Abstract
Ketoprofen is a potent non-steroidal anti-inflammatory drug (NSAID) that has been widely used in the treatment of rheumatoid arthritis and other related conditions. However, it carries the risk of undesirable systemic side effects and gastrointestinal irritation at the usual dose of oral administration. The aim of this study was to prepare and evaluate gastroresistant microcapsules containing ketoprofen. Microcapsules were obtained by a spray-drying process starting from an O/A emulsion in the presence of different pH-dependent materials (Eudragit® L100, Eudragit® S100, and stearic acid) dissolved in the external phase. The influence of formulation factors (oily phase employed for drug solubilization, type of coating) on the morphology, particle size distribution, drug loading capacity, in-vitro release, and ex-vivo permeation characteristics were investigated. Drug loading capacity was very high for all the microcapsules prepared. Formulation factors did not significatively influence the mean particle size, but modified microcapsule in-vitro and ex-vivo behavior.
Introduction
Ketoprofen is a non-steroidal anti-inflammatory drug readily absorbed from the gastrointestinal tract and peak plasma concentrations occur about 0.5–2 hr after a dose, but it causes a certain irritation in the gastrointestinal mucous membrane and possesses a bitter taste and aftertaste (Thomas and Kantar Citation1986). Great attention has been devoted to the possibility to prepare gastroresistant microcapsules or microspheres to protect the gastric mucous membrane from ketoprofen irritation or to mask its unpleasant taste (Kawashima et al. Citation1993; Giunchedi et al. Citation1994). Some pharmaceutical microparticulate systems are based on the pH-depentent solubility of Eudragit® L100 and S100, anionic copolymers of methacrylic acid, and methyl methacrylate, soluble, respectively, over pH 6 and 7 (Watts and Illum Citation1997; Chourasia and Jain Citation2003). This study concerns the use of these copolymers and stearic acid to prepare gastroresistant microcapsules containing cores of ketoprofen solubilized in different oily phases. The microencapsulation was here obtained by spray-drying process, a suitable technique to convert liquid materials into easy-to-handle solids and to produce microparticles with improved controlled release characteristics (Luppi, Bigucci, and Cerchiara Citation2006).
Materials and methods
Ketoprofen, mineral oil, olive oil, wheat germ oil, fish oil, castor oil, and mint essence were purchased from A.C.E.F. S.p.A. (Italy), stearic acid was from Fluka (Italy), Eudragit® L100 and S100 were from Röhm Pharma (Germany), while all the solvents and buffer salts employed were from Carlo Erba (Italy). All transport studies were carried out in a Ringer’s solution with the following composition (mM): 115 NaCl; 24 K2HPO4; 4 KH2PO4; 25 NaHCO3; 24 MgCl2·6H2O; 24 CaCl2·2H2O. The buffer solution was oxygenated with carbogen and pH was adjusted to 7.4.
Drug solubility determination in different oily phases
Ketoprofen solubility in different oily phases was tested in order to select the more efficient vehicle for microcapsule core. Mineral oil, olive oil, wheat germ oil, fish oil, castor oil, mint essence, and a mixture of castor oil/mint essence (2:1, w/w) were evaluated as possible exipients for the solubilization of ketoprofen in the microcapsule core. Ketoprofen was added in excess to various excipients (1.0 g). Samples were shaken at 25°C for 24 hr, ultracentrifugated (ALC 4239R, Italy) (10,000 rpm, 10 min, 25°C), and filtered through a 0.45-μm filter. Prior to the solubility study, it was concluded that the 0.45-μm filter did not adsorb/absorb the model drugs. The drug concentrations in the filtered systems were determined using high-performance liquid chromatography (HPLC) after appropriate extraction and dilution.
A combination of liquid–liquid extraction and solid-phase extraction was utilized for sample pretreatment. Filtered samples, equivalent to about 50 mg, were dissolved in 2.5 ml of methanol/dichloromethane mixture (1:4, v/v) and extracted once with 2.5 ml of phosphate buffer pH 8. The organic phase was re-extracted three times with 2.0 ml of methanol/pH 8 phosphate buffer (1:9, v/v) mixture. Finally the aqueous phases were collected from the different extractions and diluted to 10 ml with a methanol/pH 8 phosphate buffer (1:9, v/v) mixture. The samples (3 ml) were passed through an SPE cartridge (SAX Oasis Max, Waters, USA) conditioned with methanol, and the mixture of methanol/pH 8 phosphate buffer (1:9, v/v). The flow rate was adjusted to approximately 10 ml/min. After the cartridges had been allowed to dry for 30 min, the drug was eluted using an overall volume of 10 ml of a methanol/pH 4.5 phosphate buffer (9:1, v/v) mixture. The extract was finally diluted to 10 ml and employed for HPLC analysis. The HPLC system consisted of a model SPD 10A VP Shimadzu Liquid Chromatograph, using an UV detector operating at 256 nm, a Rheodyne injector, and a computerized integration system ChromatoPlus (Shimadzu, Japan). Separations were performed with a C12 reverse-phase column (Synergy MAX RP 80A; Chemtek Analitica, Italy) at room temperature. Acetonitrile and 0.02 M phosphate buffer (pH 3.5) (70:30) mixture was used as the mobile phase, at a flow rate of 1.0 ml/min and an injection volume of 20 μl. The mixture was degassed by sonication during 10 min.
Microcapsules preparation by spray-drying
Microcapsules were prepared from an O/A emulsion containing arabic gum as emulsifier agent. A solution of ketoprofene in a castor oil, mint essence, or in a castor oil/mint essence (2:1; w/w) mixture was added to a water phase containing arabic gum and homogenized with an Ultra-Turrax® T-25 homogenizer (IKA-Labortechnik, Germany) for 5 min at 3000 rpm until complete dispersion of the oily phase. This emulsion was diluted in an ethanolic solution of Eudragit® L100 or Eudragit® S100 and stearic acid in different weight and proportion. Finally all the preparations were spray-dried in a Büchi Mini Spray Dryer (Model 191, Büchi Laboratoriums-Technik, Switzerland) equipped with 0.7 mm diameter nozzle, obtaining gastroresistant microcapsules. The inlet and outlet temperature was maintained at 100 ± 2°C and 49 ± 3°C, respectively. The microcapsules prepared were collected from the collecting chamber and stored in a dessicator. reports the different formulations (A, B, C, D, E, F, G, and H) performed for this study.
Table 1. Composition (w/w percentage) of the different microcapsule formulations containing ketoprofen.
Powder particle size analysis
The size distribution of the spray-dried powders was performed by optical microscopic observation (Nikon Eclipse E400, Japan). Each sample was analyzed in triplicate and the data reported as an average.
Scanning electron microscopy (SEM)
The morphology of microcapsules was performed by SEM analysis. The microparticles were fixed on supports and coated with gold-palladium under an argon atmosphere using a gold sputter module in a high-vacuum evaporator. Samples were then observed with LEO 420 (LEO Electron Microscopy Ltd, England) at 15 kV.
Droplet size analysis after emulsion reconstitution
To determine the droplet size distribution after emulsion reconstitution, 50 mg of the microcapsules were rehydrated with 10 ml of buffer solution (pH 7.6) in a 10 ml vial by hand-shaking (10 sec), then the emulsion was withdrawn for droplet size determination. Each sample was analyzed by Photon Correlation Spectroscopy (Chu Citation1974; Berne and Pecora Citation1976), using an instrument (Brookhaven 90-PLUS, USA) equipped with a He–Ne laser beam at a wavelength of 532 nm (scattering angle of 90°). Samples were used for particle size measurement without filtering and the droplets were not flocculated when observed under the light microscope. Each sample was analyzed in triplicate and the data reported as an average.
Encapsulation efficiency
Encapsulation efficiency was evaluated by ketoprofen extraction followed by purification and HPLC analysis. The amount of ketoprofen in the microcapsules was determined supplementing 50 mg of each sample with 2.5 ml of a methanol/dichloromethane 1:4 (v/v) mixture and subsequently extracting and purifing this solution as described for the determination of drug solubility in the different oily phases.
In-vitro release studies
In vitro release profiles of ketoprofen from gastroresistant microcapsules and standard microcapsules were conducted utilizing simulated gastric fluid (USP XXIV: pH 1.2, pepsin 0.32% w/v) and intestinal fluid (USP XXIV: pH 7.6, pancreatin 1.0% w/v). Gastroresistant microcapsules (B, C, D, and E), or standard microcapsules (A) were introduced into a donor cell containing 20 ml of the aqueous phase separated by a dialysis membrane (cellulose acetate, Mw cut-off = 14,000, Delchimica Scientific Glassware, Italy) from a receiving compartment containing 5 ml of the same aqueous buffer. The studies were performed at 37°C under continuous agitation and the drug was detected in the receiving phase by HPLC analysis.
Ex-vivo permeation studies
Preparation of tissue segments
Male Sprague-Dawley rats (Iffa Credo, France), weighting 200–300 g, were used in this study. The ileum was removed, carefully washed with cold Ringer’s solution, and put in a beaker with Ringer’s solution on ice which was continuously gassed with carbogen. For the experiments, the ileum was then divided into four 3-cm long segments. Each segment was opened along the mesenteric border and the serosa was carefully removed. Care was taken to avoid Peyer’s patches. After preparation, tissues were mounted in the Ussing diffusion chamber with an exposed intestinal area of 1 cm2. The mucosal and serosal reservoirs were immediately filled with 4.500 ml of Ringer’s solution that was continuously oxygenated and maintained at 37°C by a water jacket connected to a recirculating water bath (Polystat, Fischer Bioblock Scientific). Before an experiment, the electrical parameters of the intestinal segments were allowed to stabilize for 30–40 min to allow the tissue to recover from the preparation and to equilibrate in temperature.
Transport studies
For this study, formulations F, G, and H were chosen. At the beginning of the experiments (t0), 500 μl of gastroresistant microcapsule (F, G, and H) aqueous (Ringer) suspension (100 mg/ml) were added to the mucosal bathing solution, while 500 μl of Ringer solution was added to the serosal bathing solution; 100 μl samples were taken from the serosal bathing solution at 30 min intervals for up to 180 min. To maintain a constant volume, 100 μl of Ringer’s solution containing all ingredients of the original bathing solution were added after each sample. The effect of dilution was taken into account for the calculation of fluxes. Permeation profiles were performed under ‘sink’ condition.
Data analysis
Apparent permeability coefficients (Papp) were calculated according to the equation:
Papp (cm/s) = (dQ/dt) × (1/AC0)
where dQ/dt is the steady state appearance rate on the acceptor side of the tissue, A is the exposed area of the tissue, and C0 is the initial concentration of the drug in the donor compartment.
Results and discussion
Drug solubility determination in different oily phases
Among the different excipients considered, mint essence and the mixture of castor oil/mint essence provided the more efficient solubilization of ketoprofen, allowing a higher concentration of drug in the microcapsule core material (). In fact, complete solubilization of ketoprofen in the inner oily phase of O/A emulsion is the primary requirement to obtain microcapsules with high drug loading (ratio of drug-to-excipients) and suitable availability after oral administration (the recommended dose of ketoprofen is 75 mg three times or 50 mg four times a day). Moreover, ketoprofen loading should be maximized to reduce the amount of Eudragit® under the minimum toxic daily dose (2 mg/kg/day).
Table 2. Ketoprofen (w/w percentage) solubilized in the different excipients.
Powder particle size analysis
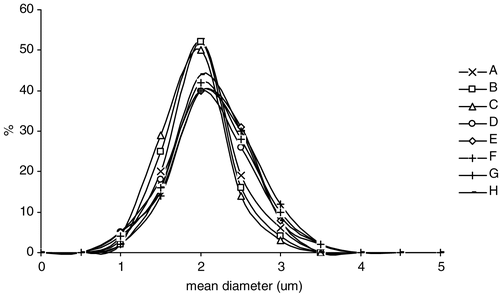
For all the microcapsules prepared, the dimensional analysis showed that the mean diameter was in the range of 1–3 μm (). Only a slight increase in microparticle polydispersity can be observed in the case of formulations D, E, F, G, and H, probably due to the presence of stearic acid in the coating material, producing less homogeneous samples.
Scanning electron microscopy (SEM)
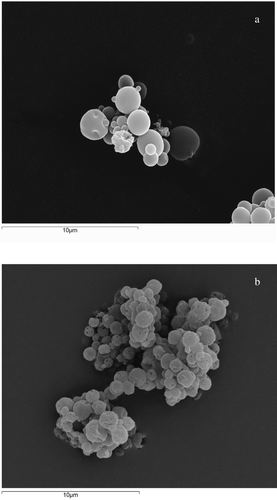
The SEM analysis () showed that microcapsules were well-separated spherical particles having similar diameters (1–3 μm). In the presence of Eudragit® L100 (Figure 2A) and S100 (data not reported) microcapsule surface appeared smooth due to the film coating ability of these kind of polymers. In the presence of stearic acid (Figure 2B) microcapsule outer surface appeared rough and a porous structure can be observed probably due to a loss of volatile flavor components of mint essence (Soottitantawat et al. Citation2005). The limited agglomeration of particles was probably due to the coating ability to diminish the degree of particle agglomeration (Pedersen et al. Citation1998) and to the storage of products in closed vials protected from humidity.
Droplet size analysis after emulsion reconstitution
The core mean diameter analyzed after emulsion reconstitution in alkaline medium (pH 7.6) was in the rage of 843–891 nm (), showing good reconstitution properties for all the microcapsules. Moreover, a mono-dispersed distribution was obtained (very low polydispersity in the range between 0.01 and 0.05) for all the formulations, suggesting that no coalescence during spray-drying occured (Dollo et al. Citation2003).
Table 3. Droplet mean diameter (± SD) after emulsion reconstitution by means of DLS analysis.
Encapsulation efficiency
The loading efficiency expressed as a percentage of the actual loading to theoretical loading was very high for all the microcapsule prepared (A, 92 ± 2; B, 94 ± 3; C, 92 ± 2; D, 96 ± 3, E, 96 ± 3; F, 94 ± 2; G, 105 ± 3; H, 95 ± 2). This data confirmed a good solubilization of ketoprofen in the inner emulsion phase and a good encapsulation during spray-drying. In the presence of stearic acid in the coating material (formulations D, E, F, G, and H), and in particular in the presence of an inner phase composed entirely of mint essence (formulation G), an increase of encapsulation efficiency can be observed, probably due to a loss of volatile components of mint essence favored by a more porous structure of the coating (see ).
In-vitro release profiles
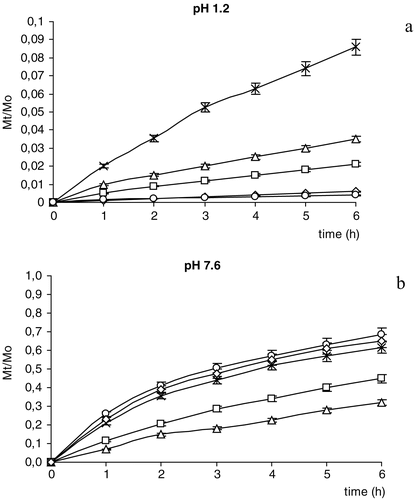
The in vitro release profiles () showed that ketoprofen availability was highly dependent on the buffer pH. Among the different coatings, Eudragit® L100 with stearic acid (formulations D and E) provided the more efficient control of the release, limiting drug availability at pH 1.2 and increasing drug availability at pH 7.6. This may be attributed to the presence of the stearic acid whose lipophilicity limits drug release in acidic medium, but favors drug solubility in alkaline medium by means of micelle formation (Cerchiara et al. Citation2003). This behavior is more pronounced in the presence of higher amounts of stearic acid in the coating materials (formulation E).
Ex-vivo permeation studies
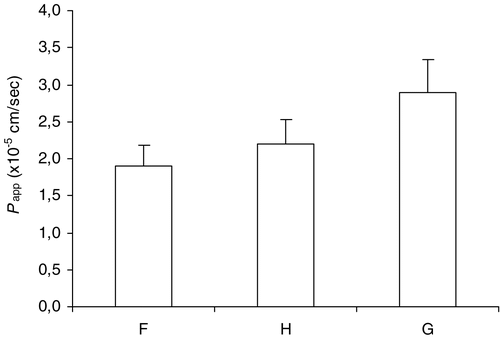
Ex-vivo intestinal permeation studies showed higher apparent permeability coefficients () across the intestinal membrane in the presence of higher amounts of mint essence (formulations H and G). This behavior can be due to an enhancing effect related to the presence of menthol in this essential oil. In fact, although menthol has been known to act as a skin absorption enhancer, it is believed that it can improve gastro-intestinal drug absorption (Flashner-Barak et al. Citation2004).
Conclusion
Microcapsules coated with Eudragit® L100 and stearic acid could serve as potent canditates for site-specific delivery of anti-inflammatory drugs. This formulation allows one to deliver high amounts of ketoprofen in the intestinal region and increases its permeation through the intestinal membrane. At last, the mint essence improves the organolectic properties of the formulation and could increase anti-inflammatory and analgesic effects of the drug.
Acknowledgment
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Berne BJ, Pecora R 1976. Dynamic Light Scattering with Application to Chemistry Biology and Physics. New York, Wiley-Interscience.
- Cerchiara T, Luppi B, Bigucci F, Petrachi M, Orienti I, Zecchi V 2003. Controlled release of vancomycin from freeze dried chitosan salts coated with different fatty acids by spray drying. J. Microencapsul. 20, 473–478.
- Chourasia MK, JainSK 2003. Pharmaceutical approaches to colon targeted drug delivery systems. J. Pharm. Sci. 6, 33–66.
- Chu B 1974. Laser Light Scattering. New York, Academic Press.
- Dollo G, Le Corre P, Guérin A, Chevanne F, Burgot JL,Le Verge R, 2003. Spray-dried redispersible oil-in-water emulsion to improve oral bioavailability of poorly soluble drugs. Eur. J. Pharm. Sci. 19, 273–280.
- Flashner-Barak M, Lerner EI, Rosenberger V, Moldavski N 2004. Menthol solutions of drugs. United States Patent 20040198646.
- Giunchedi P, Conti B, Maggi L, Conte U 1994. Cellulose acetate butyrate and polycaprolactone for ketoprofen spray-dried microsphere preparation. J. Microencapsul. 11, 381–393.
- Kawashima Y, Iwamoto T, Niwa T, Takeuchi H, Hino T 1993. Role of the solvent-diffusion-rate modifier in a new emulsion solvent diffusion method for preparation of ketoprofen microspheres. J. Microencapsul. 10, 329–340.
- Luppi B, Bigucci F, Cerchiara T 2006. Microencapsulation strategies for essential oils - A review. In: Recent Progress in Medicinal Plants, ed. Govil JN, Vol. 12, 307–332. Global Trends in Production, Utilization and Marketing of Medicinal Plants, LLC, Houston, TX, Studium Press.
- Pedersen GP, Fäldt P, Bergenstahl B, KristensenHG 1998. Solid state characterization of a dry emulsion: a potential drug delivery system. Int. J. Pharm. 171, 257–270.
- Soottitantawat A, Takayama K, Okamura K, Muranaka D, Yoshii H, Furuta T, Ohkawara M,Linko P 2005. Microencapsulation of l-menthol by spray drying and its release characteristics. Innovat. Food Sci. Emerg. Tech. 6, 163–170.
- Thomas G, and Kantar MD 1986. Ketoprofen: a review of its pharmacological and clinical properties. Pharmacotherapy 6, 93–103.
- Watts PJ, and Illum L 1997. Colonic drug delivery. Drug Dev. Ind. Pharm. 23, 893–913.