Abstract
This study systematically investigated the enhancing effect of fatty acids on the skin permeation of diclofenac. The fatty acids were evaluated in terms of their carbon-chain length, the degree of unsaturation, and their functional groups. The rat-skin permeation rates of diclofenac, saturated in propylene glycol (PG) containing 1% (w/v) fatty acid, were determined using the Keshary-Chien diffusion cells at 37°C. The effect of fatty acids on the saturated solubility of diclofenac in PG was also determined at 37°C using high-performance liquid chromatography. Among the saturated fatty acids tested, palmitic acid (C16:0) showed the most potent skin permeation-enhancing effect. A parabolic correlation was observed between the enhancement effect and the fatty acid carbon-chain length among these saturated fatty acids of C12–C20 units. For the monounsaturated fatty acid series, an increase in permeation was observed as the carbon-chain length increased, and oleic acid (C18:1) showed the highest permeation-enhancing effect. Increasing the number of double bonds in the octadecanoic acids resulted in a parabolic effect in the permeation of diclofenac, revealing oleic acid as the most effective enhancer used in this study. When the carboxylic acid moiety of oleic acid was changed to an amide (oleamide) or hydroxyl (oleyl alcohol) group, a decrease in permeation activity was observed. These results, therefore, suggest that the cis-monounsaturated configuration and the carboxylic acid moiety of an 18-carbon unit fatty acid in PG are the optimum requirements for the effective skin permeation of diclofenac.
Keywords :
Diclofenac is a strong nonsteroidal anti-inflammatory drug (NSAID) clinically used for the management of acute conditions of inflammation and pain, musculoskeletal disorders, and arthritis. Although diclofenac is a relatively safe and tolerable NSAID, the clinical use of diclofenac has been often limited because of its potential to cause irritation and ulceration of the gastrointestinal mucosa after oral administration (McCarthy Citation1999). NSAIDs are known to inhibit cyclo-oxygenase-2 at the inflammation sites, but most of them also inhibit gastric mucous cyclo-oxygenase-1, which is associated with gastrointestinal damage (Mitchell and Warner Citation1999).
The oral formulation of diclofenac is accompanied with the above-mentioned adverse effects as well as high degree of hepatic first-pass metabolism and short biological half-life (John Citation1979; Willis et al. Citation1979). Therefore, a transdermal delivery system has been attempted to overcome these disadvantages and maintain relatively consistent plasma levels for long-term therapy from a single dose (Goosen et al. Citation1998). However, because few reports on the transdermal delivery system for diclofenac have not been very satisfactory (Takahashi et al. Citation1995; Escribano et al. Citation2003), the use of an optimal chemical enhancer may be the key to a more successful transdermal delivery system for diclofenac.
The selection of suitable enhancer(s) for improving the transdermal permeation of a selected drug is a difficult task involving factors such as the nature and concentration of active ingredients and excipients, and the kind of delivery system used (Sinha and Kaur Citation2000). Fatty acids are among the most popular penetration enhancers used for the formulation of transdermal drug delivery systems (Tanojo et al. Citation1997; Oh et al. Citation1998; Santoyo and Ygartua Citation2000; Oh et al. Citation2001). They are also commonly used as adjuvants for cosmetics and pharmaceuticals because most fatty acids have the advantage of being endogenous components of human skin. Fatty acids are known to enter the hydrophobic tails of the stratum corneum lipid bilayer, disturbing their packing, increasing their fluidity, and subsequently, decreasing the diffusional resistance to permeates (Golden et al. Citation1987). The effect of varying carbon-chain lengths, degrees and types of unsaturation (position, number), the branching schema, and substituents has been reported to influence the ability of fatty acids to act as skin penetration enhancers (Elyan et al. Citation1996; Bhatia and Singh Citation1998; Takeuchi et al. Citation1998; Taguchi et al. Citation1999).
In this study, the effects of 12 fatty acids on the transdermal delivery of diclofenac in vitro have been systematically investigated. The fatty acids were grouped in terms of their carbon-chain length, cis configuration, the number of double bonds, and their functional groups.
MATERIALS AND METHODS
Materials
Diclofenac sodium was obtained from Ahn-Gook Pharmaceutical Co. (Seoul, Korea) and converted to its free-base form to be used in the current study by adjusting the pH and collecting the precipitates. The fatty acids listed in were purchased from Sigma Chemical Co. (St. Louis, MO, USA) and were of the highest available purity. Propylene glycol (PG), HPLC (high-performance liquid chromatography) grade acetonitrile, and methanol were purchased from Merck (Darmstadt, Germany). All other chemicals were reagent grade or higher.
TABLE 1 Fatty acids and analogs studied as a skin permeation enhancer
Preparation of Rat Skin
The animals used for the preparation of skin were male Sprague-Dawley (220–250 g) rats obtained from Dae-Han Laboratory Animal Research Center Co. (Taejon, Korea). The animals had free access to food and water and were kept in the Animal Experimental Center (College of Pharmacy, Seoul National University, South Korea) until they were used for the experiments. The regulations for managing and using animals have been made on the basis of the “Guide for the Care and Use of Experimental Animals (ILARM National Research Council, USA).” The study protocol was approved by the Animal Care and Use Committee of the Collage of Pharmacy, Seoul National University. Animals were killed in an ether chamber right before the experiments. The dorsal hairs were removed with a clipper and full-thickness skin was surgically removed from each rat. The skin specimen was cut into appropriate sizes after carefully removing subcutaneous fat and washing with normal saline.
Solubility of Diclofenac in PG with Various Fatty Acids
The solubility of diclofenac in PG with various fatty acids (1%, w/v) was determined at 37°C. Excess of diclofenac was added to PG and mixed by vortexing. The suspended solution was immersed in a shaking water bath at 37°C and allowed to equilibrate for 24 hr. The saturated solutions were then filtered through Minisart RC4 filters (0.45 μ m, Sartorius, Germany). Concentrations of diclofenac were analyzed by HPLC after appropriate dilution with methanol.
In Vitro Skin Permeation Study
In vitro skin permeation study across rat skin was conducted with Keshary-Chien diffusion cells at 37°C. Freshly excised rat skin was mounted between the donor and receptor cells (stratum corneum side facing the donor). The area of diffusion cell for all in vitro studies was 2.14 cm2. The donor cells, which faced the stratum corneum surface, contained a saturated solution (3.0 mL) of diclofenac in PG with 1% (w/v) fatty acids and were occluded with Parafilm. The receptor cells, which faced the dermis side, were filled with isotonic phosphate buffer (pH 7.4):PEG400 (60:40) to maintain sink condition (12.0 mL). At predetermined time intervals, 1.0 mL of the receptor solution was withdrawn and refilled with the same volume of fresh receptor solution. Samples were kept in a refrigerator until analyzed by HPLC.
HPLC Analysis of Diclofenac
Concentration of diclofenac was determined using an HPLC system equipped with a binary pump (Gilson Model 305 and 306) and automatic injector (Gilson Model 234). A LiChroCART 125-4 column (C18, 5 μ m, 125 × 4 mm, Merck, Darmstadt, Germany) was used as the stationary phase at ambient temperature. The mobile phase was an organic solvent (acetonitrile:methanol = 25:10)-acetate buffer (50 mM, pH 6.5) combination (35:65, v/v) at a flow rate of 1.0 mL/min. The variable wavelength ultraviolet detector (Gilson Model 118) was set at 275 nm. Injections of 20 μ L were made for all solutions to be analyzed. Retention time of diclofenac was at about 8 min.
RESULTS
A list of fatty acids selected as potential skin permeation enhancers, together with their melting points, is presented in . All saturated fatty acids were in solid states with melting points ranging from 44.2°C to 76.5°C, whereas the unsaturated counterparts were either oily liquid or solids at room temperature. Diclofenac was saturated in PG containing 1% (w/v) fatty acid to keep a constant driving force with the maximum thermodynamic activity for the in vitro skin permeation studies. The results of the rat skin permeation profiles using the Keshary-Chien permeation cells at 37°C are shown in , , , while the skin permeation parameters and solubility of diclofenac are listed in . An overall summary is shown in . Detailed explanations for the results are as follows.
FIG. 1 Effect of carbon-chain length of saturated fatty acids on the transdermal permeation of diclofenac through rat skin. Each point represents the mean ± S.D. of four experiments.
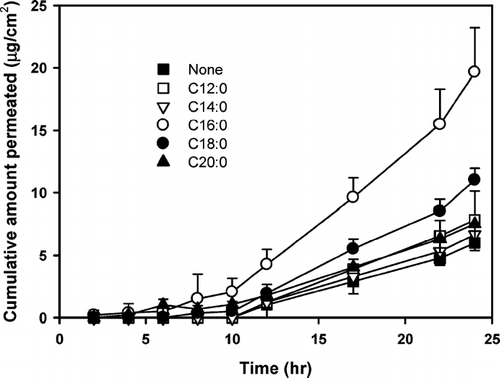
FIG. 2 Effect of carbon-chain length of cis-monounsaturated fatty acids on the transdermal permeation of diclofenac through rat skin. Each point represents the mean ± S.D. of four experiments.
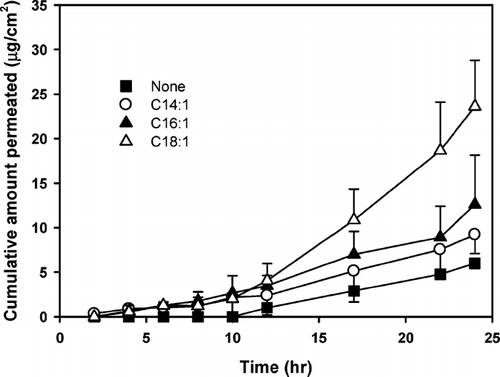
FIG. 3 Effect of number of double bonds in octadecanoic acids on the transdermal permeation of diclofenac through rat skin. Each point represents the mean ± S.D. of four experiments.
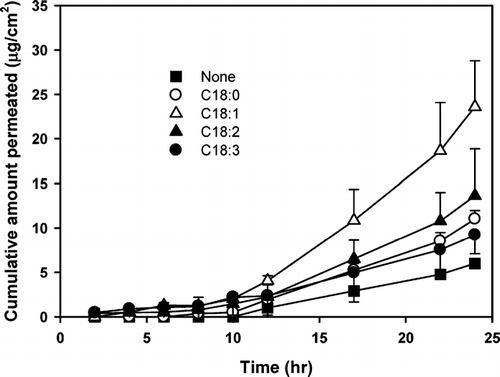
FIG. 4 Effect of various fatty acids (1.0%, w/v) on the rat skin permeation rate of diclofenac, saturated in propylene glycol at 37°C. Each point represents the mean ± S.D. of four experiments.
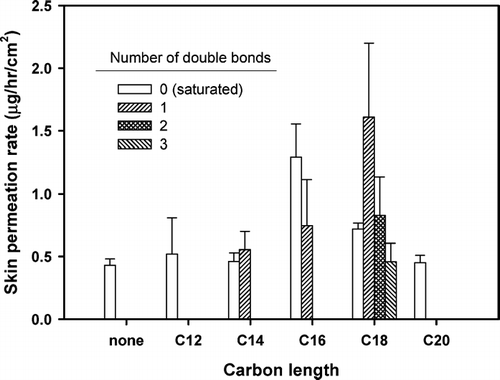
TABLE 2 In vitro rat skin permeation parameters of diclofenac, saturated in propylene glycol containing 1% (w/v) of fatty acids at 37°C
Effect of Carbon-Chain Length in Saturated Fatty Acids
Among the saturated fatty acids of 12–20 carbon units selected for this study, palmitic acid (C16:0) was most effective in increasing the permeation rate of diclofenac. Palmitic acid (1%, w/v) was able to increase the permeation of diclofenac by 3-fold when compared with diclofenac with no permeation enhancer added (). More interestingly, a parabolic correlation was observed between the enhancement effect of the fatty acids and their respective carbon-chain length (), where a gradual increase in permeation was observed up to C16 followed by a decrease as the C-units increased up to C20. Because the solubility of diclofenac in PG was not significantly affected by the addition of the saturated fatty acids, the increase in the permeation rate seems to have resulted from the increase in the permeability coefficient. On the basis of the above results, myristic acid (C14), palmitic acid (C16), and stearic acid (C18) were selected for further evaluation of their enhancing effect.
Effect of Carbon-Chain Length in cis-Monounsaturated Fatty Acids
The skin permeation-enhancing effect of cis-monounsaturated fatty acids is shown in . When compared with the addition of the corresponding saturated fatty acids, the solubility of diclofenac significantly decreased with the addition of the corresponding monounsaturated fatty acids. However, their addition increased or maintained the skin permeation rate of diclofenac compared with the rate when the saturated counterparts were added. As the carbon-chain length of the monounsaturated enhancers increased, an increase in permeation rate was observed. While the C16 analogue (palmitic acid) resulted in the highest permeation rate among the saturated analogues added, the C18 analogue (oleic acid) revealed the highest permeation among the unsaturated series as well as among all the fatty acids tested (). A comparison among the cis-monounsaturated fatty acids revealed the following order in their effect on the diclofenac permeation enhancement: oleic acid (18:1) > palmitoleic acid (16:1) > myristoleic acid (14:1) ().
Effect of Number of Double Bonds in Octadecanoic Acids
Because oleic acid (C18:1) significantly increased the skin permeation rate of diclofenac compared with its saturated form (stearic acid, C18:0), the effect of the number of double bonds in octadecanoic acid on the skin permeation of diclofenac was evaluated (). When compared with the saturated stearic acid, the addition of the unsaturated oleic acid significantly increased the permeation rate of diclofenac. However, as the number of double bonds further increased (from linoleic acid, C18:2 to linolenic acid, C18:3), a substantial decrease in the permeation rate of diclofenac was observed (). Thus, among all the saturated and unsaturated fatty acids tested in this study, oleic acid was the most potent skin permeation enhancer for diclofenac.
An increase in drug solubilization in the vehicle containing the fatty acids has been reported to result in the increase of both partitioning of drug to the skin and solvent penetration, thereby increasing the flux of drug (Aungst et al. Citation1990). However, as shown in , the solubility of diclofenac decreased as the number of the double bonds increased, yet the skin permeation rate showed the parabolic relationship. Thus, the skin permeation-enhancing effect of unsaturated octadecanoic acids, especially oleic acid, does not seem to be related to drug solubilization, but to their direct effect on the skin.
Effect of Oleic Acid Analogs
To evaluate the role of the carboxylic acid moiety in the fatty acids, oleamide and oleyl alcohol were selected for comparison with oleic acid of their enhancing effect on the skin permeation of diclofenac (). Although the solubility of diclofenac did not show any significant difference by adding 1.0% (w/v) of the oleic acid analogs, the permeation rate of diclofenac significantly increased compared with the control (i.e., without enhancer), resulting in a 3.74, 2.74, and 1.44-fold increase in the enhancing ratio with the addition of oleic acid, oleamide, and oleyl alcohol, respectively ().
FIG. 5 Effect of functional group of oleic acid analogs on the transdermal permeation of diclofenac through rat skin; (•) no enhancer, (▴) oleic acid, (▾ oleamide, (▪ oleyl alcohol. Each point represents the mean ± S.D. of four experiments.
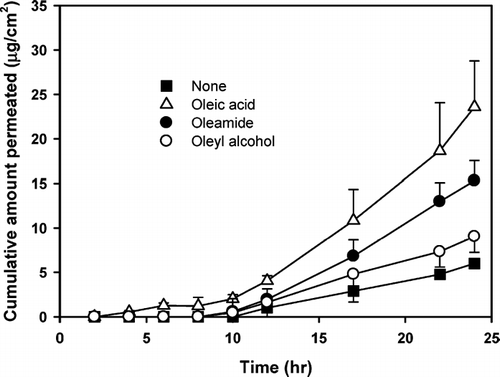
TABLE 3 In vitro rat skin permeation parameters of diclofenac, saturated in propylene glycol containing 1% (w/v) of oleic acid analogs at 37°C
It is known that the melting characteristics and solubility of a drug can have a profound effect on its permeation across a membrane (Kanikkannan et al. Citation1999; Stott et al. Citation2001). Oleyl alcohol is expected to have higher solubility parameters due to its lower melting point compared with oleic acid and oleic amide. It was, therefore, expected that oleyl alcohol would be the most effective enhancer among tested, if their enhancement was solely due to the solubility effect. However, among the three analogues, oleic acid resulted in the highest permeation rate of diclofenac ().
DISCUSSION
A parabolic correlation was observed between the enhancement effect of the saturated fatty acids and their respective carbon-chain length (). A similar relationship has been previously reported for indomethacin. Among the fatty acids tested for indomethacin, short-chain (< C6) fatty acids were less effective in enhancing the skin permeation compared with the medium-chain fatty acids (C6–C8), while a parabolic relationship between the carbon-chain length of fatty acids and skin permeation enhancement was described (Chien et al. Citation1988). It was also proposed that fatty acids with a certain chain length (around 16 carbons) possess an optimal balance between partition coefficient or solubility parameter and affinity to skin (Ogiso and Shintani Citation1990).
The results obtained in this study are consistent with those of indomethacin in that shorter-chain fatty acids result in lower skin permeation because of their insufficient lipophilicity. On the other hand, too long an alkyl chain retards permeation because of their affinity to lipids in the stratum corneum based on the hydrophobic interaction. The permeation enhancement suggests that the mode of action of the saturated fatty acids as enhancers is dependent on the permeation of the fatty acids themselves across the stratum corneum (Tanojo et al. Citation1997).
The cis configuration of unsaturated fatty acids is known to alter the phase structure of the intercellular lipid matrix, making free volumes (Harrison et al. Citation1996). The formation of the conformation “kink” isomers has been reported. This may be viewed as the mobile structural defect representing small, mobile, free volumes in the hydrocarbon phase of the membrane, resulting in increased fluidity permitting small molecules to enter the free volumes of the kinks and migrate across the membrane together with these kinks (Träuble Citation1971). Thus, it was expected that unsaturated fatty acids would exert higher enhancing effect compared with their saturated counterparts. However, in this study, this was only true for the C-18 fatty acid analogues. The enhancing effect observed in the C-14 and C-16 analogues did not reveal a statistically significant difference between the saturated and unsaturated analogues (). This may have been due to the effect of the solvent, PG, which may also have contributed in modifying the transdermal absorption (Bendas et al. Citation1995; Larrucea et al. Citation2001).
The kinks formed in the lipid structure of the skin tend to increase with more number of double bonds in the unsaturated fatty acids, which could further enhance the permeation of the drug (Tanojo et al. Citation1997; Aungst et al. Citation1986; Kandimalla et al. Citation1999; Suhonen et al. Citation1999). However, the flux of diclofenac was unaffected by the number of double bonds in the fatty acid enhancers as was reported of indomethacin (Morimoto et al. Citation1996). This may be due to the fact that the increase in the number of double bonds causes steric hindrance, limiting the partitioning of the fatty acid to the lipid bilayers of the stratum corneum. However, a more systematic study is necessary to clearly explain this phenomenon.
The results of this study demonstrated that oleic acid was the most effective enhancer in the transdermal delivery of diclofenac. In general, the permeation ability is derived from a high solubility of the solute in the donor phase and its ability to leave the formulation and move towards the absorption membrane (Minghetti et al. Citation2007). In the case of diclofenac, however, because it was already quite soluble in PG, the solubility factor did not seem to affect the permeation rate as much as the interaction between the drug and the formulation factors ( and ). The optimum permeation obtained by oleic acid may have been a result of the combination of the solubilizing effect of PG and the fatty acid (Bendas et al. Citation1995; Larrucea et al. Citation2001). This is consistent with previous reports where combining the fatty acids and PG has shown a synergistic enhancement on skin permeation rate of nicardipine (Aboofazeli et al. Citation2002), physostigmine (Wang et al. Citation2005) and ketoprofen (Bowen and Heard Citation2006). Therefore, although fatty acids have been extensively used as permeation enhancers, the choice of an optimum fatty acid depends on the drug as well as the solvent used (Aungst Citation1989; Elyan et al. Citation1996; Stott et al. Citation2001).
CONCLUSIONS
The permeation efficacy of commonly used fatty acids and its analogs was systemically evaluated using an in vitro method. In the permeation study using the saturated fatty acids, a parabolic correlation between the enhancement effect and carbon-chain length was observed, with palmitic acid (C16:0) showing the highest permeation rate. However, when unsaturated fatty acids were used, a combination effect of the solvent and fatty acid seems to have affected the skin permeation enhancement. Among all fatty acids tested, oleic acid showed the best permeation enhancement for diclofenac when solubilized in PG.
This work was supported by a grant (R01-2006-000-11230-0) from the Basic Research Program of the Korea Science and Engineering Foundation.
REFERENCES
- Aboofazeli R., Zia H., Needham T. E. Transdermal delivery of nicardipine: an approach to in vitro permeation enhancement. Drug Deliv. 2002; 9: 239–247
- Aungst B. J. Structure/effect studies of fatty acid isomers as skin penetration enhancers and skin irritants. Pharm. Res. 1989; 6: 244–247
- Aungst B. J., Blake J. A., Hussain M. A. Contribution of drug solubilization, partitioning, barrier distribution and solvent permeation to the enhancement of skin permeation of various compounds with fatty acids and amines. Pharm. Res. 1990; 7: 712–718
- Aungst B. J., Rogers N. J., Shefter E. Enhancement of naloxone penetration through human skin in vitro using fatty acids, fatty alcohols, surfactants, sulphoxides and amides. Int. J. Pharm. 1986; 33: 225–234
- Bendas B., Schmalfuss U., Neubert R. Influence of propylene glycol as cosolvent on mechanisms of drug transport from hydrogels. Int. J. Pharm. 1995; 116: 19–30
- Bhatia K. S., Singh J. Synergistic effect of iontophoresis and a series of fatty acids on LHRH permeability through porcine skin. J. Pharm. Sci. 1998; 87: 462–469
- Bowen J. L., Heard C. M. Film drying and complexation effects in the simultaneous skin permeation of ketoprofen and propylene glycol from simple gel formulations. Int. J. Pharm. 2006; 307: 251–257
- Chien Y. W., Xu H., Chiang C. C., Huang Y. C. Transdermal controlled administration of indomethacin. I. Enhancement of skin permeability. Pharm. Res. 1988; 5: 103–106
- Elyan B. M., Sidhom M. B., Plakogiannis F. M. Evaluation of the different fatty acids on the percutaneous absorption of metaproterenol sulfate. J. Pharm. Sci. 1996; 85: 101–105
- Escribano E., Calpena A. C., Queralt J., Obach R., Doménech J. Assessment of diclofenac permeation with different formulations: anti-inflammatory study of a selected formula. Eur. J. Pharm. Sci. 2003; 19: 203–210
- Golden G. M., Mckie J. E., Potts R. O. Role of stratum corneum lipid fluidity in transdermal drug flux. J. Pharm. Sci. 1987; 76: 25–28
- Goosen C., du Plessis J., Mller D. G., Janse van Rensburg L. F. Correlation between physicochemical characteristics, pharmacokinetic properties and transdermal absorption of NSAIDs. Int. J. Pharm. 1998; 163: 203–209
- Harrison J. E., Watkinson A. C., Greem D. M., Hadgraft J., Brain K. The relative effect of azone and transcutol on permeant diffusivity and solubility in human stratum corneum. Pharm. Res. 1996; 13: 542–546
- John V. A. The pharmacokinetics and metabolism of diclofenac sodium (Voltarol) in animals and man. Rheumatol. Rehabil. Suppl. 1979; 2: 22–37
- Kandimalla K., Kanikkannan N., Andega S., Singh M. Effect of fatty acids on the permeation of melatonin across rat and pig skin in vitro and on the transepidermal water loss in rats in vivo. J. Pharm. Pharmacol. 1999; 51: 783–790
- Kanikkannan N., Kandimalla K., Lamba S. S., Singh M. Structure-activity relationship of chemical penetration enhancers in transdermal drug delivery. Curr. Med. Chem. 1999; 6: 593–608
- Larrucea E., Arellano A., Santoyo S., Ygartua P. Combined effect of oleic acid and propylene glycol on the percutaneous penetration of tenoxicam and its retention in the skin. Eur. J. Pharm. Biopharm. 2001; 52: 113–119
- McCarthy D. M. Comparative toxicity of nonsteroidal anti-inflammatory drugs. Am. J. Med 1999; 107: 37S–47S
- Minghetti P., Cilurzo F., Casiraghi A., Montanari L., Fini A. Ex vivo study of transdermal permeation of four diclofenac salts from different vehicles. J. Pharm. Sci. 2007; 96: 814–822
- Mitchell J. A., Warner T. D. Cyclooxygenase-2: pharmacology, physiology, biochemistry and relevance to NSAID therapy. Br. J. Pharmacol. 1999; 128: 1121–1132
- Morimoto K., Tojima H., Haruta T., Suzuki M., Kakemi M. Enhancing effects of unsaturated fatty acids with various structures on the permeation of indomethacin through rat skin. J. Pharm. Pharmacol. 1996; 48: 1133–1137
- Ogiso T., Shintani M. Mechanism for the enhancement effect of fatty acids on the percutaneous absorption of propranolol. J. Pharm. Sci. 1990; 79: 1065–1071
- Oh S. Y., Jeong S. Y., Park T. G., Lee J. H. Enhanced transdermal delivery of AZT (Zidovudine) using inotophoresis and penetration enhancer. J. Control. Release 1998; 51: 161–168
- Oh H. J., Oh Y. K., Kim C. K. Effects of vehicles and enhancers on transdermal delivery of melatonin. Int. J. Pharm. 2001; 212: 63–71
- Santoyo S., Ygartua P. Effect of skin pretreatment with fatty acids on percutaneous absorption and skin retention of piroxicam after its topical application. Eur. J. Pharm. Biopharm. 2000; 50: 245–250
- Sinha V. R., Kaur P. M. Permeation enhancers for transdermal drug delivery. Drug Dev. Ind. Pharm. 2000; 26: 1131–1140
- Stott P. W., Williams A. C., Barry B. W. Mechanistic study into the enhanced transdermal permeation of a model β -blocker, propranolol, by fatty acids: a melting point depression effect. Int. J. Pharm. 2001; 219: 161–176
- Suhonen T. M., Bouwstra J. A., Urtti A. Chemical enhancement of percutaneous absorption in relation to stratum corneum structural alterations. J. Control. Release 1999; 59: 149–161
- Taguchi K., Fukushima S., Yamaoka Y., Takeuchi Y., Suzuki M. Enhancement of propylene glycol distribution in the skin by high purity cis-unsaturated fatty acids with different alkyl chain lengths having different double bond position. Biol. Pharm. Bull. 1999; 22: 407–411
- Takahashi K., Suzuki T., Sakano H., Mizuno N. Effect of vehicles on diclofenac permeation across excised rat skin. Biol. Pharm. Bull. 1995; 18: 571–575
- Takeuchi Y., Yamaoka Y., Fukushima S., Miyawaki K., Taguchi K., Yasuka H., Kishimoto S., Suzuki M. Skin penetration enhancing action of cis-unsaturated fatty acids with ω -9 and ω -12 chain lengths. Biol. Pharm. Bull. 1998; 21: 484–491
- Tanojo H., Bouwstra J. A., Junginger H. E., Boddé H. E. In vitro human skin barrier modulation by fatty acids: skin permeation and thermal analysis studies. Pharm. Res. 1997; 14: 42–49
- Träuble H. The movement of molecules across lipid membranes: a molecular theory. J. Membr. Biol. 1971; 4: 193–208
- Wang M. Y., Yang Y. Y., Heng P. W. S. Skin permeation of physostigmine from fatty acids-based formulations: evaluating the choice of solvent. Int. J. Pharm. 2005; 290: 25–36
- Willis J. V., Kendall M. J., Flinn R. M., Thornhill D. P., Welling P. G. The pharmacokinetics of diclofenac sodium following intravenous and oral administration. Eur. J. Clin. Pharmacol. 1979; 16: 405–410
