Abstract
The purpose of our study was to prepare liquid forms of 20% ibuprofen in 30% poloxamer 407, while avoiding gel formation and to assess their drug diffusion-penetration (permeation) into the skin. Two series of poloxamer-based formulations were prepared, both containing ibuprofen and one of two terpenes: d-limonene and 1-menthol. A rheological characterization of all preparations made allowed their grouping in two modalities: gels and fluids. Data revealed a statistically superior enhanced permeation terpene-dependent of ibuprofen in fluid preparations, specially the one containing d-limonene. Cumulative permeation in 24 hr was 2500 μ g/cm2 and 4500 μ g/cm2 for the 1-menthol and d–limonene, respectively, for fluid preparations as compared with 2000 μ g/cm2 and 1600 μ g/ cm2 for d-limonene and 1-menthol on gels and only 1200 μ g/cm2 of the control solution (p < 0.05). Results postulate that a liquid 30% poloxamer-based preparation of ibuprofen with d-limonene is possible and that it may be useful as a topical preparation of ibuprofen.
Oral therapy with nonsteroidal anti-inflammatory drugs (NSAIDs) can result in effective anti-inflammatory, analgesic, and antipyretic actions, but the clinical use of these drugs is often limited because of their potential to cause adverse effects such as gastroduodenal irritation and ulceration (Puri and Sanghavi Citation1992). Dermal administration of these agents can avoid such disadvantages (Beetge et al. Citation2000). Many topical creams and gels have been prepared utilizing standard excipients and occasionally irritating substances, which are believed to facilitate drug permeation. Yet penetration of the active principle beyond a few superficial layers of corneal cells has not been demonstrated and has been regarded as unlikely (El-Kattan et al. Citation2000).
The success of transdermal systems depends on the ability of the drug to diffuse-penetrate (permeate) into deeper layers of the skin and to reach structures such as joints and muscles. Nevertheless, the stratum corneum has been recognized as the predominant permeation-barrier of the skin (Cross, Pugh, and Hadgraft Citation2001). It has been proposed that, to surpass this obstacle, the so-called “chemical penetration enhancers” (QPE) may be included in topical formulations to achieve a real permeation of the active principle (Park et al. Citation2000; Sinha and Pal Citation2001). Various studies have shown that QPE can indeed facilitate drug permeation, and some mechanisms of action have been proposed. For example, it has been suggested that QPE interact with lipids of the outermost layer of the skin in the stratum corneum allowing, somehow, better penetration of coadministred drugs (López et al. Citation2000).
Poloxamer 407 (P-407) is a triblock copolymer with a central hydrophobic chain of polyoxypropylene (PPO) and two lateral hydrophilic chains of polyoxyethylene (PEO). It has a average molecular weight of 12,000 Daltons and a PEO/PPO ratio of 2:1 (w/w). Solutions of P-407 at concentrations of 20% show in situ thermoreversible gelation behavior (fluid when cold) (Ricci et al. Citation2002; Bohorquez et al. Citation1999) and this viscosity change can be induced many times without modifying P-407 properties. P-407 has low toxicity, making it an excellent candidate for medical and biomedical applications (Ivanova et al. Citation2002; Rowe, Sheskey, and Weller, Citation2003). The presence of inorganic salts, multivalent anions, and drugs modify, in most instances, the rheological characteristics of P-407, usually lowering its sol-gel transition temperature (Paavola et al. Citation1998; Ricci et al. Citation2005). This phenomenon is important because there is an inverse correlation between viscosity of a gel and the release of a given drug into a biological system (Barichelo et al. Citation1999). Because P-407 at 20% or more becomes gel at room temperature, the release of a drug into a biological surface will be somehow delayed (Pandit et al. Citation2000; Scherlund et al. Citation2000).
Considering the above conditions, the purpose of our study was to prepare a liquid form of 20% ibuprofen in 30% P-407. But we wanted to avoid gel formation to facilitate drug permeation into the skin, and we added either of two QPE, d-limonene or 1-menthol, to enhance permeation of ibuprofen through experimental mouse skin.
MATERIALS AND METHODS
Poloxamer 407 (Pluronic F-127) was purchased from BASF. Ibuprofen was kindly provided by Pharmacia & Upjohn, México. Propylene glycol (Fisher, USP grade) and d - limonene were purchased from Sigma and 1-menthol from Fluka. NaOH and ethanol were reactive grade (J.T. Baker). Acetonitrile and water were high-performance liquid chromatography (HPLC) grade (J.T.Baker). Skins were obtained from male CD-1 mice 6 weeks old.
Preparation of P-407 Gel Formulations
First, 30% P-407 gels were prepared using the cold method described by Schmolka (Citation1972). Ratios of ingredients for each preparation varied as described in . Considering that ibuprofen has low water solubility, pH modification was necessary to dissolve it in water (pH 8 with 0.1 M NaOH). Second, P-407 was slowly incorporated by stirring under constant temperature (6°C). When preparations appeared homogeneous after approximately 2 hr, they were refrigerated for 24 hr. After this time period, a clear solution was obtained and, as expected, gel was formed in all preparations when left at laboratory temperature (20–24°C). Incorporation of either terpene—d-limonene or 1-menthol—into these gels was easily achieved by simple stirring. Control consisted of gel formulation with no terpene incorporation.
TABLE 1 Formulation composition of poloxamer gels and solutions
Preparation of P-407 Fluid Formulations
To obtain 30% P-407-based fluid preparations with low gel-formation tendency, ibuprofen was used instead of Na.ibuprofen. This former derivative was first dissolved in ethanol followed by rapid incorporation of different amounts of propylene-glycol, adding finally either terpene. This was achieved by constant stirring, also at 6°C. When a clear fluid was obtained after approximately 1 hr, preparations were refrigerated overnight. Gel formation did not occur when left at laboratory temperature (see for ingredients). Control preparation consisted of ibuprofen phosphate buffer solution, pH 7.2 without P-407.
Rheological Measurements
Gels and fluid produced were studied using an Ares, Rheometric Fluid Spectrometer III (TA Instruments). The measuring system involved concentric double wall cylinders (Couette geometry). Fully 10 ml of cold solutions and gels were transferred to the cylinders. To measure linear viscoelastic properties, it was necessary to determine the linear viscoelastic region at a given frequency. This region was determined by measuring G′ (storage or elastic modulus) and G” (loss or viscous modulus). G′ is related to the storage of energy during the cycle or elastic energy, whereas G” is related to the dissipation of energy during the cycle or viscous energy, as a function of the strain amplitude. The strain amplitude sweep was made between 0.1 and 100%.
We observed that between 40% and 100% of strain amplitude,G′ and G” were both constant; therefore, a strain amplitude of 50% was chosen to make all the dynamic measurements. In liquid preparations G′ and G” were determined between 1 and 100 rad s− 1. The advantage of the linear viscoelastic properties to physically characterize a material is because the deformation is very small, the internal structure of the material is preserved during the measurements.
The sol-gel transition temperature of P-407 gels was determined from oscillation measurements at 6.28 rad s− 1. The sol-gel transition temperature graph was determined by sweep temperature as a function of the elastic modulus (G′) from 5°C to 55°C. The transition temperature was defined as the point where the elastic modulus (G′) and the viscous modulus (G″) curves cross for the gel. In liquid preparations the sweep temperature was determined from 0 ° to 55°C.
In Vitro Skin Permeation
Trials were carried out as described by Park et al. (Citation2000) using a section of abdominal skin, excised from male CD-1 mice 6 weeks old, and placed as membranes in vertical Franz diffusion cells (PermeGear, Riegelsville, PA, USA), with a permeation area of 1.0 cm2 and a receptor cell volume of 7.0 ml. Comparisons of fluid versus gel preparations were made. Franz cells were maintained in a water bath at 37°C ± 0.5°C. The cell receptor compartment contained phosphate isotonic buffer, pH 7.2 and was maintained under stirring at 600 rpm. First, 300 mg of each sample were placed on the epidermal side of the skin facing the donor compartment. Second, after a 60-min temperature stabilization period, 300 μ l aliquots from the receptor compartment were taken through the sampling port of the cells at scheduled intervals over a 24-hr time period. The aliquot volume withdrawn was immediately replaced by an equivalent volume of phosphate isotonic buffer, pH 7.2 also at 37°C ± 0.5°C. Samples were subject to analysis of ibuprofen as described by Haikala, Heimonen, and Vuorela (Citation1991). After plotting the cumulative amount of ibuprofen permeated through mouse skin as a function of time, permeation parameters were calculated, and the flux was calculated using the following equation (El-Kattan et al. Citation2000).
where Jt, is the flux (μ g/cm2/h), dM/dt is the slope of cumulative corrected drug amount in the receptor compartment versus time, and A is the diffusional area (cm2). The lag time (Tlag) was determined by extrapolating the linear portion of the curve to the abscissa; the cumulative amount of ibuprofen after 24 hr (Q24) was determined.
Statistics
Chemical trials as well as skin permeation studies were repeated six times and results are presented as mean ± 1 SD. Dunnet t test was performed to detect any significance difference in permeation variables between the two ibuprofen liquid preparation with P-407 and either d-limonene or 1-menthol. Also, mean rheological values were compared using the same test.
RESULTS
Rheological Measurements Studies
As stated, the gelation temperature is the point where the elastic modulus (G′) and the viscous modulus (G”) curves cross. These points for the 30% P-407 solution containing 5% ibuprofen · Na with d-limonene or l-menthol (2%) are shown in (25°C for 30% P-407 with d-limonene; 23°C for 30% P-407 with l-.menthol; and 28°C for 30% P-407 lacking terpene). Little or no change G′ values were noted from 0 to 20°C in the gel samples, but higher temperatures induce a rapid sol-gel transition. At the end of the sol-gel transition, G′ values are stable with no change.
FIG. 1 Sol-gel transition temperature for 30% P- 407, containing 5% Na.ibuprofen and either 2% of d-limonene or 2% of 1-menthol. (Means ± SE, n = 5).
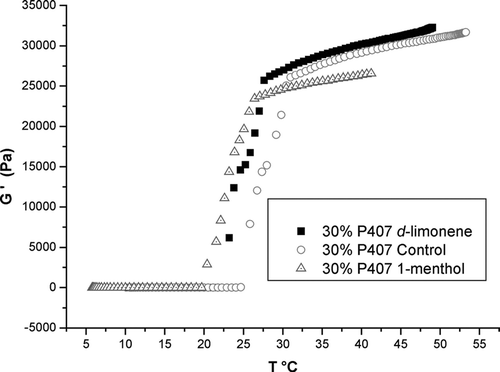
The gel-sol transition temperature is affected by the addition of propylene glycol and ethanol to 30% P-407 plus 20% ibuprofen, avoiding gel formation. The resulting preparation is a solution instead of a gel, as indicated by the G′ elastic modulus. No tangible changes in G” values are observed in these fluid samples within temperatures from 0 ° to 55°C.
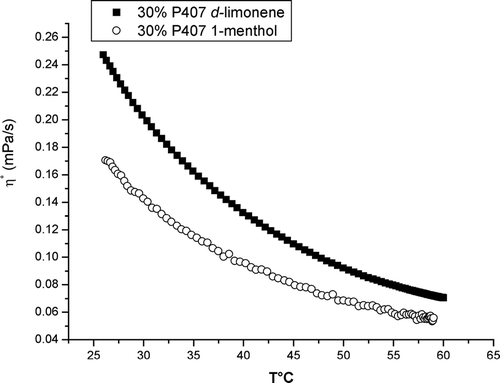
Fluid preparations viscosity decreased as temperature increased. This viscosity can be expressed as viscosity-complex (η*), which is a function of temperature. Then, at 27°C, 30% P-407 solution had a η* of 25.98 m Pa/s when 1-menthol was added and 37.41mPa/s when d-limonene was added. Viscosity–temperature tendencies are shown in .
Skin Permeation Studies
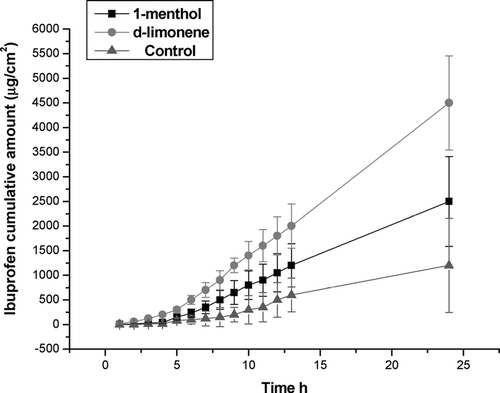
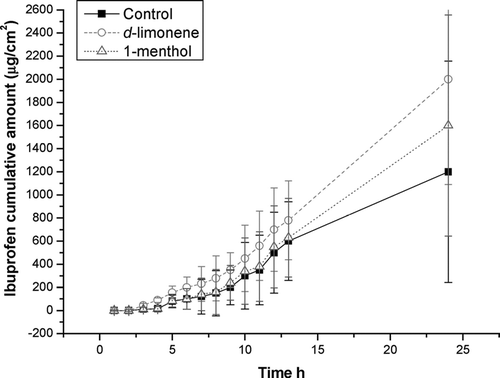
Results of permeation expressed as flux (Jt), lag time (Tlag), and cumulative amount of ibuprofen after 24 hr (Q24), are shown in (see and ). The flux rate of ibuprofen from the 30% P-407 1-menthol containing fluid preparation was 114 ± 10 μ g /cm2/hr with a Tlag of 0.89 hr and a Q24 of 2500 μ g/cm2 ± 219. For the 30% P-407 d-limonene containing preparation, the corresponding values were: flux rate = 200 ± 19 μ g/cm2/h ; Tlag = 0.30 hr; Q24 4500 ± 430 μ g/cm2. The control solution values were flux rate = 54.6 ± 17 μ g/cm2/hr; Tlag 1.67 hr and Q24 1200 ± 13 μ g/cm2. D-limonene had statistically superior ability to promote skin permeation of ibuprofen than the one containing l-menthol (p < 0.01) or the control solution (p < 0.001).
TABLE 2 Terpene effect on percutaneous permeation of ibuprofen from gel and fluid preparations
DISCUSSION
The purpose of our study was to obtain a liquid form of 20% ibuprofen in the highest possible concentration of P-407, avoiding gel formation and containing one QPE: d-limonene or 1-menthol. A liquid rather than a gel preparation is believed to facilitate ibuprofen permeation into the skin, while taking advantage of the permeation enhancing potential of the nonionic surfactant P-407 as well as of the terpenes used. These results indicate that the mixture of 30% P-407–20% ibuprofen-propylene glycol-ethanol-2% d-limonene or (2% 1-menthol) can result in a liquid- transparent formulation with a pleasant odor and is, easy to spread. Final viscosity values obtained were 25.98 mPa/s for the 1-menthol-containing preparation and 37.41 mPas/s for the d-limonene-containing one. These values are much higher than values of water (1.02 mPas/s) but can still be regarded as liquid. Fully 30% P-407 preparations are usually gels, but in this study the addition of ethanol (necessary to dissolve 20% ibuprofen), as well as the addition of propoylene glycol and d-limonene or 1-menthol, avoided gel formation within a temperature range of 25° to 50°C. It is important to emphasize that contrast with other authors (Ivanova et al. Citation2002; Choi et al. Citation1999), who suggested that the addition of glycols was sufficient to avoid gel formation in a concentration-dependant manner, in this study propylene-glycol alone was unable to prevent gel formation of 30% P-407. Furthermore the ethanol-ibuprofen containing preparation could not maintain in a liquid state the P-407-propylene glycol mixture, and a strong gel was obtained. The addition of either d-limonene or 1-menthol was necessary to obtain the fluid form of this mixture.
The rheological characteristics of a given topical formulation are important to define drug permeation; that is, a semiliquid or liquid state appears to promote permeation better than gel preparations (Barichelo et al. Citation1999). Also, the overall efficacy of a topical formulation depends on the release characteristics of the delivery vehicle and pharmacokinetics of the drug as it diffuses through the skin. The driving force for transport is the chemical potential gradient, but the penetration of topically applied drugs also depends on the thermodynamic activity of the drug within the vehicle, as well as on the presence of the so-called penetration enhancers (Djordjevic, Michniak, and Urich Citation2003). Hence, liquid preparations here described had a theoretically better permeation potential.
Skin permeation studies using Franz cells have been widely accepted (Zhao and Singh Citation2000; Djordjevic et al. Citation2003; Mohammed 2001; Park et al. Citation2000; Mortazavi and Aboofazeli, Citation2003; Fang et al. Citation2001). Permeation of ibuprofen into mouse skin section of the fluid preparations here (one with d-limonene and other with 1-menthol) were compared with the gel preparations, and a comparison was made against a control solution. Both terpene enhancers showed a significant effect on the percutaneous permeation of ibuprofen using hairless mouse skin with a Q24 of 2500 μ g/cm2 for the 1-menthol-containing preparation and a Q24 of 4500 μ g/cm2 for the d-limonene preparation, as compared with a Q24 of only 1200 μ g/cm2 of the control solution (p < 0.001 in both cases as compared with the control solution). Also, a statistically significant difference in favor of the d−limonene preparation as compared with 1-menthol, was obtained (p < 0.01). Nevertheless, we emphasize that hairless mouse skin may not reflect permeability of human skin and confirmation studies should be carried out in humans.
Many researchers have discussed the mechanism of action of percutaneous absorption enhancers. Okabe (Citation1989) and O'Hare (Citation1994) found that d-limonene and 1-menthol enhance the skin permeation of several drugs. They postulated that the permeation enhancement action of d-limonene and ethanol was achieved when d-limonene penetrates into the skin, already in contact with ethanol and both change the barrier structure of the stratum corneum. The transfer of d−limonene to the skin is thereby enhanced under the coexistence of ethanol with the skin, where it may act as the accelerating agent for the permeation promoting activity of d-limonene (Okabe et al. Citation1989).
CONCLUSION
Our work shows the manner in which a liquid form of 20% ibuprofen in 30% P-407 is made, but avoiding gel formation. The addition of promoters for skin permeation of ibuprofen, in particular d-limonene, enhances the permeation of ibuprofen in mouse skin sections.
REFERENCES
- Barichelo J. M., Morichita M., Takayama K., Nagai I. Absortion of insulin from pluronic F-127 gels following subcutaneous administration in rats. Int. J. Pharm. 1999; 184: 189–198
- Beetge E., du Plessis J., Gerbrandt D., Goosen C., Janse F. The influence of the physicochemical characteristics and pharmacokinetic properties of selected NSAIDs on their transdermal absorption. Int. J.Pharm. 2000; 193: 261–264
- Bohorquez M., Kock C., Tryastad T., Pandit N. A study of the temperature-dependent micellization of pluronic F-127. J. Colloid Interface Sci. 1999; 216: 34–40
- Choi H., Lee M., Kim M., Kim C. Effect of additives on physicochemical properties of liquid suppository bases. Int. J. Pharm. 10 1999; 190(1)13–19
- Cross S. E., Pugh J., Hadgraft J. Probing the effect of vehicles on topical delivery: understanding the basic relationship between solvent and solute penetration using silicone membranes. Pharm. Res. 2001; 18: 999–1005
- Djordjevic J., Michniak B., Urich K. E. Amphiphilic star-like macromolecules as novel carries for topical delivery of nonsteroidal anti-inflamatory drugs. AAPS Pharm. Sci. 2003; 5: 1–12
- El-Kattan A. F., Asbill C. S., Kim N., Michniak B. B. Effect of formulation variables on the percutaneous permeation of ketopropfen from gel formulations. Drug Del. 2000; 7: 147–153
- Fang J. Y., Hong Ch. T., Chiu W., Wang Y. Y. Effect of liposomes and niosomes on skin permeation of enoxacin. Int. J. Pharm. 2001; 219: 61–72
- Haikala V. E., Heimonen I. K., Vuorela H. J. Determination of ibuprofen in ointment by reverse phase liquid chromatography. J. Pharm. Sci. 1991; 456–458
- Ivanova R., Lindman B., Alexandridis P. Effect of pharmaceutically aceptable glycols on the stability of the liquid crystalline gels formed by poloxamer 407 in water. J. Colloid Interface Sci. 2002; 252: 226–235
- Jones D. S., Brown A. F., Woolfson D. A. Solute and solvent effects on the thermorheological properties of poly(oxyethylene)block copolymers: Implications for pharmaceutical dosage forms design. J. of Apllied Polymer Sci. 2002; 87: 1016–1026
- Kikuchi K., Takayama K., Nagai T. Effect of d-limonene on the amounts of ethanol and indomethacin penetrated from aqueous gel ointments to rat skin. Chem. Pharm. Bull (Tokyo) 1992; 40: 3108–3109
- López A., Llinares F., Cortell C., Herráez M. Comparative enhancer effects of span 20 with tween 20 and azone on the in Vitro percutaneous penetration of compounds with different lipophilicities. Int. J. Pharm. 2000; 202: 133–140
- Mohammed F. A. Topical permeation characteristics of diclofenac sodium from NaCMC gels in comparison with conventional gel formulations. Drug Devel. Ind. Pharm. 2000; 27: 1083–1097
- Mortazavi S. A., Aboofazeli R. An investigation into the effect of various penetration enhancers on percutaneous absorption of piroxicam. Iranian J. Pharm. Res. 2003; 2: 135–140
- Obata Y., Takayama K., Okabe H., Machida Y., Nagai T. Effect of cyclic monoterpenes on percutaneous absortion in the case of water- soluble drug (diclofenac sodium). Drug Design Del. 1990; 6: 319–328
- O'Hare N., Takayama K., Machida Y., Nagai T. Combined effect of d-limonene and temperature on the skin permeation of ketoprofen. Int. J. Pharm. 1994; 105: 31–38
- Okabe H., Takayama K., Ogura A., Nagai T. Effect of limonene and related compounds on the percutaneous absortion of indomethacin. Drug Design Del 1989; 4: 313–321
- Paavola A., Tarkkila P., Xu M., Wahlström T., Yliruusi J., Rosenberg P. Controlled release gel of ibuprofen and lidocaine in epidural use—analgesia and systemic absortion in pigs. Pharm. Res. 1998; 15: 482–487
- Pandit N., Trygstad T., Croy S., Bohorquez M., Koch C. Effect of salts on the micellization clouding and solubilization behavior of pluronic F-127 solutions. J. Colloid Interface Sci. 2000; 222: 213–220
- Park E.-S., Chang S.-Y., Hahn M., Chi S.-Ch. Enhancing effect of polyoxyethylene alkyl ethers on the skin permeation of ibuprofen. Int. J. Pharm. 2000; 209: 109–119
- Puri R. D., Sanghavi N. M. Evaluation of topical non-steroidal anti-inflammatory drugs using penetration enhancers. Ind. J. Pharmacol. 1992; 24: 227–228
- Ricci E. J., Bently M. V. L. B., Bretas R. E. S., Farah M., Marchetti J. M. Rheological characterization of poloxamer 407 lidocaine hydrochloride gels. Eur. J. Pharm. Sci. 2002; 17: 161–167
- Ricci E. J., Lunardi L. O., Nanclares D. M. A., Marchetti J. M. Sustained release of lidocaine from Poloxamer 407 gels. Int. J. Pharm. 2005; 288: 235–244
- Rowe R., Sheskey P., Weller P. Poloxamer. Handbook of Pharmaceutical excipients, R. Rowe, P. Sheskey, P. Weller. American Pharmaceutical Association, London 2003; 447–449
- Scherlund M., Malmsten M., Holmqvist P., Brodin A. Thermosetting microemulsions and mixed micellar solutions as drug delivery systems and mixed micellar solutions as drug delivery systems for periodontal anesthesia. Int. J. Pharm. 2000; 194: 103–116
- Sinha V., Pal M. K. Permeation enhancers for transdermal drug delivery. Drug Dev. Ind. Pharm. 2001; 26: 1131–1140
- Smolka I. R. Artificial skin I. Preparation and properties of pluronic F-127 gels of treatment of burns. J. Biomed. Mater. Res. 1972; 6: 571–582
- Zhao K., Singh J. Mechanism(s) of in vitro percutaneous absortion enhancement of tamoxifen by enhancers. J. Pharm. Sci. 2000; 88: 771–779