Abstract
Novel complex hydrogel beads were prepared from two edible polymers: pectin, a carbohydrate from citrus fruits, and zein, a protein from corn. The pectin/zein complex hydrogels did not swell in physiological environments, but hydrolyzed in the presence of pectinases. An in vitro study showed the capacity of the hydrogels to endure protease attack and residence time variation. The physical and biological properties of the new hydrogels were attributed to molecular entanglement of the two polymers. The pectin networks were stabilized by the bound zein molecules. In turn, the pectin networks shielded the bound zein from protease digestion.
Colon-specific drug delivery is highly desirable for the treatment of colonic diseases, such as inflammatory or infectious bowel diseases or colon cancer, and for the systemic delivery of polypeptide or protein drugs, and other drugs (Liu et al. Citation2003). Among many approaches for oral drug delivery to the colon site, pectin-based matrices show considerable promise, because they remain intact in the upper gastrointestinal (GI) tract and are degraded in the colon by colonic bacteria that are almost exclusively confined to the colon. In addition, pectin has a long history of use in the food industries; the nontoxic and edible nature of pectin make it more suitable in the construction of colon drug delivery systems compared with other polymers.
Pectin is a cell wall structural polysaccharide, distributed abundantly in such vegetables and fruits as sugar beet and citrus fruit. Pectin is enriched in blocks of (1 → 4) linked galacturonic acid and galacturonic acid methyl ester units interrupted by single (1 → 2) linked rhamnose units. The gelling and thickening properties of pectin strongly depend on the degree of esterification (D.E.) of the garacturonic acid. High D.E. pectin gels in the presence of a dehydrating agent, sugar, at a lower pH; low D.E. pectin gels by chelation with divalent ions such as calcium (BeMiller Citation1986). Pectin gels have been extensively tested for applications in oral drug administration (Liu et al. Citation2003). One obstacle in applying pectin gels for colon-specific drug delivery is their high swelling behavior in physiological environments. Drugs with high solubility in physiological conditions may display premature release kinetics due to the expanded pore size of pectin gels. To overcome this problem, pectin was used in combination with polyacrylate derivatives such as Eudragit®RL and RS (Semdé et al. Citation2000), or with hydroxypropylmethyl cellulose (Turkoglu and Ugurlu Citation2002) and chitosan (Kwabena and Fell Citation2001), or was formulated in the form of multiparticulate tablets. These strategies enhance the water resistance of resultant pectin complexes to some degree. However, improved pectin-based drug delivery systems are still desired.
In our study, zein was used with pectin to form complex hydrogel beads that were examined for potential use in colon-specific drug delivery. Zein is a major storage protein of corn kernels. The hydrophobic nature of zein and its capability to adhere to or coat other surfaces are attractive for several industrial purposes (Shukla and Munir Citation2001). For pharmaceutical applications, zein has been patented for the production of protein microspheres, which are able to protect encapsulated drugs from stomach acid and release them in the small intestines based on protease digestion (Mathiowitz et al. 1991). The development of pectin/zein complexes for colon-specific drug delivery is based on the hypothesis that the combination of zein with pectin may suppress the swelling of pectin in the stomach and limit zein degradation in the small intestine. For this initial study, pectin/zein complex hydrogel beads were prepared in the presence of calcium chloride in an ethanol solution. The resultant beads were characterized for structure and swelling properties in simulated physiological environments. The resultant beads also were examined for controlled drug release under conditions mimicking transit from the mouth to the colon.
MATERIALS AND METHODS
Sodium salts of pectin from citrus (D.E., 93%), zein (from corn), indomethacin, bovine serum albumin (BSA), fluorescein isothiocyanate-dextran (FITC-dextran), fluorescent red 646 reactive kit, Sorensen's phosphate buffer (pH 7.4), pepsin, and Pectinex 3XL were purchased from Sigma-Aldrich (St. Louis, MO, USA). All other chemicals were analytical grade and used as purchased, unless mentioned otherwise.
The sodium pectinate was de-esterified before use to create more free carboxylic groups, as described previously (Rubinstein et al. Citation1993; Liu et al. Citation2004). Fluorescent red 646 labeled BSA (f-BSA) was prepared on site by mixing the kit with BSA by the method provided by the manufacturer.
Preparation of Pectin/Zein Complex Hydrogel Beads
Pectin/zein complex hydrogel beads were prepared by pumping a pectin solution, 2.0% (w/v), into an ethanol solution (75%) containing zein and calcium chloride under constant stirring at ambient temperature. The concentrations of zein in the ethanol solution were 1, 5, 10, or 20% (w/v); the concentration of calcium chloride was 0.5%, w/v. As illustrated in , pectin droplets were produced individually through a tube (diameter, 0.8 mm) connected to a syringe pump. The droplets were allowed to cure in the reception phase for 15 min followed by washing with ethyl acetate and milliQ water, then air dried in a hood at ambient temperature. To prepare indomethacin preloaded hydrogel beads, the drug was predissolved in ethanol and mixed with 2.2% pectin solution to give a final pectin/indomethacin weight ratio of 2:0.5 (g/g) in 100 ml. To load BSA into the beads, 0.5 g of BSA with a trace of f-BSA were dissolved in 100 ml of 2.0% pectin solution prior to droplets formation. Beads loading with FITC-dextran were prepared by the same method. To prepare calcium pectinate beads, zein-free ethanol solution containing calcium chloride, 0.5%, was used.
To determine the zein content in the complex hydrogel beads and the amount of encapsulated drugs, dry beads, ∼75 mg for each type, were dissolved in 100 ml 75% ethanol solution containing 0.4 g NaOH and 0.5% Tween20. The optical density of the liquid phase was measured at 280 nm for zein and at 320 nm for indomethacin. The precipitates were separated by centrifugation, dissolved in a phosphate buffer (pH 7.2), then measured at 660 nm for f-BSA and at 495 nm for FITC-dextran. The measurements were performed using an ultraviolet-visible spectrophotometer (Shimadzu, UV-1601; Shimadzu Corp, Kyoto, Japan). Solutions with known amounts of identical chemicals were used to prepare standard curves.
Physical Characterization
Scanning Electron Microscopy (SEM)
For SEM examination, the pectin/zein complex hydrogel beads were critical-point dried from liquid carbon dioxide after being dehydrated in gradient ethanol. For examination of interior structures, some dried beads were cut in half after cooling in liquid nitrogen at −78°C. Samples were mounted on specimen stubs, coated with a thin layer of gold by dc sputtering (Edwards High Vacuum, Wilmington, MA, USA) and the edge of the samples were painted with colloidal silver adhesive. Images of topographical features of the whole beads and cut beads were obtained using a model Quata 200 FEG, scanning electron microscope (FEI, Hillsboro, OR, USA). Digital images were collected at 50, 500, and 5000x.
Fluorescence Microscopy
The structure of pectin/zein complex hydrogel beads also was analyzed by measuring the autofluorescence intensity for both zein and pectin at 425/475 nm (ex./em.) using a fluorescence stereomicroscope (Leica MZ FLIII, Leica Microsystem, Eston, PA, USA).
Swelling Characterization
The swelling behavior of pectin and pectin/zein beads was investigated by observing the change in diameter after incubating the beads in solutions at pH 3.5, 5.0 or 7.4 for desired periods, using an optical microscope equipped with a finely graduated scale. A total of 20 beads were examined for each sample.
In Vitro Drug Release
Drug-loaded calcium cross-linked pectin beads and pectin/zein complex hydrogel beads, ∼75 mg (dry weight) for each examination, were placed in a screw neck jar containing 100 ml release medium. The jars holding all contents were placed in an incubator at 37 °C and the mixtures were magnetically stirred. To mimic the conditions from the mouth to colon transit, the release media were changed in the sequence of 0.01 M KH2PO4-citrate buffer (pH 3.5) for 2 hr, Sorensen's phosphate buffer (pH 7.4) for 4 hr, and 0.05 M phosphate-citrate buffer (pH 5.0) containing Pectinex 3XL (120 ferment depectinization units, FDU, in 1.0 ml) for 8 hr. The pectinase-added buffer was saturated with CO2 to create an anaerobic environment prior to use. Then 3 ml of the release media were removed every 30 min; the amount of released indomethacin and f-BSA was determined by spectrophotometric methods. Aliquots of identical fresh medium were added to replace each withdrawn volume.
To evaluate the protease susceptibility of pectin/zein complex hydrogel beads and the subsequent drug release, pectin/zein beads containing encapsulated FITC-dextran were incubated in 0.01 M KH2PO4-citrate buffer solution, pH 3.5, containing 0.5% Tween20 and pepsin at different concentrations. The incubation was conducted at 37 °C for predetermined periods. At the end of incubation, the amount of drugs in the solution and the amount of zein remaining in the beads were measured by the method described previously in this section.
Statistical Analysis
All data are expressed as the mean ± standard deviation. Student's t-tests were performed and a value of p < 0.05 was considered significant.
RESULTS AND DISCUSSION
Preparation and Characterization
In the present study, pectin/zein complex hydrogel beads were prepared via the coacervation of pectin with calcium and the following molecular interaction between the two macromolecules. All beads had an average size of ∼2 mm (). Zein content in the complex beads could be adjusted by altering the initial zein concentration in the ethanol solutions (). The presence of zein together with calcium in the reception phase slightly enhanced drug encapsulation efficiency (). For calcium pectinate beads, only 70% of indomethacin and 60% of BSA or dextran could be encapsulated. For pectin/zein complex hydrogel beads, the encapsulation rates of the three drugs were more than 80%.
TABLE 1 Characterization of pectin and pectin/zein beads
TABLE 2 Determined amount of drugs in beads
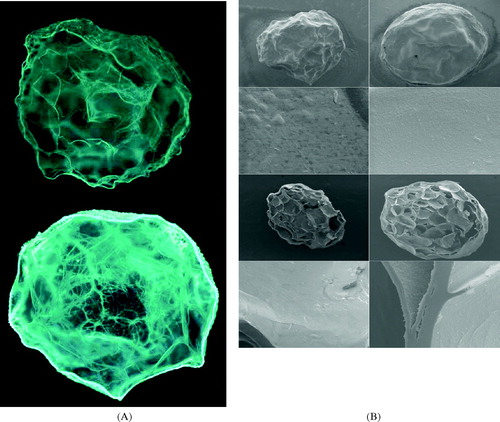
Micrographs of resultant hydrogel beads are shown in . Fluorescence microscopy () revealed the roughly spherical structures of calcium pectinate and pectin/zein hydrogels beads. Both pectin and zein have broad intrinsic fluorescence. However, casual observation reveals that the autofluorescence of zein is stronger than that of pectin. The greater autofluorescence intensity in pectin/zein beads than in calcium pectinate indicates protein uptake by the calcium pectinate structures. As shown in , zein was mainly located around the periphery of the structures but also migrated into the beads. Furthermore, the zein macromolecules were either bound to the pectin networks or aligned to densely packed fibers. shows the SEM structures of pectin and pectin/zein, both in whole bead and cut bead. All beads had well-defined internal honeycomb-like structures. For pectin/zein beads, the complexed zein formed a thin, smooth layer coating the surfaces of the beads and lining the walls of the pores. The zein layers consisted of small particles with an average size less than 2 μ m (, both the whole beads and cut beads at high magnification); the particles were tightly arranged. The appearance of the zein layer was similar to that found in a pure zein membrane cast on a glass plate (Dong, Sun, and Wang Citation2004). Furthermore, the comparison of SEM images of various pectin/zein structures revealed that beads with higher zein content had more zein located on peripheral surfaces (data not shown).
FIG. 2 (A) Autofluorescence images of pectin (top) and pectin/zein beads (bottom; field width, 2.5 mm). (B) SEM photographs of pectin (left) and pectin/zein beads (right). From top to bottom: whole beads at low magnification (field width, 2.7 mm), whole beads at high magnification (field width, 27 μm), cut beads at low magnification (field width, 2.7 mm), and cut beads with high magnification (field width, 27 μm).
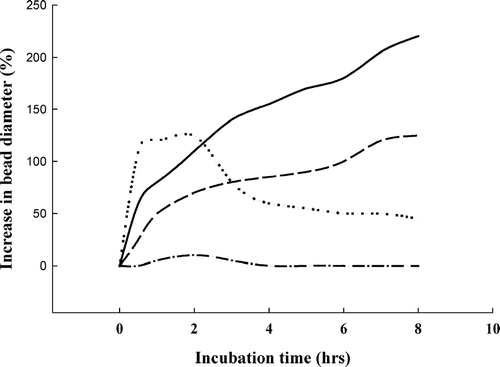
shows the swelling behavior of pectin and pectin/zein complex hydrogel beads at different solution pH. Pectin beads cross-linked with calcium ions swelled in all three solutions. The greatest increase in bead size was observed at pH 5.0, with the next greatest increase at pH 3.5. At pH 7.4, the calcium pectinate beads swelled fast at the beginning, then gradually became transparent, indicating the dissolution of the beads because of the dissociation of ionic linkages; some of the beads dissolved after 2 hr incubation. For pectin/zein, no obvious differences in bead size could be recorded before and after incubation, regardless of the solution pH. Thus, we presumed that changes in solution pH should have an impact on the porous structure of calcium pectinate beads but do not alter the porosity of pectin/zein beads.
FIG. 3 Swelling measurement of drug-free pectin hydrogel beads in solutions with different pH at ambient temperature. For calcium pectinate (sample code I), a pH-dependent swelling behavior could be clearly seen at pH 3.5 (dash line), pH 5.0 (solid line), and pH 7.4 (dotted line). For pectin/zein complex (sample code II; dash-dotted line), no obvious changes in beads size could be recorded at pH 7.4; the same at pH 3.5 and pH 5.0 (data not shown).
Controlled Drug Release
The in vitro drug release study was conducted under conditions mimicking the GI tract. A KH2PO4-citrate buffer solution with pH 3.5 was chosen to represent the acidic condition in the stomach; Sorensen's phosphate buffer with pH 7.4 represents the condition in the small intestine. Although the pH value in the colon is approximately 7, it can be acidic if prebiotics are used and digested by probiotic bacteria (Hotchkiss et al. Citation2003). In this study, a phosphate-citrate buffer with pH 5.0 was a compromise between the actual pH in the lower GI tract and the optimum pH for the Pectinex activity (Rubinstein Citation1993). Indomethacin was used as a model low molecular weight drug; BSA as a model protein drug. The release of indomethacin is shown in . For calcium pectinate beads, only a small portion of the drug could be detected in the stomach simulation (, zone I). About 95% of encapsulated indomethacin were detected in the small intestine simulation (, zone II). The drug remaining in the beads, ∼3.5% of the total encapsulation, was released in the colon simulation (, zone III). The solubilities of indomethacin in aqueous solutions at pH 3.5, 7.4, and 5.0 were ∼1.5, 130, and 3.0 mg/100 ml, respectively (Munjeri, Collett, and Fell Citation1997), which matches well with the variation of drugs found in the three release media.
FIG. 4 Cumulative release of indomethacin from pectin beads (•-sample code I) and pectin/zein beads (○-sample code II) at 37°C. The release media were changed in the sequence of (zone I) 0.01 M KH2PO4-citrate buffer (pH 3.5) for 2 hr, (zone II) Sorensen's phosphate buffer (pH 7.4) for 4 hr, and (zone III) 0.05 M phosphate-citrate buffer (pH 5.0) containing Pectinex 3XL (120 FDU/ml) for 8 hr.
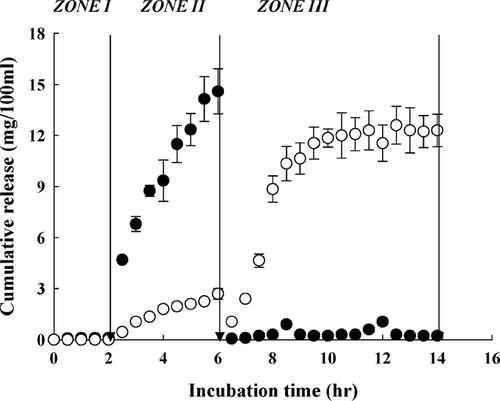
These results indicate a dissolution-control pattern for indomethacin released from calcium pectinate. In contrast, the release of indomethacin from pectin/zein complexes into 0.01 M KH2PO4-citrate buffer (, zone I) and Sorensen's phosphate buffer (, zone II) was strongly suppressed, while it was promoted by the presence of pectinolytic enzymes (, zone III). These results suggest that the drug release from pectin/zein beads depends on the enzymatic degradation of pectin.
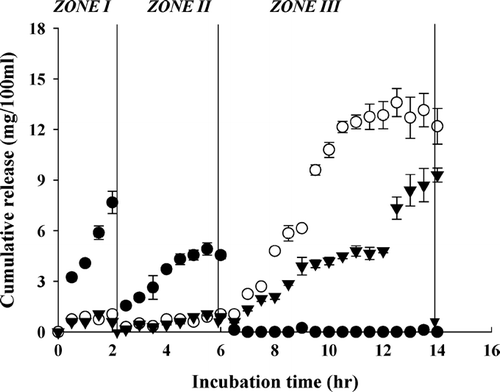
The BSA release profiles are shown in . For calcium pectinate, the release curve indicates a diffusion-control pattern. Most of the encapsulated BSA leached out from the calcium pectinate during their residence in the upper GI simulations (, zone I and II) before being transferred to zone III. This could be attributed to the high solubility of BSA and the high swelling property of the beads at pH 3.5 and pH 7.4, as indicated in . For pectin/zein beads, again, the inclusion of zein suppressed BSA release when the beads were resident in pectinase-free media (, zone I and II), whereas a highly concentrated BSA was detected in the buffer containing Pectinex 3XL (, zone III). However, the rapid and complete release of BSA in colon simulation was only seen for beads with lower zein content (, sample code II). The increase in zein content reduced BSA release from the beads (, sample code IV). This could be attributed to the steric hindrance of the bound zein macromolecules to Pectinex-pectin interaction.
FIG. 5 Cumulative release of bovine serum albumin from pectin beads (○-sample code I) and pectin/zein beads (○-sample code II; ∇-sample code IV) at 37°C. The release media were changed in the sequence of (zone I) 0.01 M KH2PO4-citrate buffer (pH 3.5) for 2 hr, (zone II) Sorensen's phosphate buffer (pH 7.4) for 4 hr, and (zone III) 0.05 M phosphate-citrate buffer (pH 5.0) containing Pectinex 3XL (120 FDU/ml) for 8 hr.
Resistance to Protease Attack and Residence Time Variation
Although the inclusion of zein offered some advantageous properties to resultant complex hydrogel beads such as more water resistance and suppressing premature drug release, several concerns were raised. One of the concerns is that zein may degrade in the upper GI tract by protease digestion. How fast does the digestion occur and how thick a zein layer is required to ensure the integrity of the beads to travel through the upper GI tract? These questions should be addressed before the beads could be considered for potential application in colon-specific drug delivery. Therefore, we investigated the protease resistance of pectin/zein complexes and the tolerance of the beads to residence time variation in the upper GI tract. Pepsin was used as a model protease. Since BSA is a substrate of pepsin, dextran, a protease-resistant macromolecule, was chosen to replace BSA to simplify the study.
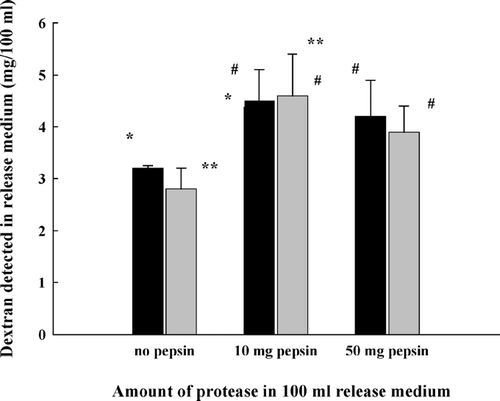
As shown in , for the complex beads with a lower zein content (sample II), about two-thirds of the initially complexed zein, ∼ 4 mg/100 mg, remained in the beads after incubation with pepsin for 4 hr. The increase of pepsin concentration from 2 to 50 mg/100 ml had no influence on zein retention. Furthermore, the increase in incubation time from 4 to 20 hr also did not affect the zein degradation (). For beads with a higher zein content (sample III), the increase of pepsin concentration () or incubation time () was able to remove more zein from the beads at beginning; however, once the zein content in beads reached about ∼ 4 mg/100 mg, neither the increase of protease nor the extension of incubation time affected zein retention. Consistent with the results shown in and , drug released from the complex beads was slightly stimulated by the presence of pepsin but independent of the protease concentration and incubation time ().
FIG. 6 Effect of pepsin on zein degradation. Pectin/zein complex beads (sample II ○ and sample III ○) were incubated in KH2PO4-citrate-pepsin buffer containing pepsin of different concentrations at pH 3.5 at 37°C for 4 hr.
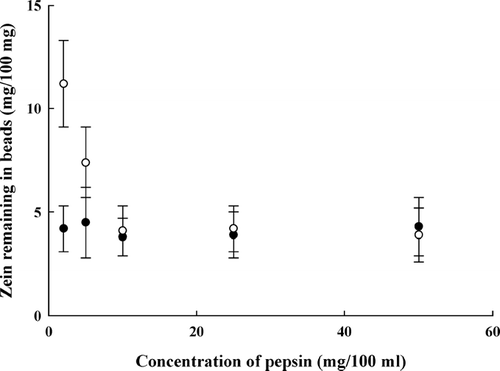
FIG. 7 Effect of incubation time on zein digestion of sample II (○) and sample III (○). Concentration of pepsin, 2 mg/100 ml.
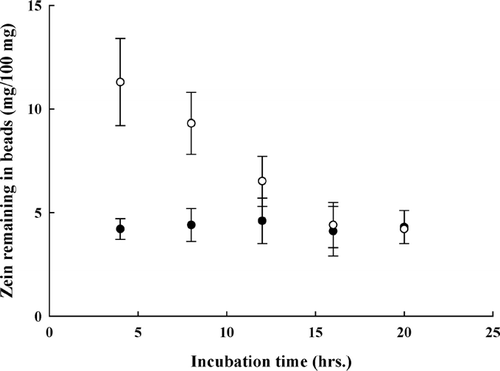
FIG. 8 Dextran released from pectin/zein beads after incubation with pepsin for 4 hr (black bar) or 20 hr (gray bar). Concentration of pepsin, 2 mg/100 ml. *, ** p < 0.05; # not significant.
We hypothesized that there were two types of bound zein in the complex beads: strongly bound zein and loosely associated zein. Supposedly, the final small amount of zein remaining in the beads after pepsin digestion was bound to the pectin networks by strong molecular entanglements. The structure of pectin networks was therefore stabilized by the bound zein. Furthermore, the beads with the larger portion of pectin protected the zein from protease attack better than the beads with less amount of pectin. Above this amount, the additional zein molecules may adhere to the bound zein; thus they were easy to submit to pepsin digestion.
The capability of pectin/zein beads to endure protease attack and residence time variation in the upper GI tract is important. Several parameters determine the duration of a solid dosage form in the stomach and the small intestine, such as fed/fast patterns, intensity of peristalsis, and meal compositions. As a result, residence time of a solid drug formulation in the stomach could vary from a few minutes to 8 hr and in the small bowel from 3 to 5 hr (Meyer et al. Citation1985; Davis, Hardy, and Farad Citation1986). Due to the difficulty in predicting the exact residence time, many colon-specific drug delivery systems have failed to be developed from a concept to practice. A delivery system with strong tolerance to the variations of protease concentrations and residence times in the upper GI tract, while being degraded in the presence of colonic bacteria, would be an ideal candidate for colon-specific drug delivery.
CONCLUSIONS
Pectin/zein complex hydrogel beads have shown the capability to protect incorporated drugs from premature release into physiological environments similar to the stomach and small intestines as demonstrated in vitro. The inclusion of a small portion of zein into the pectin networks effectively suppressed the swelling behavior of pectin, thus stabilizing the structural property of the pectin networks. Likewise, the pectin networks protected the bound zein from protease digestion. These properties make pectin/zein complex beads a promising system for colon-specific drug delivery. Based on this primary study, a new experiment is planned to determine optimal formulation conditions, that can permit efficient drug encapsulation and drug release.
The authors gratefully acknowledge Dr. Peter H. Cooke and Pamela Rockwell-Warner for their technical assistance and Wendy H. Kramer for technical editing.
Mention of trade names or commercial products in this article is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the U.S. Department of Agriculture.
REFERENCES
- BeMiller J. N. An introduction to pectin's: structure and properties. Chemistry and Function of Pectin's. ACS symposium series 310, American Chemical Society, Washington, DC 1986; 2–12
- Davis S. S., Hardy J. G., Farad J. W. Transit of pharmaceutical dosage forms through the small intestine. Gut 1986; 27: 886–892, [INFOTRIEVE], [CSA]
- Dong J., Sun Q., Wang J-Y. Basic study of corn protein, zein, as a biomaterial in tissue engineering, surface morphology and biocompatibility. Biomaterials 2004; 25: 4691–4697, [INFOTRIEVE], [CSA], [CROSSREF]
- Hotchkiss A. T., Loan-Martin E., Grace W. E., et al. Peptic oligosaccharides as prebiotics. Oligosaccharides in Food and Agriculture, G. Eggleston, G. L. Cote. ACS Symposium Series 849, American Chemical Society, Washington, DC 2003; 54–62
- Kwabena O. K., Fell J. T. Biphasic drug release: the permeability of films containing pectin, chitosan and HIM. Int. J. Pham. 2001; 226: 139–145, [CSA], [CROSSREF]
- Liu L. S., Won Y. J., Cooke P. H., et al. Pectin/poly(lactide-co-glycolide) composite matrices for biomedical applications. Biomaterials 2004; 25: 3201–3210, [INFOTRIEVE], [CSA], [CROSSREF]
- Liu L. S., Fishman M. L., Kost J., Hicks K. B. Pectin-based systems for colon-specific drug delivery via oral route. Biomaterials 2003; 24: 3333–3343, [INFOTRIEVE], [CSA], [CROSSREF]
- Mathiowitz E., Bernstein H., Morrel E., Schwaller K. Method for producing protein microspheres. U.S. Patent No. 5,271,961, 1993
- Meyer J. H., Dressman J., Fink A., Amidon G. Effect of size and density on canine gastric emptying of non digestible solids. Gastroenterology 1985; 89: 805–813, [INFOTRIEVE], [CSA]
- Munjeri O., Collett J. H., Fell J. T. Hydrogel beads on amidated pectins for colon-specific drug delivery: the role of chitosan in modifying drug release. J. Control. Rel. 1997; 46: 273–278, [CSA], [CROSSREF]
- Rubinstein A., Radai R., Ezra M., et al. In vitro evaluation of calcium pectinate: a poteintial colon-specific drug delivery carrier. Pharm. Res. 1993; 10: 258–263, [INFOTRIEVE], [CSA], [CROSSREF]
- Semdé R., Amighi A., Devleeschouwer M. J., Moës A. J. Studies of pectin HM/Eudragit® RL/Eudragit® NE film-coating formulations intended for colonic drug delivery. Int. J. Pharm. 2000; 197: 181–192, [CSA], [CROSSREF]
- Shukla R., Munir C. Zein: the industrial protein from corn. Ind. Crops Prod. 2001; 13: 171–192, [CSA], [CROSSREF]
- Turkoglu M., Ugurlu T. In vitro evaluation of pectin-HPMC compression coated 5-aminosaclicylic acid tablets for colonic delivery. Eur. J. Pharm. Biopharm. 2002; 53: 65–73, [INFOTRIEVE], [CSA], [CROSSREF]

