Abstract
Inhalable messenger RNA (mRNA) has demonstrated great potential in therapy and vaccine development to confront various lung diseases. However, few gene vectors could overcome the airway mucus and intracellular barriers for successful pulmonary mRNA delivery. Apart from the low pulmonary gene delivery efficiency, nonnegligible toxicity is another common problem that impedes the clinical application of many non-viral vectors. PEGylated cationic peptide-based mRNA delivery vector is a prospective approach to enhance the pulmonary delivery efficacy and safety of aerosolized mRNA by oral inhalation administration. In this study, different lengths of hydrophilic PEG chains were covalently linked to an amphiphilic, water-soluble pH-responsive peptide, and the peptide/mRNA nano self-assemblies were characterized by dynamic light scattering (DLS) and transmission electron microscopy (TEM). The in vitro mRNA binding and release, cellular uptake, transfection, and cytotoxicity were studied, and finally, a proper PEGylated peptide with enhanced pulmonary mRNA delivery efficiency and improved safety in mice was identified. These results showed that a proper N-terminus PEGylation strategy using 12-monomer linear monodisperse PEG could significantly improve the mRNA transfection efficiency and biocompatibility of the non-PEGylated cationic peptide carrier, while a longer PEG chain modification adversely decreased the cellular uptake and transfection on A549 and HepG2 cells, emphasizing the importance of a proper PEG chain length selection. Moreover, the optimized PEGylated peptide showed a significantly enhanced mRNA pulmonary delivery efficiency and ameliorated safety profiles over the non-PEGylated peptide and LipofectamineTM 2000 in mice. Our results reveal that the PEGylated peptide could be a promising mRNA delivery vector candidate for inhaled mRNA vaccines and therapeutic applications for the prevention and treatment of different respiratory diseases in the future.
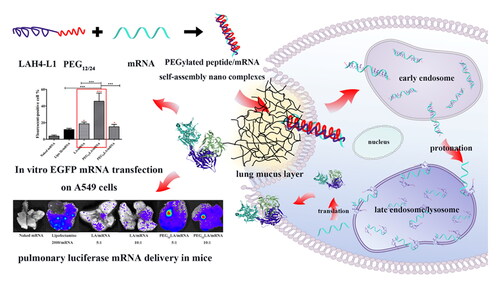
Graphical abstract (TOC)
Schematic illustration of proper PEGylation strategy of pH-responsive LAH4-L1 peptide-mRNA nano self-assemblies enhances the pulmonary delivery efficiency of aerosolized mRNA.
1. Introduction
Nowadays, messenger RNA (mRNA) can be synthesized by in vitro transcription technology, and once it is delivered into the cytoplasm, the exogenous mRNA can be rapidly translated into different therapeutic molecules such as antigens, antibodies or other proteins to achieve different clinical purposes (Berraondo et al., Citation2019; Lin et al., Citation2020; Weng et al., Citation2020; Qin et al., Citation2022). In the past decade, a series of advances have been accomplished in treating lung diseases such as monogenetic disorders such as cystic fibrosis (CF) and surfactant protein B (SP-B) deficiency and multifactorial diseases such as asthma and chronic obstructive pulmonary disease (COPD) using mRNA-based protein supplementation therapies or mRNA-based CRISPR/Cas stem cell therapies (Sahu et al., Citation2019). Apart from this, the sudden outbreak of the COVID-19 pandemic has pushed mRNA vaccines into the first line of novel vaccine development. Unlike conventional vaccines, mRNA vaccines can be rapidly designed once the target gene sequence is defined, prepared in a fraction of time, and do not require a series of complicated procedures such as protein purification, pathogen inactivation, or detoxification treatments (Vetter et al., Citation2018). Moreover, mRNA vaccines are more effective than protein subunit or inactivated vaccines at inducing cellular immune responses and are safer than live-attenuated vaccines to eliminate the safety concerns of virus mutation risk (Lauring et al., Citation2010). In addition, compared to the approved injection vaccines, inhalation vaccines were more effective in preventing airborne transmitted diseases by inducing not only the conventional systemic immune responses but also quick and strong mucosal immunity (Zasheva et al., Citation2020) by producing secretory immunoglobulins such as IgA, IgM, and IgG at respiratory tract mucosa surfaces (Hellfritzsch & Scherließ, Citation2019). For instance, the production of dimeric secretory IgA (sIgA) in the airways is of the utmost importance to respiratory system immune defense (Kato et al., Citation2013), and the mucosal dimeric sIgA has been proven more effective in neutralizing SARS-CoV-2 particles (Wang et al., Citation2021) than the monomeric IgA in blood, demonstrating the broad application prospects of inhaled mRNA vaccines to confront the highly infectious respiratory viruses such as influenza and COVID-19 (Heida et al., Citation2022).
To date, some mRNA therapies such as cancer immunotherapy (Faghfuri et al., Citation2021), CF treatment (Sahu et al., Citation2019), and infectious disease-relevant mRNA vaccines (Maruggi et al., Citation2019) have entered clinical stages. Benefits of mRNA vaccines and therapies include mRNA can be easily synthesized by a cell-free process which enables fast production at a large scale and relatively low cost (Weng et al., Citation2020). Furthermore, compared to foreign DNA, which needs to enter the cell nucleus for transcription but the nuclear envelopes of non-dividing or slow-dividing cells such as antigen-presenting cells and somatic cells are formidable foreign gene entry barriers, mRNA initiates protein translation once it reaches the cytoplasm (Sahin et al., Citation2014) and thereby the transfection efficiency of mRNA is usually higher than plasmid DNA and without the potential concerns of chromosomal gene insertion risk (Gomez-Aguado et al., Citation2020).
Nevertheless, the long single-stranded mRNA is much more unstable than the double-stranded plasmid DNA or siRNA. This highly water soluble and negatively charged biomacromolecule is also unable to pass through the lipophilic plasma membrane unfacilitated. Therefore, developing stable and cost-effective mRNA delivery vectors to prevent it from premature degradation, overcome the airway drug delivery barriers, and promote its cellular uptake and endosomal escape in a simple formulation plays an indispensable role in promoting the clinical applications of inhaled mRNA therapeutics and vaccines. Protamine and cell-penetrating peptides (CPPs) are two kinds of peptide-based mRNA vectors that are already in clinical phases for cancer immunotherapy and mRNA vaccine development (Kubler et al., Citation2015; Armbruster et al., Citation2019; Sebastian et al., Citation2019; van den Brand et al., Citation2019). However, none of them is given by inhalation route. In 2019, Qiu et al. first reported the feasibility of using a PEGylated synthetic KL4 peptide to achieve effective mRNA pulmonary delivery in dry powder formulations (Qiu et al., Citation2019), which could be stored in a desiccator at ambient temperature rather than the harsh mRNA preservation condition such as −60 °C or −80 °C ultralow temperature (Crommelin et al., Citation2021; Uddin & Roni, Citation2021), thereby increasing the clinical applicability of peptide-based mRNA delivery vector for inhalable mRNA therapy and vaccine development.
Previous studies have reported a water-soluble, lysine and histidine-rich cationic peptide LAH4-L1 (abbreviated as LA) is an efficient DNA and siRNA delivery vector (Lam et al., Citation2012) that could be formulated into inhalable dry powders (Liang et al., Citation2014, Citation2015). This amphiphilic, pH-responsive LA peptide could mediate efficient plasmid DNA transfection on a range of airway and alveolar epithelial cell lines and antigen-presenting cells (APCs) without noticeable toxicity (Liang et al., Citation2014, Citation2015; Xu et al., Citation2016). In this study, its potential for pulmonary mRNA delivery was first investigated. Although the self-assembly process of this peptide with mRNA is quick and efficient in vitro, the in vivo gene delivery efficiency of the LA peptide is unsatisfactory after injection or inhalation administration in mice. Given the successful application of PEGylation strategies for other transgene vectors, such as lipid nanoparticles and quantum dots for pulmonary drug delivery (Li et al., Citation2020; Lokugamage et al., Citation2021), the strategy of conjugating different chain lengths of hydrophilic polyethylene glycol (PEG) to the N-terminal of LA peptide was employed in this study. As polydisperse PEG polymers such as PEG2000 are subjected to batch-to-batch variation, monodisperse PEG with precise molecular weight and better homogeneity (Qiu et al., Citation2019, Citation2021) is adopted in this study. Our results first revealed the peptide vector modified with different lengths of monodisperse linear PEG chain led to significantly different mRNA transfection results, shedding light on the possibility of developing more safe and efficient self-assembly polypeptides, peptide-linked polymeric or other particulate nano delivery systems to speed up the translation of inhalable mRNA therapies and vaccines into the clinic.
2. Materials and methods
2.1. Chemicals and reagents
All peptides in this study () were purchased from ChinaPeptides (Shanghai, China). CleanCap® firefly luciferase mRNA, EGFP mRNA, and cyanine-5 EGFP mRNA were purchased from TriLink Bio Technologies (San Diego, CA, USA). LipofectamineTM 2000 transfection reagent was purchased from Invitrogen (Carlsbad, USA). Luciferin potassium salt was purchased from Promega (Madison, WI, USA). Dulbecco’s modified Eagle’s medium (DMEM), 0.25% Trypsin-EDTA, penicillin/streptomycin antibiotics, and PBS (pH 7.2–7.4) were purchased from Genview (Florida, USA). Fetal Bovine Serum (FBS, South America origin) and Opti-MEM I reduced serum medium were purchased from Gibco (New York, USA). DNA Gel Loading Dye (6 ×) was purchased from Biosharp (Anhui, China). GelRed nucleic acid stain was purchased from US Everbright (California, USA). 1 × Tris-acetate EDTA (TAE) buffer was diluted from 50 × TAE buffer (Sangon Biotech, Shanghai, China) using distilled water. A549 and HepG2 cells were purchased from China Center for Type Culture Collection (CCTCC, Shanghai, China). Other reagents were obtained from Sigma-Aldrich (Saint Louis, MO, USA) of analytical grade or better grade.
Table 1. Purity, sequence, and molecular weight (MW) of the LAH4-L1 and PEGylated peptides.
2.2. Synthesis of peptides
Polypeptides were synthesized by Fmoc solid phase synthesis method by Rink amide-MBHA Resin. Some potential active sites of the LA polypeptide chain were chemically protected before PEGylation, including the histidine, protected by the Trt group, and the Boc group, protected by the lysine. The leucine and alanine side chains were not chemically protected since no reactive functional groups existed. Afterward, the N-terminal amino group of the LA peptide was connected with a monodisperse PEG of different polymerization degrees by dehydration condensation. Specifically, 1.5 eq Boc-NH2-PEG12-CH2CH2COOH was dissolved in DMF, then 3 eq HBTU and 10 eq DIEA were added and reacted for 60 min at room temperature. PEG12/PEG24 modified LAH4-L1 polypeptide was obtained by lysing TFA/TIS/H2O/EDT (92.5:2.5:2.5:2.5% v/v) for 2 h at room temperature and precipitated by ether. The products were purified by HPLC and lyophilized. The purity of PEG-modified polypeptides was verified by HPLC (purity > 90%), and their molecular weight was confirmed by ESI-MS.
2.3. Experimental methods
The particle size and morphology study of peptide/mRNA nano self-assemblies were tested by dynamic light scattering (DLS) and transmission electron microscope (TEM). The vector-mRNA binding and the mRNA release were measured by gel retardation assay, and the cytotoxicity assay of the non-PEGylated or PEGylated peptide vectors and their mRNA nano self-assemblies were examined on A549 and HepG2 cell lines by MTT assay. Next, the mRNA cellular uptake and mRNA transfection in vitro was measured by flow cytometry. Afterward, in vivo luciferase mRNA transfection was carried out by intratracheal delivery of different vector/mRNA aerosols to mice. In vivo safety evaluation of peptide/mRNA nano self-assemblies was tested by mice’s weight change and lung histological manifestations. BALB/c mice of both sexes with an average age of 8 to 12 weeks and body weight between 18 to 22 g were used. The mice were housed under a 12 h dark-light cycle at a constant temperature and with ad libitum feeding on tap water and standard chow. The experiment was carried out after one week of adaptation. All mice were obtained from Shanghai SLAC Laboratory Animal Co., Ltd, and the experimental design, treatment, and disposal process followed the relevant animal ethical standards and approved experimental animal procedures of the Animal Ethics Committee of Fujian Medical University, PR China (the approved animal ethical number is IACUC FJMU 2022-Y-0683). A full description of experimental methods is provided in Supplementary Information (DOC).
2.4. Statistical analysis
Data were presented as mean ± SEM. GraphPad Prism software version 5.0 (GraphPad Software Inc., San Diego, CA, USA) was used for statistical analyses. Tukey’s post-hoc multiple comparison tests were carried out for one-way analysis of variance (ANOVA) analysis (P-value: *P < 0.05, **P < 0.01, ***P < 0.001). Differences were considered as statistically significant at P < 0.05.
3. Results
3.1. Particle size and morphology study of peptide/mRNA nano self-assemblies
The self-assembly process of peptide and mRNA is quick in less than 20 min, and the results of hydrodynamic diameters and polydispersity indexes (PDIs) showed both non-PEGylated, and PEGylated LA peptides could form stable nano self-assemblies with mRNA (). The hydrodynamic diameters of different peptide/mRNA self-assemblies were increased when the peptide and mRNA binding ratio was increased from 5:1, 10:1 to 15:1 mass ratios (w/w), leading to the incremental mean hydrodynamic diameters from around 96 nm to 152 nm for LA/mRNA self-assemblies, from around 146 nm to 180 nm for PEG12LA/mRNA self-assemblies and from around 158 nm to 229 nm for PEG24LA/mRNA self-assemblies. The PDIs of all peptide/mRNA self-assemblies were similar (ranging from 0.20 to 0.28), and the relatively uniform size distribution of these nano self-assemblies could decrease the risk of forming large aggregates that might impede cellular uptake. Among these peptide/mRNA nano self-assemblies, the hydrodynamic diameters of PEGylated LA/mRNA self-assemblies were larger than the non-PEGylated LA/mRNA self-assemblies, implying the PEGylated peptides tend to form looser nanoparticles which might promote the intracellular release of mRNA (Qiu et al., Citation2019, Citation2021). The morphologies of different peptide/mRNA self-assemblies were visualized by transmission electron microscopy (). In short, all peptides could form similar compact, spherical or oval-shaped nanoparticles with mRNA of less than 200 nm, and the sizes agreed with their hydrodynamic diameters.
Figure 1. The physicochemical characterization of different peptide/mRNA nano self-assemblies. (A) Hydrodynamic diameters of different peptides/luciferase mRNA nano self-assemblies prepared at a 5:1 to 15:1 ratio (w/w) were measured by dynamic light scattering (DLS). (B) The polydispersity indexes (PDIs) of peptides/EGFP mRNA self-assemblies prepared at a 5:1 to 15:1 ratio (w/w) measured by DLS. Data are plotted as mean ± SEM (n = 3). (C–E) Transmission electron microscopy (TEM) images of LA/mRNA, PEG12LA/mRNA, and PEG24LA/mRNA nano self-assemblies. Scale bar = 200 nm.

3.2. mRNA binding and release study
The agarose gel electrophoresis assay was carried out to evaluate the binding and release difference between the non-PEGylated and PEGylated peptide vectors to mRNA. The mRNA band intensity decreased as the peptide-to-mRNA binding ratio (w/w) increased, and the disappearance of the mRNA band in the gel indicated a complete binding between mRNA and peptides. As shown in , complete bindings were observed from a 5:1 mass ratio onwards for both non-PEGylated and PEGylated peptide/mRNA self-assemblies because the mRNA band was no longer visible at these ratios. Based on this, 5:1 and higher peptide/mRNA binding ratio (w/w) were chosen for subsequent cellular uptake and transfection research. Although a previous study demonstrated that the steric hindrance of the hydrophilic PEG chain slightly impeded the binding of another PEGylated KL4 peptide vector with mRNA (Qiu et al., Citation2019), our result found the proper N-terminal PEGylation of LA peptide would not noticeably interfere its binding with mRNA. For the mRNA release study, different polyanionic surfactant sodium dodecyl sulfonate (SDS) concentrations were added to dissociate the peptide/mRNA self-assemblies by competing for binding mRNA with the cationic peptides (). The reappearance of the mRNA band in the gel indicated the dissociation of mRNA from the peptide/mRNA self-assemblies, which was used to mimic the mRNA release differences in the presence of cytoplasm polyanions (Qiu et al., Citation2019). Although the mRNA affinity of PEG12LA and PEG24LA peptides were comparable to the non-PEGylated LA peptide, both the PEG12LA/mRNA and PEG24LA/mRNA nano self-assemblies were completely dissociated in the presence of 8 mM SDS, whereas the dissociation of the non-PEGylated LA/mRNA self-assemblies required a higher concentration of at least 10 mM SDS, suggesting the PEGylated peptide/mRNA self-assemblies are more easily dissociated than the non-PEGylated counterparts, which could facilitate the target mRNA release in the cytoplasm and contribute to the enhanced mRNA transfection of PEG12LA peptide.
Figure 2. Agarose gel electrophoresis assay to evaluate the ability of EGFP and luciferase mRNA binding (A–C) and release (D) of the non-PEGylated and PEGylated peptide vectors. For mRNA binding, peptides/mRNA self-assemblies were prepared from a 0.5:1 to 15:1 ratio (w/w). For mRNA release, peptides/luciferase mRNA self-assemblies were prepared at a 5:1 ratio (w/w) and different concentrations of sodium dodecyl sulfate (SDS) solutions were added to dissociate luciferase mRNA from the self-assemblies. Unbound mRNA and peptide/mRNA self-assemblies without SDS treatment were used as controls. Electrophoresis was carried out at 140 V for 40 min, and the gel was visualized under UV illumination.

3.3. In vitro cell viability assay
The cytotoxicity assay of the non-PEGylated or PEGylated peptide vectors and their mRNA nano self-assemblies were examined on A549 and HepG2 cell lines by MTT assay. A549 is a human alveolar epithelial adenocarcinoma cell line commonly used to evaluate mRNA delivery agents’ performance through intranasal or pulmonary administration (Qiu et al., Citation2021). HepG2 is a representative human hepatocellular carcinomas cell line that may influence mRNA metabolism (Zheng et al., Citation2021). As shown in , the cytotoxicity of PEGylated peptides (PEG12LA and PEG24LA) were significantly lower than the non-PEGylated LA peptide at high concentrations (50 and 25 μg/mL), indicating PEGylation could decrease the vector’s cytotoxicity in vitro. The better biocompatibility of these PEGylated peptide vectors might be due to the PEG chain’s partially charge shielding effect of the high positive charges of LA peptide, as high positive charge vectors have been reported to produce strong interaction with the negatively charged cell membranes and endosomal membranes to cause cells death (Muqier et al., Citation2022). Similarly, the cytotoxicity of PEGylated peptides/mRNA nano self-assemblies was significantly lowered at all test w/w ratios (). Therefore, a proper peptide N-terminus PEGylation strategy could efficiently reduce the toxicity of the transgene peptide vector without decreasing its mRNA binding ability.
Figure 3. MTT cytotoxicity assay of peptides and peptides/mRNA self-assemblies on A549 cells (A,C) and HepG2 cells (B,D). (A) MTT results of different concentrations of peptides on A549 cells. (B) MTT results of different concentrations of peptides on HepG2 cells. (C) MTT results of peptides/mRNA self-assemblies prepared at a 5:1 to 15:1 ratio (w/w) on A549 cells. (D) MTT results of peptides/mRNA self-assemblies prepared at a 5:1 to 15:1 ratio (w/w) on HepG2 cells. The cell viability (%) was calculated by comparing the absorbance of cells treated with different peptides or peptides/mRNA self-assemblies to the untreated cells. The data were analyzed by one-way ANOVA followed by tukey’s post-hoc multiple comparison tests (*P < 0.05, **P < 0.01, ***P < 0.001). data are plotted as mean ± SEM (n = 3).

3.4. In vitro mRNA cellular uptake study
The cellular uptake of fluorescent cyanine-5 (Cy5) labeled mRNA was measured by flow cytometry on A549 human lung epithelial cells and HepG2 human hepatoma cells (). The trends were similar on both cell lines. At 5:1 peptide/mRNA binding ratio (w/w), over 95% of treated cells showed efficient cellular uptake of mRNA mediated by LA and PEG12LA peptide, while there was only around 70% or 50–60% of treated cells showed efficient mRNA uptake mediated by PEG24LA or LipofectamineTM 2000, respectively. The w/w ratio of 2:1 was the optimal nontoxic and best transfection ratio for LipofectamineTM 2000 and the tested mRNA. Although the gel electrophoresis assay demonstrated PEGylation did not impede the binding of peptide and mRNA, it is interesting to find the cellular uptake efficiency of different PEGylated peptides varied largely. Specifically, the relatively short PEG12 chain modification barely influences the alveolar and hepatic cellular uptake of peptide/mRNA nano self-assemblies, while a longer PEG chain modification significantly reduces the cellular uptake on both cell lines, emphasizing the importance of proper PEG chain length optimization. The reason is speculated that the overlong PEGylation could lead to a strong inhibition of the vector’s adhesion to the cell membranes by surface charge shielding, together with the increased particle sizes that may decrease the cellular internalization ability (Pozzi et al., Citation2014; Mehta et al., Citation2018).
Figure 4. Cellular uptake study of Cy5 fluorescently labeled mRNA by flow cytometry. The percentage of Cy5 fluorescent positive cells (A,B), the representative histograms (C,D) that displayed Cy5 positive cells (red) compared to untreated cells (blue), and the mean fluorescence intensity of A549 and HepG2 cells treated with different peptide/Cy5-mRNA self-assemblies at 5:1 w/w ratio (E,F) were shown. Data are plotted as mean ± SEM (n = 3). The data were analyzed by one-way ANOVA followed by tukey’s post-hoc multiple comparison test. Black *P < 0.05, **P < 0.01 and ***P < 0.001 comparisons among different peptides/mRNA self-assemblies at the same ratio (w/w); blue *P < 0.05 and ***P < 0.001 significant difference between the peptide/mRNA self-assemblies and LipofectamineTM 2000/mRNA lipoplexes; red *P < 0.05 and ***P < 0.001 vs. the naked mRNA group.

3.5. In vitro mRNA transfection study
The potential of non-PEGylated and PEGylated peptides to mediate efficient green fluorescent protein (EGFP) mRNA transfection on A549 and HepG2 cells was evaluated by flow cytometry. The highest transfection efficiencies were achieved at 5:1 peptide/mRNA binding ratio (w/w), and the transfection efficiencies were decreased when the peptide to mRNA binding ratios (w/w) was increased from 5:1 to 10:1 and 15:1 for all peptide vectors (), as the excessive cationic peptides might inhibit the intracellular mRNA release and increase the cytotoxicity. Notably, both the mean fluorescence intensity of all transfected cells () and the EGFP fluorescent-positive cell percentage (i.e., the successfully transfected cell percentage, ) were significantly higher when the transfection was mediated by PEG12LA peptide comparing to the PEG24LA peptide, non-PEGylated LA peptide or LipofectamineTM 2000, because the factors influencing the final transfection efficiency are related to both cellular uptake and the intracellular disposing process (Mehta et al., Citation2018). Once inside the acidic endosomal environment, the LA peptide’s histidines become protonated and induce the peptide to undergo a conformational change from an α-helix to a disordered conformation. The allosteric peptide could thus bind, insert into and destabilize the neutral and anionic endosomal membrane by hydrophobic and electrostatic interaction to promote the escape of the dissociated nucleic acids (Iacobucci et al., Citation2012; Lam et al., Citation2012). However, the protonation of peptide histidine under the endosomal environment will produce a more positive charge to prevent the follow-up release of negatively charged mRNA from the peptide. On the contrary, the gel electrophoresis assay has proved the proper PEG12 chain modification would not noticeably hinder the binding of LA peptide and mRNA but facilitate the release and the follow-up endosomal escape of dissociated mRNA, accounting for the enhanced transfection ability of PEG12LA peptide vector.
Figure 5. EGFP mRNA transfection study using flow cytometry. The transfection efficiencies of naked mRNA or different vector/mRNA self-assemblies prepared at different mass ratios (from 5:1 to 15:1) on A549 cells (A) and HepG2 cells (B). (C and D) Representative histograms show the percentage of cells that displayed positive EGFP fluorescence signals (red) compared to untreated cells (blue) using 2 μg EGFP mRNA at a 5:1 w/w ratio of peptide/mRNA self-assemblies or 2:1 w/w ratio of LipofectamineTM 2000/mRNA lipoplexes on A549 cells (C) and HepG2 cells (D). (E) and (F) the EGFP fluorescent-positive cell percentage of different vector/mRNA self-assemblies compared to naked mRNA on A549 (E) and HepG2 cells (F). Data are plotted as mean ± SEM (n = 3). The data were analyzed by one-way ANOVA followed by tukey’s post-hoc multiple comparison test. Black **P < 0.01 and ***P < 0.001 comparisons among different peptides/mRNA self-assemblies at the same ratio (w/w); blue *P < 0.05 and ***P < 0.001 comparisons between LipofectamineTM 2000/mRNA lipoplexes and peptide/mRNA self-assemblies; red *P < 0.05 and ***P < 0.001 comparisons between different vector/mRNA self-assemblies and the naked mRNA group.

Different from the PEG12LA peptide, the transfection efficiency of a longer PEG chain modified PEG24LA peptide/mRNA nano self-assemblies was slightly lower than that of non-PEGylated LA/mRNA nano self-assemblies since the inhibition of cellular uptake has overweighed the benefits of sufficient intracellular mRNA dissociation for the PEG24LA peptide mediated mRNA delivery. In summary, these results indicated that an appropriate modification site, such as the peptide’s N-terminal and a proper PEG chain length optimization, is important to realize the enhanced mRNA transfection for pH-responsive peptide-based mRNA delivery vectors.
3.6. In vivo mRNA delivery to lung tissues in mice
Due to the anatomical differences in the respiratory tract structures between humans and mice, intratracheal administration of an mRNA formulation in aerosol from a specific drug delivery device (YAN-30012 micro-sprayer aerosolizer, Yuyan Instruments Co. Ltd., Shanghai, China) was used in this study to ensure a sufficient dose of the drug could be deposited in trachea and lungs. The mean aerosol diameter of YAN-30012 aerosolized droplets is around 18 μm, with 90% aerosol droplet diameters below 30 μm, and all aerosol diameters are below 50 μm with the smallest sprayed droplet diameter of 5 μm (data provided by Yuyan Instrument Co. Ltd, https://www.yuyanbio.com/). Although several publications have provided evidences about the highly efficient pulmonary drug deposition effect achieved by this instrument (Wang et al., Citation2020; Wu et al., Citation2020; Gu et al., Citation2021; Su et al., Citation2021; Tian et al., Citation2021; Wu et al., Citation2021; Li et al., Citation2022; Peng et al., Citation2022a, Citation2022b), we reconfirmed the aerosol’s deposition performance using fluorescent in vivo imaging technology after intratracheal administration of Cy5.5 fluorescent dye to mice. The fluorescent in vivo live imaging results revealed the Cy5.5 fluorescent signal is exclusively distributed in mouse trachea, whole lungs and the oral-nasal regions after 5 min of intratracheal administration (Figure S1). Afterwards, the in vivo mRNA transfection efficiency of the aerosolized PEG12LA/mRNA and the non-PEGylated LA/mRNA self-assemblies were evaluated at 5:1 and 10:1 w/w ratios following non-invasively intratracheal delivery of 10 μg luciferase mRNA in mice. After 24 h, the luciferase protein expression in the lungs of mice treated with naked mRNA () and PEG24LA/mRNA complexes (Figure S2 and S3) was invisible, while the bioluminescence was detectable in lung tissues of mice treated with mRNA delivered by LipofectamineTM 2000, LA peptide and PEG12LA peptides (), confirming that the liquid aerosol formulations could reach the deep lung area and highlighting the indispensable role of a transgene vector for efficient pulmonary mRNA delivery.
Figure 6. Pulmonary mRNA transfection and safety studies in mice. (A) Representative white light and bioluminescent images of mouse lungs after pulmonary delivery of 10 μg luciferase mRNA using different transgene vectors. BALB/c mice were intratracheally administered with naked mRNA, LipofectamineTM 2000/mRNA lipoplexes at 2:1 ratio (w/w), LA/mRNA or PEG12LA/mRNA self-assemblies at 5:1 and 10:1 w/w ratios, respectively. At 24 h post-administration, the mice were euthanized, and the lungs were isolated for bioluminescent imaging. (B) The average radiant efficiency (i.e., luminescent value) was calculated from the lung IVIS bioimaging of mice with a statistical analysis of different treatment groups. Data are plotted as mean ± SEM (n = 5) and analyzed by one-way ANOVA followed by tukey’s post-hoc multiple comparison tests (*P < 0.05, **P < 0.01, ***P < 0.001). (C and D) The weight change of mice at 24 h after intratracheal delivery of 5 μg luciferase mRNA (C) or 10 μg luciferase mRNA (D) to mice with or without (naked) a gene delivery vector. Data are plotted as mean ± SEM (n = 4) and analyzed by one-way ANOVA followed by tukey’s post-hoc multiple comparison tests (*P < 0.05, **P < 0.01, ***P < 0.001). (E) The entire lung tissues of BALB/c mice following pulmonary delivery of 75 μL PBS, naked mRNA or different peptide/mRNA liquid aerosol formulations containing 10 μg luciferase mRNA for 24 h (n = 3 per group) were histologically examined and the representative images of mouse lung histological study were shown. H&E staining slides were observed under a microscope at 200× magnification (scale bar = 100 μm).
Compared to the non-PEGylated peptide/mRNA self-assemblies, PEG12LA mediated in vivo mRNA transfection is significantly better than that of LA, PEG24LA and LipofectamineTM 2000 (), the reason may be ascribed to the charge and steric shielding of the proper length of hydrophilic PEG chain modification, which improves the nanoparticles colloidal stability, thereby reducing the particle aggregation and entrapment by negatively charged airway mucus (Huckaby & Lai, Citation2018). Moreover, the recognition and clearance of alveolar macrophages (Osman et al., Citation2018; Moreno-Fierros et al., Citation2020) and the accessibility of antibodies and degradative enzymes (Qiu et al., Citation2019) were also reduced by the PEGylation of transgene vectors, which prolongs the lung retention time of the delivered mRNA and thus enhances its bioavailability. However, the pulmonary mRNA transfection efficiencies of PEG12LApeptide/mRNA self-assemblies were not further enhanced when increasing the peptide-to-mRNA binding ratio (w/w) from 5:1 to 10:1, which is consistent with the in vitro mRNA transfection result since more peptide will result in an excessive positive charge that might increase the possibility of being captured by the airway mucus mesh and the early recognition and clearance by the alveolar macrophages. Although PEG12LA peptide mediated the highest luciferase mRNA transfection among all tested treatments, the in vivo transfection efficiency of LipofectamineTM 2000 is significantly better than LA and PEG24LA peptides and there is not a significant difference between the in vivo mRNA transfection efficiency of LA and PEG24LA peptide (), emphasizing the importance of a proper PEG chain selection.
Apart from the significantly higher lung luciferase mRNA expression midiated by PEG12LA over LipofectamineTM 2000, it is also interesting to find only mice treated with PEG12LA/mRNA self-assemblies demonstrated enhanced bioluminescence in the tracheal and bronchial region. Because tracheal and bronchial epithelial cells are one of the main targets to treat respiratory diseases and defense against the invasion of respiratory pathogens, the improved mRNA transfection efficiency in this area is hopeful to enhance therapeutic effects for diseases such as asthma and chronic bronchitis and improve the immune protection effects of inhaled mRNA vaccines against airborne diseases as well (Moreno-Fierros et al., Citation2020). Last but not least, following intratracheal delivery of peptide/mRNA self-assemblies in mice, the bioluminescent signals were only localized in the lung but not heart, liver, spleen, and kidney at 24 h post-administration (data not shown), which suggested the inhalation administration could indeed improve the therapeutic effect of delivered mRNA in lung tissues and reduce the off-target side effects to other major organs.
3.7. In vivo safety evaluation after pulmonary mRNA delivery in mice
The weight change of mice after pulmonary delivery of different peptide/mRNA nano self-assemblies was shown in (n = 4). At 24 h post-administration, the pulmonary delivery of aerosolized LipofectamineTM 2000/mRNA lipoplexes (2:1 w/w ratio) and LA/mRNA self-assemblies (5:1 and 10:1 w/w ratios) containing 5 μg mRNA all resulted in a significant decrease of mouse average body weight by more than 5%, and the weight loss of mice became more pronounced at a higher dose of 10 μg mRNA per mice, causing 10.41%, 6.28% and 8.56% of mouse average weight loss when treated with LipofectamineTM 2000/mRNA lipoplexes (2:1 w/w ratio) or LA/mRNA self-assemblies (5:1 or 10:1 w/w ratio), respectively. However, there was no significant body weight loss for the PEG12LA/mRNA self-assemblies treated mice receiving either 5 μg or 10 μg luciferase mRNA (at both 5:1 and 10:1 w/w ratios) when comparing to the PBS-treated mice, suggesting the in vivo biosafety of the PEGylated peptide vector was significantly improved after PEGylation of the peptide.
The lung histopathological study of mice after intratracheal delivery of different aerosol formulations containing 10 μg luciferase mRNA was also investigated (). At 24 h post-transfection, the H&E staining results showed the lungs treated with PBS, naked mRNA, or PEG12LA/mRNA nano self-assemblies (at both 5:1 and 10:1 w/w ratios) all presented healthy histological manifestations, including intact alveolar and bronchiolar structure, regular air space distribution without edema or inflammatory cell infiltration in the alveolar, interstitial or bronchiolar spaces. However, the lungs treated with LA/mRNA self-assemblies at a 5:1 w/w ratio showed signs of slight lung inflammation, such as mild edema and a small number of inflammatory cell infiltration. What is more, signs of severer inflammation were observed in mouse lungs treated with LipofectamineTM 2000/mRNA lipoplexes at 2:1 w/w ratio and LA/mRNA self-assemblies at 10:1 w/w ratio, including severe interstitial pulmonary edema, inflammatory exudates in the alveolar cavity and a large number of inflammatory cell infiltration and hemorrhage, indicating the nonnegligible toxicity of these vector at an effective dose to mediated detectable luciferase mRNA expression after intratracheal administration. The histopathological analysis showed the proposed PEGylated strategy could eradicate the potential inflammatory risk in the lungs and improve the inhalation safety of cationic peptide vectors.
4. Discussion
In this study, a combinational mRNA chemically modification strategy including proper 5′-capping modification producing naturally occurring Cap1 structure, 120 A poly(A) tail elongation, optimization of the 5′ and 3′ untranslated region (UTR), and the 5-methoxy uridine modification that has been successfully used in the Pfizer/BioNTech’s approved injectable COVID-19 vaccine BNT162b2139 (Elkhalifa et al., Citation2022) was adopted to improve the stability and transgene expression of mRNA and decrease its nonspecific inherent immunogenicity (Kormann et al., Citation2011; Wadhwa et al., Citation2020; Leppek et al., Citation2021). Although the lung H&E staining results proved the designed naked mRNA could evade the innate immune responses in mice, the nearly invisible luciferase protein expression in lungs after the intratracheal administration of naked mRNA emphasizes the indispensable role of a transgene vector to achieve efficient pulmonary mRNA delivery.
Commonly used mRNA delivery vectors include viral and non-viral vectors (Ibba et al., Citation2021). Despite the advantages of viral vectors in high delivery efficacy, their clinical applications are limited due to the immunogenicity problem, restricted tissue tropism, restricted nuclear acid size, high production costs, and biosafety constraints (Bulcha et al., Citation2021). Non-viral vectors offer several advantages, such as lower immunogenicity, ease of large-scale production at lower cost, a broad range of plasmid sizes, and better safety profiles. Non-viral vectors successfully entering clinical phases include polymers, lipid nanoparticles, exosomes, and peptides (Ibba et al., Citation2021). Although the transfection efficiencies of some cationic polymers are high, there are some safety concerns about their inherent cytotoxicity problem, as some synthetic polymers are not easily or completely biodegradable (Kargaard et al., Citation2019). The most widely studied mRNA vaccine delivery vectors are lipid nanoparticles (LNPs), including liposomes, solid lipid nanoparticles, and nanostructured lipid carriers (Tenchov et al., Citation2021; Weng & Huang, Citation2021). Two authorized COVID-19 vaccines, Moderna mRNA-1273 and Pfizer/BioNTech BNT162b21, use LNPs to deliver antigen mRNA (Anderson et al., Citation2020; Polack et al., Citation2020). However, some adverse effects such as morbilliform rash, pain, swelling, fever, sleepiness, Bell’s palsy even death have been reported, which may be related to the immunogenicity of LNP materials (Klimek et al., Citation2021; Moghimi, Citation2021; Ndeupen et al., Citation2021). A recent study provided evidence about the highly inflammatory nature of some vaccine-relevant LNP components (Ndeupen et al., Citation2021), as the intranasal administration of 10 μg LNPs triggered rapid and robust inflammatory responses in lungs and resulted in a high mortality rate of mice. Our results also showed the nonnegligible toxicity of a commonly used LNP-mRNA formulation, as more than 10% weight loss and signs of apparent lung inflammation were observed after intratracheal delivery of LipofectamineTM 2000/mRNA lipoplexes at an effective dose to induce luciferase mRNA expression in mice. Furthermore, most mRNA-LNP formulations have complicated compositions, strict storage temperature requirements, and limited shelf-life since some lipids are liable to hydrolysis and oxidation (Schoenmaker et al., Citation2021). In contrast, peptide-based gene delivery vectors have cheap and simple synthesis advantages, stable for long-term storage in lyophilized powders, and safe degradation products of amino acids. However, previously very limited peptide vectors have been reported to achieve efficient lung mRNA transfection.
Here, the feasibility of safe and efficient pulmonary mRNA delivery by water-soluble pH-responsive peptide-mRNA nano self-assemblies was investigated for the first time. As far as we know, it is also the first study to investigate the influence and mechanism of different PEG chain-modified cationic polypeptide vectors for mRNA delivery. Different from a number of previous studies showing that the in vitro cellular uptake and transfection efficiency of most PEGylated transgene vectors are obviously decreased (Fant et al., Citation2010; Gjetting et al., Citation2010; Wang et al., Citation2014; Veiman et al., Citation2015; Khalil & Harashima, Citation2018), our research found the in vitro PEGylated peptide mediated mRNA delivery efficiency enhancement could be accomplished in a simpler way by merely optimizing the PEGylation site and the length of PEG chain, thereby avoiding some more complicated strategies such as mixing the non-PEGylated vectors with their PEGylated counterparts at a specific proportion (Gjetting et al., Citation2010; Wang et al., Citation2014; Veiman et al., Citation2015; Osman et al., Citation2018; Kunnapuu et al., Citation2019), optimizing the PEG grafting ratio to the vectors (Ke et al., Citation2020) or conducting the PEG post-modification after the vector’s complexation with nucleic acids (Rao et al., Citation2008) to compensate the transfection inhibition by PEGylation of the transgene vector. In this study, different chain lengths of monodisperse PEG chain were covalently linked to the N-terminal of LA peptide at a 1:1 molar ratio, resulting in minimal interference to the peptide-mRNA self-assembly process. Besides, it was also found that the significant transfection efficiency improvement was only realized by the 12-monomer PEG-modified peptide rather than the 24-monomer PEG-modified peptide, which shows the balancing of mRNA’s binding with peptide and its intracellular release performance is important. The proposed PEGylation strategy shows great potential for developing more peptide-based mRNA delivery systems such as cationic peptides, fusogenic peptides or cell-penetrating peptides, peptide-linked polymeric nanoparticles or lipid nanoparticles to realize the broad applications of inhalant mRNA vaccines and pulmonary therapeutic mRNA in the near future.
5. Conclusions
In summary, this work describes an innovative approach that combines a novel pH-responsive cationic peptide with an optimized PEG chain length modification to realize safe and efficient pulmonary mRNA delivery in a simple liquid aerosol formulation. The modification of peptide by different lengths of monodisperse PEG chains at the N-terminal could either improve or decrease its mRNA delivery ability, indicating the fine-tuning of the PEG chain length is crucial to maintain the favorable nucleic acid condensation and transfection capacity while reducing the toxicity of cationic peptide-based transgene vectors, which should be taken into consideration in peptide-based transgene vector design. Finally, the proper PEGylated peptide-mRNA nano self-assemblies could result in successful respiratory mRNA delivery without visible safety risk in mice and thus hold great promise for future clinical translation.
Authors’ contributions
Yingying Xu: conceptualization, methodology, analysis and interpretation of the data, the drafting of the paper, and revising it critically for intellectual content. Yijing Zheng: writing-original draft, investigation, validation, data analysis, software, and editing. Xuqiu Ding: methodology, investigation, data analysis, visualization. Chengyan Wang: animal study, investigation and data analysis. Bin Hua, Shilian Hong, Xiaoman Huang, and Jiali Lin: investigation, validation. Peng Zhang: resources, funding acquisition, writing-review. Wei Chen: project administration, funding acquisition, supervision, writing-review, and editing. All authors read and approved the final draft to be published.
Ethical approval statement
The justification of intratracheally delivery of mRNA in mice is carefully evaluated and approved by the Laboratory Animal Welfare and Ethics Committee of Fujian Medical University, PR China. Although a A549 human lung epithelial cell model has been used in this study, a in vivo delivery safety and effectiveness evaluation of the vulnerable lung tissues is indispensable because a representative cell model could not fully disclosure the peptide/mRNA self-assemblies’ interaction with airway mucus and nuclease environment, as well as the potential toxicity toward different types of pulmonary epithelial cells and immune cells. A total of 144 BALB/c male mice with an average age of 8 to 12 weeks and body weight between 18 to 22 g were used. All mice were purchased from Shanghai SLAC Laboratory Animal Co. Ltd. The mice were housed under a 12 h dark-light cycle at a constant temperature and with ad libitum feeding on water and standard laboratory chow. The experiment was carried out after one week of adaptation. Before intratracheal administration, the mice were anesthetized with intra-peritoneal injection of anesthetics (80 mg/kg ketamine and 4.5 mg/kg xylazine). A guiding cannula was gently intubated inside the trachea non-invasively to minimize the suffering of mice. The animal care and experimental protocols (the experimental design, justification for use of animals, animal treatment and disposal process) were all performed in accordance with protocols reviewed and approved by the Laboratory Animal Welfare and Ethics Committee of Fujian Medical University, PR China (the approved animal ethical number is IACUC FJMU 2022-Y-0683).
Supplementary information
A full description of detailed experimental methods, including the gel electrophoresis assay, particle size and morphology measurement, the MTT cytotoxicity assay, the mRNA cellular uptake and transfection, and in vivo mRNA delivery and biosafety studies, the fluorescent in vivo images of intratracheal administration of Cy5.5 fluorescent dye to mice (Figure S1) and pulmonary mRNA transfection study of PEG24LA/mRNA treated mice at 5:1 and 10:1 weight ratios (Figure S2 and S3) are provided in Supplementary Information (DOC).
Supplemental Material
Download MS Word (4.4 MB)Acknowledgments
We thank Cailing Yan, Shuping Zheng, and Minxia Wu of the Public Technology Service Center, Fujian Medical University, for the assistance in the IVIS bioluminescent imaging, flow cytometry, and transmission electron microscopy experiments and the assistance of the Laboratory Animal Center of Fujian Medical University for animal studies.
Data availability statement
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Anderson EJ, Rouphael NG, Widge AT, et al. (2020). Safety and immunogenicity of SARS-CoV-2 mRNA-1273 vaccine in older adults. N Engl J Med 383:1–14.
- Armbruster N, Jasny E, Petsch B. (2019). Advances in RNA vaccines for preventive indications: a case study of a vaccine against rabies. Vaccines 7:132.
- Berraondo P, Martini PGV, Avila MA, et al. (2019). Messenger RNA therapy for rare genetic metabolic diseases. Gut 68:1323–30.
- Bulcha JT, Wang Y, Ma H, et al. (2021). Viral vector platforms within the gene therapy landscape. Signal Transduct Target Ther 6:53.
- Crommelin DJA, Anchordoquy TJ, Volkin DB, et al. (2021). Addressing the cold reality of mRNA vaccine stability. J Pharm Sci 110:997–1001.
- Elkhalifa D, Rayan M, Negmeldin AT, et al. (2022). Chemically modified mRNA beyond COVID-19: potential preventive and therapeutic applications for targeting chronic diseases. Biomed Pharmacother 145:112385.
- Faghfuri E, Pourfarzi F, Faghfouri AH, et al. (2021). Recent developments of RNA-based vaccines in cancer immunotherapy. Expert Opin Biol Ther 21:201–18.
- Fant K, Esbjörner EK, Jenkins A, et al. (2010). Effects of PEGylation and acetylation of PAMAM dendrimers on DNA binding, cytotoxicity and in vitro transfection efficiency. Mol Pharm 7:1734–46.
- Gjetting T, Arildsen NS, Christensen CL, et al. (2010). In vitro and in vivo effects of polyethylene glycol (PEG)-modified lipid in DOTAP/cholesterol-mediated gene transfection. Int J Nanomedicine 5:371–83.
- Gomez-Aguado I, Rodriguez-Castejon J, Vicente-Pascual M, et al. (2020). Nanomedicines to deliver mRNA: state of the art and future perspectives. Nanomaterials 10:364.
- Gu P, Wang D, Zhang J, et al. (2021). Protective function of interleukin-22 in pulmonary fibrosis. Clin Transl Med 11:e509.
- Heida R, Hinrichs WL, Frijlink HW. (2022). Inhaled vaccine delivery in the combat against respiratory viruses: a 2021 overview of recent developments and implications for COVID-19. Expert Rev Vaccine 21:957–74.
- Hellfritzsch M, Scherließ R. (2019). Mucosal vaccination via the respiratory tract. Pharmaceutica 11:375.
- Huckaby JT, Lai SK. (2018). PEGylation for enhancing nanoparticle diffusion in mucus. Adv Drug Deliv Rev 124:125–39.
- Iacobucci V, Di Giuseppe F, Bui TT, et al. (2012). Control of pH responsive peptide self-association during endocytosis is required for effective gene transfer. Biochim Biophys Acta 1818:1332–41.
- Ibba ML, Ciccone G, Esposito CL, et al. (2021). Advances in mRNA non-viral delivery approaches. Adv Drug Deliv Rev 177:113930.
- Kargaard A, Sluijter JPG, Klumperman B. (2019). Polymeric siRNA gene delivery – transfection efficiency versus cytotoxicity. J Control Release 316:263–91.
- Kato A, Hulse KE, Tan BK, et al. (2013). B-lymphocyte lineage cells and the respiratory system. J Allergy Clin Immunol 131:933–57; quiz 958.
- Ke X, Shelton L, Hu Y, et al. (2020). Surface-functionalized PEGylated nanoparticles deliver messenger RNA to pulmonary immune cells. ACS Appl Mater Interfaces 12:35835–44.
- Khalil IA, Harashima H. (2018). An efficient PEGylated gene delivery system with improved targeting: synergism between octaarginine and a fusogenic peptide. Int J Pharm 538:179–87.
- Klimek L, Novak N, Hamelmann E, et al. (2021). Severe allergic reactions after COVID-19 vaccination with the Pfizer/BioNTech vaccine in Great Britain and USA. Allergo J Int 30:51–5.
- Kormann MS, Hasenpusch G, Aneja MK, et al. (2011). Expression of therapeutic proteins after delivery of chemically modified mRNA in mice. Nat Biotechnol 29:154–7.
- Kubler H, Scheel B, Gnad-Vogt U, et al. (2015). Self-adjuvanted mRNA vaccination in advanced prostate cancer patients: a first-in-man phase I/IIa study. J Immunother Cancer 3:1–14.
- Kunnapuu K, Veiman KL, Porosk L, et al. (2019). Tumor gene therapy by systemic delivery of plasmid DNA with cell-penetrating peptides. FASEB Bioadv 1:105–14.
- Lam JK, Liang W, Lan Y, et al. (2012). Effective endogenous gene silencing mediated by pH responsive peptides proceeds via multiple pathways. J Control Release 158:293–303.
- Lauring AS, Jones JO, Andino R. (2010). Rationalizing the development of live attenuated virus vaccines. Nat Biotechnol 28:573–9.
- Leppek K, Byeon GW, Kladwang W, et al. (2021). Combinatorial optimization of mRNA structure, stability, and translation for RNA-based therapeutics. Nat Commun 13:1–22.
- Li Z, Luo G, Hu WP, et al. (2020). Mediated drug release from nanovehicles by black phosphorus quantum dots for efficient therapy of chronic obstructive pulmonary disease. Angew Chem 132:20749–57.
- Li C, Zhan W, Yang Z, et al. (2022). Broad neutralization of SARS-CoV-2 variants by an inhalable bispecific single-domain antibody. Cell 185:1389–401.e18.
- Liang W, Chow MY, Lau PN, et al. (2015). Inhalable dry powder formulations of siRNA and pH-responsive peptides with antiviral activity against H1N1 influenza virus. Mol Pharm 12:910–21.
- Liang W, Kwok PC, Chow MY, et al. (2014). Formulation of pH responsive peptides as inhalable dry powders for pulmonary delivery of nucleic acids. Eur J Pharm Biopharm 86:64–73.
- Lin YX, Wang Y, Blake S, et al. (2020). RNA nanotechnology-mediated cancer immunotherapy. Theranostics 10:281–99.
- Lokugamage MP, Vanover D, Beyersdorf J, et al. (2021). Optimization of lipid nanoparticles for the delivery of nebulized therapeutic mRNA to the lungs. Nat Biomed Eng 5:1059–68.
- Maruggi G, Zhang C, Li J, et al. (2019). mRNA as a transformative technology for vaccine development to control infectious diseases. Mol Ther 27:757–72.
- Mehta D, Leong N, McLeod VM, et al. (2018). Reducing dendrimer generation and PEG chain length increases drug release and promotes anticancer activity of PEGylated polylysine dendrimers conjugated with doxorubicin via a cathepsin-cleavable peptide linker. Mol Pharm 15:4568–76.
- Moghimi SM. (2021). Allergic reactions and anaphylaxis to LNP-based COVID-19 vaccines. Mol Ther 29:898–900.
- Moreno-Fierros L, Garcia-Silva I, Rosales-Mendoza S. (2020). Development of SARS-CoV-2 vaccines: should we focus on mucosal immunity? Expert Opin Biol Ther 20:831–6.
- Muqier M, Xiao H, Yu X, et al. (2022). Synthesis of PEGylated cationic curdlan derivatives with enhanced biocompatibility. J Biomater Sci Polym Ed 33:465–80.
- Ndeupen S, Qin Z, Jacobsen S, et al. (2021). The mRNA-LNP platform’s lipid nanoparticle component used in preclinical vaccine studies is highly inflammatory. Iscience 24:103479.
- Osman G, Rodriguez J, Chan SY, et al. (2018). PEGylated enhanced cell penetrating peptide nanoparticles for lung gene therapy. J Control Release 285:35–45.
- Peng J, Cai Z, Wang Q, et al. (2022a). Carboxymethyl chitosan modified oxymatrine liposomes for the alleviation of emphysema in mice via pulmonary administration. Molecules 27:3610.
- Peng B, Nguyen TM, Jayasinghe MK, et al. (2022b). Robust delivery of RIG-I agonists using extracellular vesicles for anti-cancer immunotherapy. J Extracell Vesicles 11:e12187.
- Polack FP, Thomas SJ, Kitchin N, et al. (2020). Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med 383:2603–15.
- Pozzi D, Colapicchioni V, Caracciolo G, et al. (2014). Effect of polyethyleneglycol (PEG) chain length on the bio-nano-interactions between PEGylated lipid nanoparticles and biological fluids: from nanostructure to uptake in cancer cells. Nanoscale 6:2782–92.
- Qin S, Tang X, Chen Y, et al. (2022). mRNA-based therapeutics: powerful and versatile tools to combat diseases. Signal Transduct Target Ther 7:1–35.
- Qiu Y, Clarke M, Wan LTL, et al. (2021). Optimization of PEGylated KL4 peptide for siRNA delivery with improved pulmonary tolerance. Mol Pharm 18:2218–32.
- Qiu Y, Man RCH, Liao Q, et al. (2019). Effective mRNA pulmonary delivery by dry powder formulation of PEGylated synthetic KL4 peptide. J Control Release 314:102–15.
- Rao GA, Tsai R, Roura D, et al. (2008). Evaluation of the transfection property of a peptide ligand for the fibroblast growth factor receptor as part of PEGylated polyethylenimine polyplex. J Drug Target 16:79–89.
- Sahin U, Kariko K, Tureci O. (2014). mRNA-based therapeutics-developing a new class of drugs. Nat Rev Drug Discov 13:759–80.
- Sahu I, Haque A, Weidensee B, et al. (2019). Recent developments in mRNA-based protein supplementation therapy to target lung diseases. Mol Ther 27:803–23.
- Schoenmaker L, Witzigmann D, Kulkarni JA, et al. (2021). mRNA-lipid nanoparticle COVID-19 vaccines: structure and stability. Int J Pharm 601:120586.
- Sebastian M, Schroder A, Scheel B, et al. (2019). A phase I/IIa study of the mRNA-based cancer immunotherapy CV9201 in patients with stage IIIB/IV non-small cell lung cancer. Cancer Immunol Immunother 68:799–812.
- Su R, Wang H, Xiao C, et al. (2021). Venetoclax nanomedicine alleviates acute lung injury via increasing neutrophil apoptosis. Biomater Sci 9:4746–54.
- Tenchov R, Bird R, Curtze AE, et al. (2021). Lipid nanoparticles-from liposomes to mRNA vaccine delivery, a aandscape of research diversity and advancement. ACS Nano 15:16982–7015.
- Tian X, Bera H, Guo X, et al. (2021). Pulmonary delivery of reactive oxygen species/glutathione-responsive paclitaxel dimeric nanoparticles improved therapeutic indices against metastatic lung cancer. ACS Appl Mater Interfaces 13:56858–72.
- Uddin MN, Roni MA. (2021). Challenges of storage and stability of mRNA-based COVID-19 vaccines. Vaccines 9:1033.
- van den Brand D, Gorris MAJ, van Asbeck AH, et al. (2019). Peptide-mediated delivery of therapeutic mRNA in ovarian cancer. Eur J Pharm Biopharm 141:180–90.
- Veiman KL, Kunnapuu K, Lehto T, et al. (2015). PEG shielded MMP sensitive CPPs for efficient and tumor specific gene delivery in vivo. J Control Release 209:238–47.
- Vetter V, Denizer G, Friedland LR, et al. (2018). Understanding modern-day vaccines: what you need to know. Ann Med 50:110–20.
- Wadhwa A, Aljabbari A, Lokras A, et al. (2020). Opportunities and challenges in the delivery of mRNA-based vaccines. Pharmaceutics 12:102.
- Wang Y, Li J, Oupicky D. (2014). Polymeric plerixafor: effect of PEGylation on CXCR4 antagonism, cancer cell invasion, and DNA transfection. Pharm Res 31:3538–48.
- Wang Z, Lorenzi JCC, Muecksch F, et al. (2021). Enhanced SARS-CoV-2 neutralization by dimeric IgA. Sci Transl Med 13:eabf1555.
- Wang P, Zhang L, Liao Y, et al. (2020). Effect of intratracheal instillation of ZnO nanoparticles on acute lung inflammation induced by lipopolysaccharides in mice. Toxicol Sci 173:373–86.
- Weng Y, Huang Y. (2021). Advances of mRNA vaccines for COVID-19: a new prophylactic revolution begins. Asian J Pharm Sci 16:263–4.
- Weng Y, Li C, Yang T, et al. (2020). The challenge and prospect of mRNA therapeutics landscape. Biotechnol Adv 40:107534.
- Wu L, Rodríguez-Rodríguez C, Cun D, et al. (2020). Quantitative comparison of three widely-used pulmonary administration methods in vivo with radiolabeled inhalable nanoparticles. Eur J Pharm Biopharm 152:108–15.
- Wu L, Wu LP, Wu J, et al. (2021). Poly(lactide-co-glycolide) nanoparticles mediate sustained gene silencing and improved biocompatibility of siRNA delivery systems in mouse lungs after pulmonary administration. ACS Appl Mater Interfaces 13:3722–37.
- Xu Y, Liang W, Qiu Y, et al. (2016). Incorporation of a nuclear localization signal in pH responsive LAH4-L1 peptide enhances transfection and nuclear uptake of plasmid DNA. Mol Pharm 13:3141–52.
- Zasheva S, Gugleva V, Andonova V. (2020). Aerosol vaccines–perspectives and therapeutic impact. Scripta Sci Pharmaceut 7:18–25.
- Zheng Q, Qin F, Luo R, et al. (2021). mRNA-loaded lipid-like nanoparticles for liver base editing via the optimization of central composite design. Adv Funct Mater 31:2011068.