 500 ohm × cm2.
As shown in , the transport profiles of both 14C-mannitol and budesonide across the nasal epithelial cell monolayers cultured by LCC or ALI method for 7 days were linear for up to 60 min. Because mucin and/or cilia developed in ALI condition may work as a barrier of drug transport, lower Papp value in ALI than that in LCC method was expected. However, it was interesting to note that no significant difference in the Papp value was observed between the two methods in this study (), unlike what had been reported previously on solute permeability of LCC and ALI cell layers in rabbit (Mathias et al. Citation1995; Yang et al. Citation2000). Yang et al. (Citation2000) reported that the Papp of the lipophilic solutes (β-blockers) across primary conjunctival epithelial cell monolayer was about 3-fold lower in ALI than in LCC condition. They suggested that the permeability of ALI generally better reflects that of the excised tissue than LCC. Mathias et al. (Citation1995) also reported that the permeability of hydrophilic solutes across the rabbit tracheal epithelial cell monolayer grown under ALI culture was only half of that under LCC condition, whereas the permeability of the lipophilic solutes was similar in both culture conditions. They explained that the tight junctional arrangement (e.g., number of strands, junctional length, presence of pores or channels within the junction, or length of lateral intercellular space) might be widely different depending on the culture conditions. It may be possible that the higher than expected Papp of mannitol in this study may be due to factors related to characteristics of the human nasal cells that differ from the conjunctial cells used by CitationYang et al. (2002) or tracheal cells used by Mathias et al. (Citation1995). However, what exactly is the factor that would cause this different type of result would need further investigation. Nevertheless, these results imply that the intact tight junction was well developed in both culture methods and that the monolayers can be used for transport studies of both hydrophilic and lipophilic drugs.
FIG. 4. Transport profiles of (A) 14C-mannitol and (B) budesonide across the passage cultured human nasal cell monolayer using ALI (•) or LCC (▴) condition for 7 days (passage 2 and 3). Each value is the mean ± SD of 3 determinations.
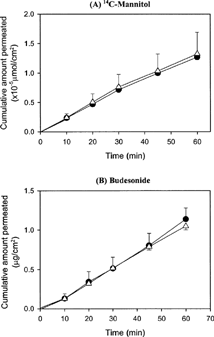
TABLE 1 Apparent permeability coefficients (Papp) of 14C-mannitol and budesonide across the passage cultured human nasal epithelial cell monolayers grown under the air-liquid interface or liquid-covered culture condition for 7 days
CONCLUSIONS
It was possible to culture HNE cell monolayers under ALI conditions that resembles the in vivo situation. Serially passaged culture under ALI condition allows the preparation of a large number of highly differentiated epithelial cell monolayers that developed cilia on the apical surface and mucin gene expression better than LCC condition. Higher TEER value in ALI was maintained for longer duration than that in LCC. Thus, the ALI culture of passaged HNE cell monolayer seems to be an appropriate in vitro model for nasal secretion and differentiation studies, and more specifically for nasal drug transport studies.
This study was supported by the Medical Research Institute Grant (2001-23), Pusan National University Hospital.
REFERENCES
- Agu R. U., Jorissen M., Willems T., Angustijins P., Kinget R., Verbeke N. In vitro nasal drug delivery studies: comparison of derivatised, fibrillar and polymerized collagen matrix-based human nasal primary culture systems for nasal drug delivery studies. J. Pharm. Pharmacol. 2001; 53: 1447–1456, [PUBMED], [INFOTRIEVE], [CSA]
- Agu R. U., Jorissen M., Willems T., Kinget R., Verbeke N., Augustijns P. Alternatives to in vivo nasal toxicological screening for nasally-administered drugs. S.T.P. Pharm. Sci. 2002; 12: 13–22, [CSA]
- Agu R., Dang H. V., Jorissen M., Willems T., Vandoninck S., Van Lint J., Vandenheede J. V., Kinget R., Verbeke N. In vitro polarized transport of L-phenylalanine in human nasal epithelium and partial characterization of the amino acid transporter involved. Pharm. Res. 2003; 20: 1125–1132, [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Buisine M. P., Devisme L., Copin M. C., Durand-Reville M., Gosselin B., Aubert J. P., Porchet N. Developmental mucin gene expression in the human respiratory tract. Am. J. Respir. Cell Mol. Biol. 1999; 20: 209–218, [PUBMED], [INFOTRIEVE], [CSA]
- Cornaz A. L., Burri P. Nasal mucosa as an absorption barrier. Eur. J. Pharm. Biopharm. 1994; 40: 261–270, [CSA]
- Dormer R. L., Harris C. M., Clark Z., Pereira M. M., Doull I. J., Norez C., Becq F., McPherson M. A. Sildenafil (Viagra) corrects DeltaF508-CFTR location in nasal epithelial cells from patients with cystic fibrosis. Thorax. 2005; 60: 55–59, [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Faraj J. A., Hussain M. R., Aramake Y., Iseki K., Kagoshima M., Dittert L. W. Mechanism of nasal absorption of drugs, ø. Nasal absorption of leucine enkephalin. J. Pharm. Sci. 1990; 79: 698–702, [PUBMED], [INFOTRIEVE], [CSA]
- Gray T. E., Guzman K., Davis C. W., Abdullah L. H., Nettesheim P. Mucociliary differentiation of serially passaged normal human tracheobronchial epithelial cells. Am. J. Respir. Cell Mol. Biol. 1996; 14: 104–112, [PUBMED], [INFOTRIEVE], [CSA]
- Gruenert D. C., Basbanm C. B., Widdicombe J. H. Long-term culture of normal and cystic fibrosis epithelial cells grown under serum-free condition. In Vitro Cell Dev. Biol. 1990; 26: 411–418, [PUBMED], [INFOTRIEVE], [CSA]
- Hanamaure Y., Deguchi K., Ohyama M. Ciliogenesis and mucus synthesis in cultured human respiratory epithelial cells. Ann. Otol. Rhinol. Laryngol. 1994; 103: 889–895, [CSA]
- Harris C. M., Mendes F., Dragomir A., Doull I. J., Carvalho-Oliveira I., Bebok Z., Clancy J. P., Eubanks V., Sorscher E. J., Roomans G. M., Amaral M. D., McPherson M. A., Penque D., Dormer R. L. Assessment of CFTR localisation in native airway epithelial cells obtained by nasal brushing. J. Cyst. Fibros. 2004; 43–48, Suppl 2[CROSSREF], [CSA]
- Hoverberg H. W., Davies J. R., Carlstedt I. Different mucins are produced by the surface epithelium and submucosa in human trachea: identification of MUC 5AC as a major mucin form the goblet cells. Biochem. J. 1996; 318: 319–324, [CSA]
- Hussain A. A., Hirai S., Bawarshi R. Nasal absorption of natural contraceptive steroids in rats-progesterone absorption. J. Pharm. Sci. 1981; 70: 466–467, [PUBMED], [INFOTRIEVE], [CSA]
- Kaartinen L., Nettesheim P., Adler K. B., Randell S. H. Rat tracheal epithelial cell differentiation in vitro. In Vitro Cell Dev. Biol. 1993; 29A: 481–492, [CSA]
- Kissel T., Werner U. Nasal delivery of peptides: an in vitro cell culture model for the investigation of transport and metabolism in human epithelium. J. Control Rel. 1998; 53: 195–203, [CROSSREF], [CSA]
- Krishnamoorthy R., Mitra A. K. Prodrugs for nasal drug delivery. Adv. Drug Deliv. Rev. 1998; 29: 135–146, [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Marttin E., Schipper N. G. M., Verhoef J. C., Merkus F. W. H. M. Nasal mucociliary clearance as a factor in nasal drug delivery. Adv. Drug Deliv. Rev. 1998; 29: 13–38, [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Mathias N. R., Kim K. J., Robison T. W., Lee V. H. L. Development and characterization of rabbit tracheal epithelial cell monolayer models for drug transport studies. Pharm. Res. 1995; 12: 1499–1505, [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Mattinger C., Nyugen T., Schafer D., Hormann K. Evaluation of serum-free culture conditions for primary human nasal epithelial cells. Int. J. Hyg. Environ. Health 2002; 205: 235–238, [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Merkkus F. W. H. M., Schipper N. G. M., Verhoef J. C. The influence of absorption enhancers on intranasal insulin absorption in normal and diabetic subjects. J. Control Rel. 1996; 41: 69–75, [CROSSREF], [CSA]
- Remigius U. A., Mark J., Tom W., Patrick A., Renaat K., Norbert V. In vitro nasal drug delivery studies: comparison of derivatised, fibrillar and polymerized collagen matrix-based human nasal primary culture systems for nasal drug delivery studies. J. Pharm. Pharmacol. 2001; 53: 1447–1456, [CSA]
- Roh H. J., Goh E. K., Wang S. G., Chon K. M., Yoon J. H., Kim Y. S. Serially passaged normal human nasal epithelial cells, morphology and mucous secretory differentiation. J. Rhinol. 1999; 6: 107–112, [CSA]
- Schipper N. G. M., Verhoef J. C., Merkus F. W. H. M. The nasal mucociliary clearance: relevance to nasal drug delivery. Pharm. Res. 1991; 81: 807–814, [CROSSREF], [CSA]
- Usui S., Shimizu T., Kishioka C., Fujita K., Sakadura Y. Secretory cell differentiation and mucus secretion in cultures of human nasal epithelial cells: use of a monoclonal antibody to study human nasal mucin. Ann. Otol. Rhinol. Laryngol. 2000; 109: 271–277, [PUBMED], [INFOTRIEVE], [CSA]
- Wu R., Yankaskas J., Cheng E., Knowles M. R., Boucher R. Growth and differentiate of human nasal epithelial cells in culture, serum free, hormone-supplemented medium and proteoglycan synthesis. Am. Rev. Respir. Dis. 1985; 132: 311–320, [PUBMED], [INFOTRIEVE], [CSA]
- Werner U., Kissel T. Development of a human nasal cell culture model and its suitability for transport and metabolism studies under in vitro conditions. Pharm. Res. 1995; 12: 565–571, [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Yang J. J., Ueda H., Kim K. J., Lee V. H. L. Meeting future challenges in topical ocular drug delivery: development of an air-interfaced primary culture of rabbit conjunctival epithelial cells on a permeable support for drug transport studies. J. Control Rel. 2000; 65: 1–11, [CROSSREF], [CSA]
- Yoo J. W., Kim Y. S., Lee S. H., Lee M. K., Roh H. J., Jhun B. H., Lee C. H., Kim D. D. Serially passaged human nasal epithelial cell monolayer for in vitro drug transport studies. Pharm. Res. 2003; 20: 1690–1696, [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Yoon J. H., Kim K. S., Kim S. S., Lee J. G., Park I. Y. Secretory differentiation of serially passaged normal human nasal epithelial cells by retonic acid: expression of mucin and lysozyme. Ann. Otol. Rhinol. Laryngol. 2000; 109: 594–601, [PUBMED], [INFOTRIEVE], [CSA]
- Yoon J. H., Moon H. J., Seong J. K., Kim C. H., Lee J. J., Choi L. Y., Song M. S., Kim S. H. Mucociliary differentiation according to time in human nasal epithelial cell culture. Differentiation 2003; 70: 77–83, [CROSSREF], [CSA]