Abstract
Metoprolol tartrate sustained-release tablets (100 mg) were prepared using xanthan/guar gums and also hydroxypropyl methyl cellulose (HPMC) carboxymethyl-Cellulose (CMC) polymers by direct compression method. Physical characteristics of the tablets and water uptake in addition to their dissolution profiles were compared with standard (Lopressor® SR) tablets. Dissolution test was performed in the phosphate buffer solution (pH 6.8) and the samples were analyzed spectrophotometerically in 275.7 nm. Dissolution studies showed that formulations containing 100 and 80% of HPMC, 100% of guar, and 20% of xanthan followed the Higuchi model, while those containing 60 and 40% HPMC and 100 and 80% xanthan followed a zero-order model. The tablets with 40% xanthen followed a Hixon-Crowell model. In cellulose derivatives the highest MDT and dissolution efficiency until 8 hr (DE8%) belonged to tablets with 40% HPMC, increasing the amount of CMC decreased the drug release rate, and formulations containing 60 and 40% of HPMC had the USP dissolution standards. While, in the gum formulations, the highest mean dissolution time and the lowest DE8% belonged to tablets with 100% xanthan, increasing the xanthan decreased the release rate of metoprolol, and formulations containing 80 and 100% xanthan had the USP dissolution standards. Results showed that natural gums are suitable for production of sustained-release tablets of metoprolol.
Hydrophilic polymers are widely used in the formulation of modified–release oral dosage forms. Various synthetic polymers (HPMC, NaCMC, polymethacrylale, etc.) and natural materials (xanthan, guar gum, and chitosan) have been tried by various researchers. It has been shown that in hydrophilic matrices, swelling as well as erosion of the polymer occurs simultomeously, and both of them contribute to the overall drug release rate (Sujja-areevath et al. Citation1998).
Guar gum, a natural macromolecular galactomannan, and xanthan gum, a macromolecule product of Xanthomana campestris composed of glucose, mannose, and glucorunic acid (Castro, Tirapegui, and Benedicto Citation2003), are significantly less expensive than hydroxypropyl-methylcellulose (HPMC) and other cellulosic derivatives (Altaf et al. Citation1998a). Recently the potential of guar gum as an inexpensive and flexible carrier for oral extended release drug delivery has been highlighted. In vitro and in vivo release of a highly soluble drug (diltiazem) from guar gum matrix tablets was found to be similar to that of a commercial reference product (Altaf et al. Citation1998b). Guar formulations are relatively insensitive to changes in stirring speed of dissolution test, compaction pressure, or storge under accelerated stress conditions. These tablets also showed good in vitro, and in vivo correlation (Yu et al. Citation1998). Guar-based tablets of low soluble drugs (ketoprofen and nifedipine) have shown also comparable release to commercial products (Oruvail® and Procardia XL®) (Yu et al. Citation1997; Friend et al. Citation1997). However, guar gum alone may not sustain drug release satisfactorily requiring the addition of other hydrocolloids like HPMC in relatively large amounts (Bhalla and Sanzgiri Citation1987).
The aim of our study was to assess drug release from guar gum and xanthan-based matrix tablet formulations of metoprolol and the capability of these natural gums in production of sustained–release tablets in comparison with cellulosic derivatives. The potential advantages of natural gums as sustained–release excipients are their high viscosity, low cost, and commercial availability (Altaf et al. Citation1998a). The ultimate goal of our work is to provide natural gum based sustained-release systems that are inexpensive, in terms of raw material and manufacturing coasts, and suitably robust to accommodate a variety of drugs.
MATERIALS AND METHODS
Metoprolol tartrate was gift from Alborz Daru, (Iran). Other materials were Lopressor® SR 100 mg tablets (Novartis, Swithzerland), hydroxypropyl methylcellulose (HPMC K4M) (Shinetsu, Japan), sodium carboxymethyl cellulose (NaCMC), xanthan gum (Arthur Branwell, England), guar gum (Hercules, USA), microcrystalline cellulose (Avicel PH101) (FMC, USA), titanium dioxide (Quitrous, Germany), magnisum stearate (Merck Chemical Company, Germany), and colloidal silicon dioxide (Aerosil) (Aldrich, Germany).
Preparation of Matrix Tablets
Round tablets were prepared by a direct compression method with a single punch tableting machine (Kilian Co, GmbH, Koln-Niehl, Germany). The total weight of the tablets was 320 mg, hardness 70 N, and diameter was 12 mm. Different formulations based on natural gums (xanthan/guar) or cellulose derivatives (HPMC/CMC) are shown in .
TABLE 1 Different formulations of metoprolol matrix tablets based on natural gums or cellulose derivatives
Drug Release Studies
Dissolution test was carried out according to method II of USP (USP 25, 2002) in 500 ml of phosphate buffer solution (pH 6.8) at 37 ± 0.5°C. Rotating speed of the paddle was 50 rpm. At 0.5, 1, 2, 4, 6, 8, 10, and 11 hr after starting the test, 3-ml samples of the dissolution medium were withdrawn and analyzed spectrophotometrically at 275.7 nm for metoprolol.
Analysis of Release Data
Dissolution efficiency (DE) (Banakar Citation1992) after 8 hr of release test was used to compare the results of dissolution tests of different formulations:
The other dissolution parameter used for comparing the different formulations was mean dissolution time (MDT), that is calculated from the amount of drug released to the total cumulative drug. MDT is a measure of the rate of the dissolution process: the higher the MDT, the slower the release rate. The following equation was used to calculate the MDT from the mean dissolution data:
Where i is the dissolution sample number, n is the number of dissolution sample time, tmid is the time at the midpoint between i and i − 1, and Δ M is the additional amount of drug dissoloved between i and i − 1 (Gohel and Panchal Citation2002).
The similarity factor f2 was calculated from the mean dissolution data according to the following equation:
Where n is the number of pull points, wt is an optional weight factor, Rt is the reference profile at time point t, and Tt is the test profile at the same time point, the value of f2 should be between 50 and 100 (CitationFDA Guidance 1997). An f2 value of 100 suggests that the test and reference profiles are identical, and as the value becomes smaller, the dissimilarity between release profiles increases.
Erosion and Water-Uptake of Tablets
The matrix erosion and water-uptake studies were carried out by the method of Dürig and Fassihi (Citation2002) to check the effect of formulation ingredients on matrix erosion and to establish a correlation among matrix erosion, water uptake, and drug released. In brief, tablets were subjected to identical conditions as described for dissolution testing. Fully 3 tablets were used per time point. At times 0.5, 1, 2, 4, 6, 8, 10, and 11 hr, the tablets were carefully removed and patted with tissue paper to remove excess surface water. After determining the wet weight, the tablets were dried at 70°C for 10 days, before reweighing to determine the remaining dry weight. Placebo tablets consisting of pure polymers were treated in the same way.
Water uptake and mass loss were determined gravimetrically according to the following equations:
RESULTS
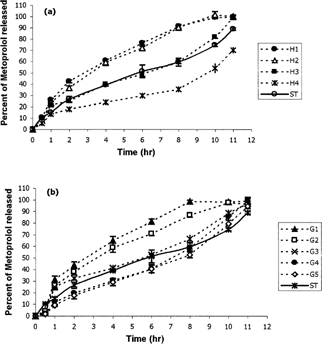
Metoprolol release profiles from different tablets based on cellulose derivatives with different ratios of HPMC and CMC compared with the standard Lopressor tablets are shown in , whereas compares the drug release from tablets based on mixtures of natural gums (xanthan/guar).
FIG. 1 Metoprolol release profiles from different tablets based on (a) cellulose derivatives and (b) mixtures of natural gums, compared with the standard Lopressor tablets (ST) (n=3).
Comparison of release parameters and diffusion exponent of Peppas equation (Mt/M∞ = Ktn) for different formulations are shown in . The results of regression analysis of release data based on best curve-fitting method are shown in .
TABLE 2 Comparison of release parameters and diffusion exponent (n) of Peppas equation for Lopressor (ST) and different formulations of metoprolol matrix tablets based on natural gums or cellulose derivatives
TABLE 3 Regression analysis (r2) of release data based on best curve-fitting method for Lopressor (ST) and different formulations of metoprolol matrix tablets based on natural gums or cellulose derivatives (n = 3)
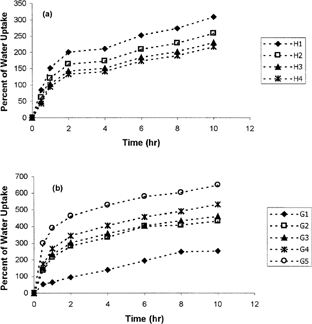
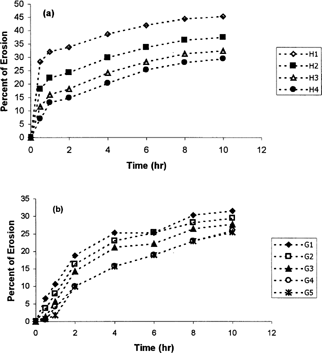
shows water uptake of metoprolol matrix tablets (a) based on cellulose derivatives and (b) based on natural gums. Erosion percentage of metoprolol tablets with cellulosic matrix and natural gums are shown in and , respectively.
DISCUSSION
Cellulosic derivatives like HPMC and CMC are among the most popular hydrophilic polymers used in production of sustained-release (SR) matrix tablets. This is because of their facility for compaction, capability in loading with high percentages of drugs and negligible effect of processing variables on the drug release characteristics from their matrices (Rao, Devi, and Buri Citation1988; Hogan Citation1989).
These polymers have been used for preparation of SR matrix tablets like indomethacin (Talukdar et al. Citation1996), 4-aminopyridin (Juarez, Rico, and Villafuerte Citation2001), mixture of alpernolol and metoprolol (Rao et al. Citation1988) and prednisolone (Rao, Haslam, and Stella Citation2001). However, natural gums like xanthan and guar gum are gaining the researchers' interest in tableting because of their swelling capability, simplicity, and cheap production process (Munday and Cox Citation2000). Xanthan gum specially has better flow properties than HPMC (Talukdar et al. Citation1996). These gums have been reported to be used in production of matrix tablets of indomethacin (Talukdar et al. Citation1996: Talukdar, Rombaut, and Kinget Citation1998), theophylline (Khullar, Khar, and Agarwal Citation1998), diltiazem (Altaf et al. Citation1998a), sodium diclofenac (Munday and Cox Citation2000), and trimethazidin HCl (Krishniah et al. 2002).
As shows Tablet H1 that contains only HPMC as the cellulose derivative releases ∼ 90% of the drug during 8 hr while, H4 that contains 60:40 percent ratio of CMC:HPMC releases only 70% after 11 hr. Considering the USP25 standards (USP25, Citation2002) for SR metoprolol tablets, H1 nd H2 are not suitable while H3 and H4 provide the USP standards. shows that increasing the CMC of tablets increases the MDT while decreasing the DE8%. This table also shows that MDT of tablet H4 is significantly (p < 0.05) higher than other formulations containing cellulosic mixtures and also its DE8% significantly lower (p < 0.05) than others. Rao et al. (Citation1988) and Juarez et al. (Citation2001) also reported that increasing the CMC content of the SR tablets prolonged the dissolution time. and also show that increasing the CMC ratio decreases the water uptake and erosion of the tablets, which describes the slower drug release with increasing the CMC ratio.
In natural gums series, reveals that tablet G1 that contains only guar gum as the retarding polymer, releases ∼80% of the drug after 6 hr and ∼ 100% after 8 hr. Meanwhile, G4 and G5 that release 85 and 80% of the drug, respectively, after 10 hr, have the slowest release rate among natural gums series. MDT of these tablets also is significantly (p<0.05) higher than other tablets of these series (). also indicates that MDT is increased while DE8% decreases by increasing the xanthan content of tablets.
Sinha et al. (Citation2004) also reported that increasing the xanthan gum content of 5-flurouracil tablets decreased the drug release rate. The higher water uptake () but lower erosion () of tablets containing greater xanthan content, may describe the lower release rate of metoprolol in these tablets ().
Considering that f2 or similarity factor for tablets H3, G3, G4, and G5 are more than 50, these tablets may be the most similar to standard tablets (). However, H3 has greater f2 (CitationFDA Guidance 1997) that makes it more similar to the Lopressor tablets compared with natural gums.
According to Peppas equation (Chueh, Zia, and Rhodes Citation1995), Lopressor tablets show a non-Fickian release mechanism () and a zero-order kinetic release (). Tablets H1 and H2 release metoprolol with a Fickian mechanism () and a Higuchi kinetic (). However, tablets H3 and H4 with higher percentage of CMC show a non-Fickian mechanism () and a zero-order release kinetic (). As tablet H4 has the lowest erosion compared to other formulation of cellulosic derivatives, Hixon-Crowell mechanism which is basically according to matrix erosion (Ritger et al. Citation1987a, b), unlikely plays an important role in drug release from this tablet.
In natural gums, for G1 and G2 Higuchi kinetic is dominant ( and ). In G3 tablets that show greater erosion compared with G1 and G2 (), Hixon-Crowell mechenism plays an important role in drug release. However, in tablets G4 and G5 containing higher percentage of xanthan, n exponent is greater than 1 and definitely drug release is a function of chain relaxation of macromolocules (Ritger and Peppas Citation1987a, Citation1987b).
In general, drug release from compact matrices follows a Fickian mechanism, but some-times because of the presence of other additives, a mixture of diffusion (Case I) and chain relaxation (Case II) is seen. However, when drug diffusion does not follow a Fickian mechanism, it is too similar to a zero-order continuous and heomogeneous release kinetic (Catellani et al. Citation1988).
CONCLUSION
Tablets H3 and H4 with 60:40 and 40:60 ratio HPMC:CMC, respectively, from cellulosic derivatives meet the USP25 requirements for SR metoprolol tablets and release the drug with a zero-order kinetic. However, just H3 is smiliar to Lopressor from f2 point of view. From natural gums, formulations G4 and G5 with 80:20 and 100:0 ratio of xanthan:guar, respectively, have the greatest similarity factor to Lopressor and release the drug with a zero-order kinetics. Results show that natural gums with high percentage of xanthan are suitable and promising for production of SR matrix tablets of metoprolol.
REFERENCES
- Altaf S. A., Yu K., Parasrampuria J., Friend D. R. Guar gum-based sustained release diltiazem. Pharm. Res. 1998; 15(8)1196–1201, [PUBMED], [INFOTRIEVE], [CSA]
- Altaf S. A., Wong D., Gebert M., Larrabee S., Yu K, Friend D. R. Drug release profiles of swellable hydrocolloid formulations: USP apparatus 2 versus apparatus 3, in vitro correlation. Pharm. Technol. 1998b; 22: 62–70, [CSA]
- Banakar U. V. Pharmaceautical Dissolution Testing, 1st ed. Marcel Dekker, New York 1992; 191–194
- Bhalla H. L., Sanzgiri Y. D. An improved controlled release tablet of salbutamol sulphate. Ind. J. Pharm. Sci. 1987; 49: 22–25, [CSA]
- Castro I. A., Tirapegui J., Benedicto M. L. Effects of diet supplementation with three soluble polysaccharides on serum lipid levels of hypercholesterolemic rats. Food Chem. 2003; 80: 323–330, [CROSSREF], [CSA]
- Catellani P. L., Vaona C., Plazzi P., Colombo P. Compressed matrices, formulation and drug release kinetics. Acta Pharm.Tech. 1988; 34(1)38–41, [CSA]
- Chueh H. R., Zia H., Rhodes C. T. Optimization of sotalol floating and bioadhesive extended release tablet formulations. Drug Dev. Ind. Pharm. 1995; 21(15)1725–1747, [CSA]
- Dürig T., Fassihi R. Guar-based monolithic matrix systems: effect of ionizable and non-ionizable substances and excipients on gel dynamics and release kinetics. J. Control. Rel. 2002; 80(1–3)45–56, [CSA]
- FDA Guidance for industry. Dissolution Testing of immediate and Modified Release Solid Oral Dosage Forms. U.S. Department of Health and Human Services, Food and Drug Administration, Washingten, DC August, 1997; 32
- Friend D. R., Altaf S. A., Yu K., Gerbert M. Development of a zero-order nifedipine dosage form using COSRx technology. Proc. Int. Symp. Control. Rel. Bioact. Mater. 1997; 24: 311–312, [CSA]
- Gohel M. C., Panchal M. K. Novel use of similarity factors f2 and Sd for the development of diltiazem HCl modified-release tablets using a 3(2) factorial design. Drug Dev. Ind. Pharm 2002; 28(1)77–87, [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Hogan J. E. Hydroxypropylmethylcellulose sustained release technology. Drug Dev. Ind. Pharm. 1989; 15: 975–999, [CSA]
- Juarez H., Rico G., Villafuerte L. Influence of admixed carboxymethylcellulose on release of 4-aminopyridine from hydroxypropyl methylcellulose matrix tablets. Int. J. Pharm. 2001; 216(1–2)115–125, [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Khullar P., Khar R. K., Agarwal S. P. Evaluation of guar gum in the preparation of sustained-release matrix tablets. Drug Dev. Ind. Pharm. 1998; 24(11)1095–1099, [PUBMED], [INFOTRIEVE], [CSA]
- Krishnaiah Y. S., Karthikeyan R. S., Gouri S. V., Satyanarayana V. Three-layer guar gum matrix tablet formulations for oral controlled delivery of highly soluble trimetazidine dihydrochloride. J. Control. Rel. 2002; 81(1–2)45–56, [CROSSREF], [CSA]
- Munday D. L., Cox P. G. Compressed xanthan and karaya gum matrices: hydration, erosion and drug release mechanisms. Int. J. Pharm. 2000; 203: 179–192, [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Rao K. V.R., Devi K. P., Buri P. Cellulose matrices for zero-order release of soluble drugs. Drug Dev. Ind. Pharm. 1988; 14(15–17)2299–2320, [CSA]
- Rao V. M., Haslam J. L., Stella V. J. Controlled and complete release of a model poorly water-soluble drug, prednisolone, from hydroxypropyl methylcellulose matrix tablets using (SBE) (7m)-beta-cyclodextrin as a solubilizing agent. J. Pharm. Sci. 2001; 90(7)807–816, [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Ritger P. L., Peppas N. A. A simple equation for description of solute release. I. Fickian and non-Fickian release from non-swellable devices in the form of slabs, sphere, cylinders or discs. J. Control. Rel. 1987a; 59: 23–36, [CROSSREF], [CSA]
- Ritger P. L., Peppas N. A. A simple equation for description of solute release. II. Fickian and anomalous release from swellable devices. J. Control. Rel. 1987b; 5: 37–42, [CROSSREF], [CSA]
- Sinha V. R., Mittal B. R., Bhutani K. K., Kumria R. Colonic drug delivery of 5-flurouracil: an in vitro evaluation. Int. J. Pharm. 2004; 269: 101–108, [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Sujja-areevath J., Munday D. L., Cox P. J., Khan K. A. Relationship between swelling, erosion and drug release from hydrophilic natural gum mini-matrix formulations. Eur. J. Pharm. Sci. 1998; 6: 207–217, [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Talukdar M. M., Michoel A., Rombaut P., Kinget R. Comparative study on xanthan gum and hydroxypropylmethylcellulose as matrices for controlled-release drug delivery. I. Compaction and in vitro drug release behavior. Int. J. Pharm. 1996; 129: 233–241, [CROSSREF], [CSA]
- Talukdar M. M., Rombaut P., Kinget R. The release mechanism of an oral controlled-release delivery system for indomethacin. Pharm. Dev.Technol. 1998; 3(1)1–6, [PUBMED], [INFOTRIEVE], [CSA]
- USP 25 USP/NF. United States Pharmacopeia/National Formulary (USP25/NF20). United States Pharmacopeial Convention, New York 2002; 1142–1142
- Yu K., Altaf S. A., Parasramuria J., Friend D. R. Sustained release ketoprofen: formulation development. Proc. Int. Symp. Control. Rel. Bioact. Mater. 1997; 24: 16–17, [CSA]
- Yu K., Gerbert M., Altaf S. A., Wong D, Friend D. R. Optimization of sustained release diltiazem formulations in man by use of an in vitro/in vivo correlation. J. Pharm. Pharmacol. 1998; 50: 845–850, [PUBMED], [INFOTRIEVE], [CSA]