Abstract
Pantoprazole is a proton pump inhibitor prodrug used in the treatment of gastric ulcers and gastroesophageal disease. Pantoprazole must be absorbed in the gastrointestinal tract and because it is unstable under acidic conditions, enteric delivery systems are required. The purpose of this study was to prepare pantoprazole-loaded microspheres by emulsion-solvent evaporation technique using two different types of enteric-coating polymers: Eudragit S 100 and hydroxypropyl methylcellulose phtalate. The microspheres have been characterized in terms of their morphology, encapsulation efficiency, and ability of stabilizing pantoprazole in acidic media. Pantoprazole determinations were carried out using a validated spectrophotometric method for the analysis of drug in dissolution media. All microspheres, except F2 formulation, were successfully obtained. The in vitro assay showed that especially F1 and F4 microspheres were more effective in protecting the drug than F3 microspheres in acidic media.
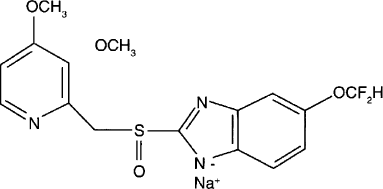
Pantoprazole () is a proton pump inhibitor prodrug, which is important in the treatment of acid-related disorders. Sesquihydrate sodium pantoprazole is used in the treatment of gastric and duodenal ulcers and gastroesophageal reflux disease accompanied by esophagitis (CitationFitton and Wiseman 1996; CitationShin and Sachs 2004). Pantoprazole suppresses parietal cell production of gastric acid, which results in the healing of ulcers and esophagitis. Pantoprazole also exerts antibacterial effects on Helicobacter pylori infections associated with other drugs, such as metronidazole, clarithyromycin, or amoxicillin, which effectively eradicate this organism from the gastric mucosa (CitationRaffin et al. 2006; CitationColome et al. 2007). Like other proton pump inhibitors, pantoprazole is substituted pyridil methyl sulfinyl benzimidazole that accumulates in the acidic secretory caniliculus of the parietal cell (luminal surface of the gastric ATPase) due to protonation of the pyridine. In this compartment, it then undergoes an acid-catalyzed chemical rearrangement, which is necessary for its activity that follows a second protonation on the benzimidazole at a much lower pKa(CitationAvner 2000). After intestinal absorption, this drug accumulates in the highly acidic environment of the parietal-cell canalicular lumen for its activation. The active form reacts with the thiol group of cysteins 813 and 822 of the transmembranal H+/K+ ATPase (Beil et al. 1992). Because this conversion must occur inside the gastric parietal cells, pantoprazole must be absorbed intact by gastrointestinal tract (CitationCheer et al. 2003; CitationRaffin et al. 2006). Therefore, pantoprazole is administered as an enteric coated, delayed release formulation.
In the last few decades, oral multiunit dosage forms such as microspheres have received much attention as drug delivery systems. These systems distribute more uniformly in the gastrointestinal tract, they are less affected by pH, and there is a minor risk of dose dumping thus resulting in a more uniform drug absorption and reducing patient-to-patient variability (CitationÇelik 1994; CitationBhalerao et al. 2001; CitationPoole 2001; CitationSoppimath et al. 2001; CitationVarde and Pack 2004; CitationŞengel et al. 2006). Besides, these drug delivery systems are also proposed to improve distribution and bioavailability of acid labile drugs (CitationRaffin et al. 2006).
This study concerns the development of microsphere formulations containing sesquihydrate sodium pantoprazole with the goal of protecting the drug from the acidic environment of the gastrointestinal system. A solvent evaporation technique was used to prepare the matrix-type microspheres loaded with pantoprazole. Two different types of enteric-coating polymers—Eudragit S 100 and hydroxypropyl methylcellulose phtalate (HPMCP)—were chosen as the matrix-forming polymers. The effect of the polymer and drug/polymer ratio variations on the preparation of microspheres was determined and evaluated.
MATERIALS AND METHODS
The materials used were sesquihydrate sodium pantoprazole, which is kindly gifted by Fargem Pharmaceutical Co., Turkey, Eugragit S 100 (Röhm Pharma, Germany), HPMCP (Shin Etsu, Japan), distilled acetylated monoglycerides (Myvacet 9-45K) (Shin Etsu, Japan), polyvinyl alcohol (PVA) 72.000 (Fluka, Switzerland), and corn oil (Turkey). All other chemicals were analytical grade.
Analytical Method Validation
In this study, a spectrophotometric method reported for the determination of pantoprazole in tablets (CitationSuslu et al. 2003) was modified and validated for the analysis of pantoprazole in dissolution medium and methanol. Due to the matrix and analyst change, determination method was partially validated in terms of stability, linearity, precision, repeatability, accuracy, and specificity and selectivity (CitationICH 1996).
A stock solution of pantoprazole (1000 μ g/ml) was prepared in distilled water. Working standard solutions were diluted from the stock solution range from 10 to 60 μ g/ml. Spectrophotometric determinations were performed by an Agilent 8453 combined with DAD UV-VIS spectrophotometer at 290 nm using 1-cm quartz cells.
Preparation of Microspheres
The microspheres were prepared by emulsion-solvent evaporation technique (CitationO'Donell and Mc Ginity 1997; CitationComoglu et al. 2003; CitationŞengel et al. 2006). Polymer was dissolved in alcohol–acetone mixture by stirring at 500 rpm with a magnetic stirrer. Accurately weighed amounts of pantoprazole and Myvacet 9-45K were dispersed in this solution and stirred at the same rate with magnetic stirrer at a temperature of less than 20°C. Then the mixture was rapidly poured into corn oil. The resultant emulsion was continuously agitated at room temperature using a three-blade propeller stirrer (Stir-Pak®, USA) at 1200 rpm for 5 hr and the solvent was removed completely by evaporation. The solidified microspheres were filtered and washed twice with 200-ml hexane and then dried at room temperature for 12 hr. Final microspheres were stored in a desiccator.
In this study, the drug/polymer ratio (1/1 and 2/1) was varied for each polymer, maintaining a constant polymer and solvent volume. The composition of each microsphere formulation prepared is given in . PVA 72.000 was used as an emulsifier for stabilizing the outer phase. The concentration of Myvacet 9-45K (10%), which was used as plasticizer, was calculated from the polymer amount (w/v%).
TABLE 1 Composition of microspheres
Determination of Production Yield and Encapsulation Efficiency
Production yield of the microspheres was determined by accurately calculating the initial weight of the raw materials (WR) and the last weight of the microspheres (WM) obtained. The ratio of WM to WR was then calculated and multiplied by 100 and expressed as a percentage. Triplicate data were used.
Encapsulation efficiency of the microspheres was estimated by dissolving accurately weighed portions from each batch in methanol, and the actual drug content was determined using a UV-DAD spectrophotometer (Agilent 8453) at a wavelength of 290 nm (n = 6).
The percentage of encapsulation efficiency was calculated using equations 1 and 2 (CitationHasçiçek et al. 2003; CitationŞengel et al. 2006).
Particle Size Analysis of Microspheres
The mean particle size and size distribution of microspheres were determined by laser diffractometry (Sympatec HELOS, H0728, particle size analyzer, Germany). Small amounts of microspheres were dispersed in distilled water and then analyzed. Each determination was carried out in triplicate.
Scanning Electron Microscopy
Scanning electron microscopy (Jeol JSM-6490 LV, Japan) was used to examine the shape and surface morphology of the microspheres. Samples of microspheres were dusted on to double-sided tape on an aluminum stub. The stubs were then coated with gold using a cold sputter coater (Polaran E 5100) to a thickness of 400A°. The samples were imaged using 15-kV electron beam.
Differential Scanning Calorimetry (DSC)
The DSC was performed (DSC-60 Schimadzu, Japan) after sealing the samples (pantoprazole, Eudragit S 100, HPMCP, physical mixtures of each polymer with pantoprazole and microspheres) in aluminum pans. Calibration was carried out using indium. The DSC tracings were performed from –140 to 250°C at a rate of 10°C/min.
Fourier Transform Infrared (FTIR) Spectroscopy Analysis
The FTIR spectroscopy analysis (Jasco FT/IR-420) of raw materials, their physical mixtures, and microparticles were carried out using KBr pellets. Each sample was recorded at a resolution of 2 cm−1 over the wavenumber region of 4.000–400 cm−1.
In Vitro Release Study
The dissolution test was performed according to the USP 24 paddle method (Aymes, D96D) at a rotation speed of 50 rpm. Pantoprazole loaded microspheres were poured into a vessel containing 900 ml of phosphate buffer pH 6.8. The samples were collected at predetermined time intervals (5, 10, 15, 30, 45, 60, 90, 120, and 150 min) and then analyzed. The test was conducted in triplicate.
The gastro-resistance study was performed in triplicate using the same technique. Microspheres were poured into a vessel containing 300 ml of 0.1 mol/l HCl (acid stage). After 30 min, 600 ml of phosphate buffer containing an excess amount of NaOH was added into the vessel to neutralize the acid medium. Samples were collected at the same time intervals of the dissolution study and then analyzed.
RESULTS AND DISCUSSION
Analytical Validation
Selectivity
Comparison of the standard pantoprazole solution and microspheres solutions showed that the microspheres did not give any absorbance at 290 nm. Similarly, pantoprazole absorbance at 290 nm was not interfered by the tested phosphate buffers and methanol.
Linearity Range and Calibration Curve
Absorbance values of pantoprazole-working standards prepared in distilled water, methanol, pH 4.5, pH 6.8, and pH 7.4 phosphate buffers at the concentrations of 5, 10, 20, 30, 40, 50, 60, 70, 80, and 90 μ g/ml were measured at 290 nm. Calibration curves in the media indicated above were constructed by plotting absorbance values versus pantoprazole concentrations. Regression analysis indicated linear relationships between absorbance and concentration and there was not any significant difference observed in five different media. Therefore, water was chosen in rest of the study. The calibration curve was linear in the range of 5–90 μ g/ml with a regression equation of y = 0.0334x + 0.0047 (R2 = 0.9999).
Stability
The stability of pantoprazole stock solutions was tested in dark, daylight, and 4°C for the periods of 0, 8, 24, and 48 hr. The results demonstrated that the pantoprazole solution in water was stable only in the first 8 hr at 4°C at dark with a 98.5% recovered pantoprazole. In freeze-thaw cycle study in −23°C and room temperature, the ratio of recovered pantoprazole was, respectively, 98.5, 97.5, and 96.30% in three cycles. Thus, pantoprazole solutions were prepared daily and kept in cold and dark during analysis.
Accuracy
To examine the accuracy of the method, recovery experiments were performed at 10, 30, and 60 μ g/ml standard solutions of pantoprazole (n = 6). Closeness of the recovery results to 100% showed that the accuracy of the method was acceptable (). The recovery percentage values ranged between 99.90 and 101.13% indicating the high accuracy of the method.
TABLE 2 Accuracy and repeatability results of the spectrophotometric method for pantoprazole determination
Repeatability
Standard solutions containing 10, 30, and 60 μ g/ml pantoprazole were analyzed in the same day (n = 6). Found amounts of pantoprazole are given in indicating that the relative standard deviation did not exceed 1% stating the high precision of the method.
Ruggedness
The ruggedness of the method was controlled by two different operators for pantoprazole solution at a concentration of 30 μ g/ml, and mean values of found pantoprazole concentrations were statistically evaluated using t test (p = .05, n = 6). Test results were found as tcalculated (0.43) < ttable (2.23), indicating that there was no significant difference between the mean values, so that the method was rugged ().
TABLE 3 Ruggedness of spectrophotometric method for pantoprazole determination
Preparation of Microspheres
The emulsion-solvent evaporation technique was used to prepare sodium pantoprazole sesquihydrate-loaded microspheres. Corn oil was selected as an outer phase because pantoprazole, Eudragit S 100, and HPMCP were not soluble in corn oil (CitationŞengel et al. 2006). Acetone and alcohol mixture with dielectric constants of 20.7 and 22.0, respectively, was chosen as a disperse (inner) phase because solvents with dielectric constants between 10 and 40 show poor compatibility with hydrophobic substances like corn oil, and the system of these solvents with hydrophobic liquids was reported to be applicable to the microencapsulation process (CitationKawata et al. 1986; CitationKim et al. 1994; CitationMateovic et al. 2002; CitationŞengel et al. 2006). An emulsifying agent, PVA 72.000 was used in this study to reduce the interfacial tension and prevent electrification and flocculation during the preparation of the microspheres (CitationHoroz et al. 2004; CitationŞengel et al. 2006). PVA 72.000, which is an anionic emulsifier, was added to the outer phase because of its hydrophobic structure. Myvacet 9-45K was used as a plasticizer in the microsphere formulations and incorporated in the inner phase to improve the flexibility of the polymer chains and form a less porous network (CitationHuang and Ghebre-Sellassie 1989; CitationŞengel et al. 2006).
Production Yield and Encapsulation Efficiency
The encapsulation efficiency and production yield of pantoprazole-loaded microspheres are shown in . All microsphere formulations are produced with high production yield and encapsulation efficiency. Production yield ranged from 50.94 to 91.45%. The encapsulation efficiency of microspheres was between 60.31 and 98.64%. The overall results have shown that both Eudragit S 100 and HPMCP are suitable polymers for the encapsulation of a hydrophilic drug.
TABLE 4 Properties of microsphere formulations
When the production yield and encapsulation efficiency of the microspheres were investigated on the basis of variation of the drug/polymer ratio, it was suggested that the production yield was not much affected by the drug/polymer ratio (p > .05), especially for F1- and F3-coded formulations. However, the encapsulation efficiency of the microspheres was found to be affected by the drug/polymer ratio. When the drug amount was increased, the encapsulation efficiency of the microspheres decreased. This phenomenon was due to the fact that there was not sufficient polymer in the media to produce microspheres containing higher pantoprazole contents at high drug concentration levels. More of the drug is lost, resulting in the formation of the microspheres with lower drug contents (CitationŞengel et al. 2006).
However, for F2 formulation, which has a drug/polymer ratio of 2/1 (pantoprazole/HPMCP), particle formation was not observed. It was suggested that polymer amount was not enough to produce microspheres.
Particle Size Analysis of Microspheres
Mean particle sizes of microspheres are shown in . Particle size analysis of pantoprazole microspheres showed that the mean particle size was affected both by the polymer type and the drug/polymer ratio (p < .01). A reduction in microsphere size was observed with decreasing drug amount. When the drug amount was increased, a more viscous internal phase occurred. During the emulsification process, the internal phase was hardly dispersed in the outer phase and larger microspheres were produced. When the drug amount was decreased, the size of microspheres decreased due to the reduced viscosity of the internal phase. These findings are similar to the results reported previously in the literature (CitationKim et al. 1994; CitationŞengel et al. 2006).
Particle size of the microspheres was also affected by the polymer type. Microspheres, which were prepared with HPMCP (drug/polymer ratio of 1/1), had a smaller mean diameter when they were compared with Eudragit S 100 microspheres. This result may be due to different chemical structures of the two polymers.
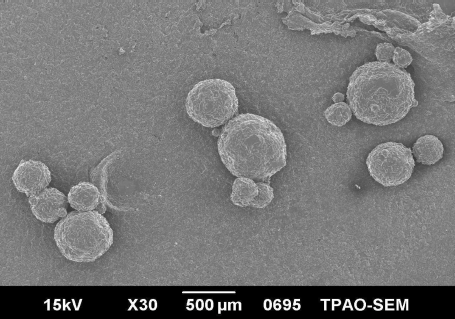
Scanning Electron Microscopy
The shape and surface characteristics of the microsphere formulations coded F1, F3, and F4 are shown in , respectively. Microspheres prepared with HPMCP were more spherical and had a smaller particle size than Eudragit S 100 microspheres. In Eudragit S 100 microspheres, microspheres prepared with a drug/polymer ratio of 1/1, shape of the microspheres was more irregular. These results showed that both the drug/polymer ratio and type of the polymer extensively affected the morphological characteristics of the microspheres.
Differential Scanning Calorimetry
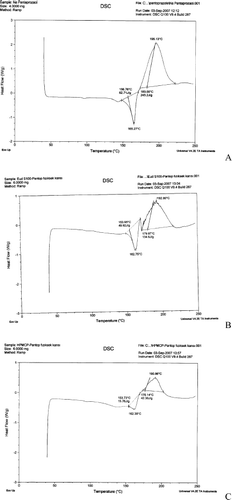
Concerning the DSC analysis (), pantoprazole showed an endothermic peak at 156.76°C (heat of fusion 245.2 J/g), followed by its degradation at 195.13°C. The peak at 156.76°C corresponds to the melting and the dehydration of pantoprazole (), which are parallel processes (CitationZupancic et al. 2005; CitationColome et al. 2007). Regarding the physical mixtures of drug and polymers, tracings showed the heat of fusion for pantoprazole–Eudragit S 100 (49.92 J/g) and pantoprazole–HPMCP mixture (134.6 J/g) () as well as the absence of fusion event of pantoprazole for the F3 and F4 formulations (). These results suggest that pantoprazole-loaded microspheres are composed by a homogeneous phase, in which the drug is encapsulated by dissolution or molecularly dispersed in the polymer or blend. According to the literature, the disappearance of any event indicates drug encapsulation (CitationColome et al. 2007). The trace presented in F1 () showed the drug encapsulation, which was not observed in F3 and F4 ().
FIG. 5. Differential scanning calorimetry (DSC) tracings of (A) sodium pantoprazole sesquihydrate, (B) physical mixture of sodium pantoprazole sesquihydrate and Eudragit S 100, (C) physical mixture of sodium pantoprazole sesquihydrate and hydroxypropyl methylcellulose phtalate (HPMCP), (D) F1 microspheres, (E) F3 microspheres, and (F) F4 microspheres.
FTIR Spectroscopy Analysis
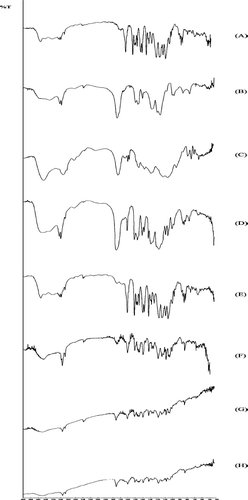
The FTIR analysis () was performed to explain the possible interactions between the polymer and the drug in pantoprazole microspheres. The spectra of the raw materials showed their characteristic, expected bands (CitationColome et al. 2007). The microsphere spectra () showed a new band at 1560 cm−1. This shift was observed as a new antisymmetrical vibration of carboxylic group. When Eudragit S was ionized, COO− band shifted from 1728 to 1560 cm−1. For HPMCP, COO− band shifted from 1715 to 1560 cm−1. This new band was sharper in drug/Eudragit S 100 (1:1) (). This new band was not observed in the drug–polymer physical mixtures ().
FIG. 6. Infrared spectra: (A) sodium pantoprazole sesquihydrate, (B) Eudragit S 100, (C) hydroxypropyl methylcellulose phtalate (HPMCP), (D) physical mixture of sodium pantoprazole sesquihydrate and Eudragit S 100, (E) physical mixture of sodium pantoprazole sesquihydrate and HPMCP, (F) F3 microspheres, (G) F4 microspheres, and (H) F1 microspheres.
In Vitro Release Study of Microspheres
Regarding the dissolution profiles (), the percentage of pantoprazole released was determined (mean ± SD) 55.82 ± 0.63%, 88.11 ± 0.73%, and 98.34 ± 0.87% for F1, F3, and F4 microspheres, respectively, after 120 min.
FIG. 7. Dissolution profiles of sodium pantoprazole sesquihydrate-loaded microspheres in pH 6.8 phosphate buffer.
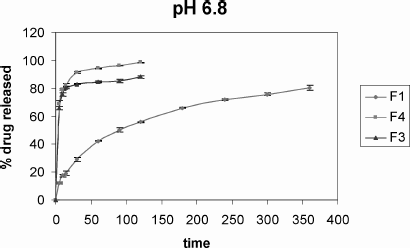
Mathematical modeling for pantoprazole from the microspheres was given in . Considering the mathematical modeling for pantoprazole microspheres, the best model was that of Weibull kinetics presenting sigmoidal curves. As the slope of the line is less than 1 (β ≤ 1), this means that there is a faster drug release at the beginning, which is followed by a first-order release profile that reaches the plateu.
TABLE 5 Kinetic results and fitting criteria of microspheres in pH 6.8 phosphate buffer
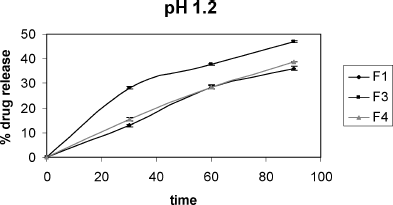
The acid resistance experiment () carried out with microspheres showed that (mean ± SD) 64.15 ± 1.25%, 54.26 ± 0.87%, and 61.11 ± 1.53% of pantoprazole remained stable for F1, F3, and F4 microspheres, respectively, presenting the stability of drug protection (CitationRaffin et al. 2006).
REFERENCES
- D. Avner. (2000). Clinical experience with pantoprazole in gastro esophageal reflux disease. Clin. Ther. 22 (10):1170–1185.
- W. Beil, U. Staar, and K. Sewing. Pantoprazole: a novel H+/K+-ATPase inhibitor with an improved pH stability. Eur. J. Pharmacol. 218:265–271.
- S. S. Bhalerao, J. K. Lalla, and M. S. Rane. (2001). Study of processing parameters influencing the properties of diltiazem hydrochloride microspheres. J. Microencapsul. 18:299–307.
- M. Çelik. (1994). Compaction of multiparticulate oral dosage forms: in Multiparticulate Oral Drug Delivery, ed. I. Ghebre-Sellassie., 181–215. New York: Marcel Dekker Inc.
- S. Cheer, A. Prakash, D. Faulds, and H. Lamb. (2003). Pantoprazole—an update of its pharmacological properties and therapeutic use in the management of acid-related disorders. Drugs 63 (1):101–132.
- L. M. Colome, R. P. Raffin, D. S. Jornada, A. R. Pohlmann, and S. S. Guterres. (2007). Pantoprazole-loaded Eudragit blended microparticles: preparation, characterization, in vitro gastro-resistance and in vivo anti-ulcer evaluation. J. Drug Del. Sci. Tech. 17 (2):113–118.
- T. Comoglu, N. Gonul, and T. Baykara. (2003). Preparation and in vitro evaluation of modified release ketoprofen microsponges. Farmaco 58:101–106.
- A. Fitton, and L. Wiseman. (1996). Pantoprazole—a review of its pharmacological properties and therapeutic use in acid-related disorders. Drugs 51 (3):460–482.
- C. Hasçiçek, N. Gonul, and N. Erk. (2003). Mucoadhesive microspheres containing gentamicin sulfate for nasal administration: preparation and in vitro characterization. Farmaco 58:11–16.
- B. B. Horoz, M. Kılıçarslan, N. Yüksel, and T. Baykara. (2004). Effect of different dispersing agents on the characteristics of Eudragit microspheres prepared by a solvent evaporation method. J. Microencapsul. 21:191–202.
- H. Huang, and I. Ghebre-Sellassie. (1989). Preparation of microspheres of water-soluble pharmaceuticals. J. Microencapsul. 6:219–225.
- ICH Guideline. (1996). Validation of analytical procedures: text and methodology Q2 (R1).
- M. Kawata, M. Nakamura, S. Goto, and T. Aoyama. (1986). Preparation and dissolution pattern of Eudragit RS microcapsules containing ketoprofen. Chem. Pharm. Bull. 34:2618–2623.
- C. K. Kim, M. J. Kim, and K. H. Oh. (1994). Preparation and evaluation of sustained release microspheres of terbutaline sulfate. Int. J. Pharm. 106:213–219.
- T. Mateovic, B. Kriznar, M. Bogataj, and A. Mrhar. (2002). The influence of stirring rate on biopharmaceutical properties of Eudragit RS microspheres. J. Microencapsul. 19:29–36.
- P. B. O'Donell, and J. W. Mc Ginity. (1997). Preparation of microspheres by the solvent evaporation technique. Adv. Drug Deliv. Rev. 28:25–42.
- P. Poole. (2001). Pantoprazole. Am. J. Health Syst. Pharm. 58 (11):999–1008.
- R. P. Raffin, L. M. Colome, A. R. Pohlmann, and S. S. Guterres. (2006). Preparation, characterization, and in vivo anti-ulcer evaluation of pantoprazole-loaded microparticles. Eur. J. Pharm. Biopharm. 63:198–204.
- T. Şengel, C. Hasçiçek, and N. Gönül. (2006). Development and in-vitro evaluation of modified release tablets including ethylcellulose microspheres loaded with diltiazem hydrochloride. J. Microencapsul. 23 (2):135–152.
- J. M. Shin, and G. Sachs. (2004). Differences in binding properties of two proton pump inhibitors on the gastric H+/K+-ATPase in vivo. Biochem. Pharmacol. 68:2117–2127.
- K. S. Soppimath, A. R. Kulkarni, and T. M. Aminabhavi. (2001). Encapsulation of antihypertensive drugs in cellulose-based matrix microspheres: characterization and release kinetics of microspheres and tableted microspheres. J. Microencapsul. 18:397–409.
- I. Suslu, S. Altinoz, and E. Yildiz. (2003). Determination of pantoprazole in tablet dosage forms by two different spectrophotometric methods. FABAD J. Pharm. Sci. 28:85–92.
- N. K. Varde, and D. W. Pack. (2004). Microspheres for controlled release drug delivery. Expert Opin. Biol. Ther. 4 (1):1–17.
- V. Zupancic, N. Ograjsek, B. Kotar-Jordan, and F. Vrecer. (2005). Physical characterization of pantoprazole sodium hydrates. Int. J. Pharm. 291:59–68.