Abstract
Platinum-based drugs are widely used as first-line anti-tumor chemotherapy agents. However, they also have nonnegligible side effects due to the free drugs in circulation. Therefore, it is necessary to develop efficient and safe delivery systems for better tumor cell targeting. Hydrogel is a promising anti-tumor drug carrier that can form a platinum/hydrogel combination system for drug release, which has shown better anti-tumor effects in some studies. However, there is a lack of systematic summary in this field. This review aims to provide a comprehensive overview of the platinum/hydrogel combination system with the following sections: firstly, an introduction of platinum-based drugs; secondly, an analysis of the platinum/hydrogel combination system; and thirdly, a discussion of the advantages of the hydrogel-based delivery system. We hope this review can offer some insights for the development of the platinum/hydrogel combination system for better cancer therapy.
Graphic Abstract
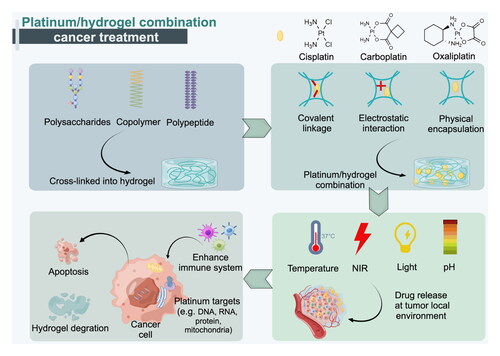
The common raw materials used in the formation of hydrogels include polysaccharides, copolymers, and polypeptides, which can be cross-linked to create hydrogels. In the case of platinum-based anti-cancer drugs such as cisplatin, carboplatin, and oxaliplatin, they can be combined with hydrogels through physical or chemical forces, resulting in a platinum/hydrogel combination.Hydrogels play a crucial role in cancer treatment by enabling the controlled and gradual release of platinum-based drugs in response to stimuli, thereby enhancing the efficacy of cancer treatment through immune system enhancement, targeted administration, and promotion of cell apoptosis. Once the drug delivery is achieved, the hydrogel can degrade naturally in the body within a few days without exhibiting side effects.
Introduction
Since the anti-cancer action of cisplatin (CDDP) was discovered in 1965, CDDP and other platinum-based drugs have been widely used for the treatment of various cancers such as head and neck cancer, colorectal cancer, and lung cancer (Lu, Citation2007). The main mechanism of intracellular cytotoxicity of platinum-based drugs is the formation of DNA and RNA adducts (Tchounwou et al., Citation2021). In clinical settings, platinum-based drugs are commonly administered intravenously due to their ability to rapidly reach systemic circulation. However, this mode of administration is associated with significant side effects. Ototoxicity, nephrotoxicity, and hair loss are common serious adverse effects caused by the presence of free drugs in circulation. Additionally, platinum-based drugs face limitations in terms of their ability to penetrate tissues. These drugs exhibit poor permeability across biological barriers, including blood vessel walls, cellular membranes, and the extracellular matrix. As a result, their accumulation at the tumor site is often insufficient, limiting their efficacy in targeting and destroying cancer cells. Moreover, platinum-based drugs demonstrate poor histocompatibility, which means that they can trigger adverse reactions and tissue damage when they come into contact with normal tissues. This lack of selectivity further reduces their antitumor efficacy.
To overcome these challenges, researchers are actively exploring innovative drug delivery systems. These systems aim to enhance the permeability of platinum-based drugs and minimize their off-target effects. Examples of such delivery systems include nanoparticles, liposomes, and hydrogel (Dobrucka et al., Citation2019; Němec et al., Citation2020; El-Shafie et al., Citation2020; Abdel-Bar et al., Citation2014). These approaches have the potential to improve drug distribution, increase tumor selectivity, and ultimately enhance the therapeutic outcomes of platinum-based chemotherapy. Among them, the platinum/hydrogel combination system is a promising drug delivery method. Hydrogels that are properly designed are nontoxic, biodegradable, and biocompatible. Furthermore, hydrogel-based drug delivery systems achieve targeted administration and prolonged/controlled drug release (Li & Mooney, Citation2016). Due to the unique characteristics of this combination system, it is necessary to summarize the current research. In this review, we firstly provide an overview of the platinum-based drugs; then we discuss the hydrogels loaded with platinum; and finally we highlight the improved antitumor efficacy of the platinum/hydrogel combination approach. In conclusion, hydrogel is a potential vehicle for the delivery of platinum-based drugs.
Overall, while platinum-based drugs are valuable in clinical practice for their anticancer properties, their administration intravenously can lead to severe side effects. The limitations in permeability and histocompatibility further impede their efficacy in specifically targeting tumors. Ongoing research into novel drug delivery strategies offers promising avenues to mitigate these challenges, improve drug performance, and ultimately enhance patient outcomes in cancer treatment.
The history of platinum-based drugs
The evolution of the platinum-based drugs
Platinum-based drugs have a long history since 1845 when Peyrone et al. successfully synthesized cisplatin (CDDP), a coordination molecule with a planar tetragonal structure also known as Peyrone’s salt (Rosenberg et al., Citation1965; Muggia et al., Citation2015). More than a century later, Rosenberg’s team first reported the anti-tumor effect of CDDP in two mouse tumor cell lines sarcoma 180 and leukemia L1210, thereby initiating the research on the anti-cancer capabilities of CDDP (Rosenberg et al., Citation1969). In 1978, the Food and Drug Administration (FDA) approved CDDP as the first choice for testicular cancer. In the following 40 years, CDDP was widely used to treat various solid cancers, including ovarian cancer and bladder cancer (Ghosh, Citation2019). Meanwhile, scientists continued to develop new platinum-based drugs, such as carboplatin and oxaliplatin, which were approved by FDA in 1989 and 2002, respectively. Japan, China, and South Korea have licensed nedaplatin, lobaplatin, and heptaplatin, respectively. Newly designed platinum complexes, such as platinum (IV) prodrugs and polynuclear platinum show anti-cancer properties in clinical or preclinical trials as well (Dilruba & Kalayda, Citation2016).
Platinum-based drugs have a range of physicochemical properties that are important to consider. Platinum-based drugs are typically administered as salts, such as cisplatin and carboplatin. They exhibit some solubility in water and are influenced by pH. Generally, platinum-based drugs have higher solubility at lower pH values (Mitsushima et al., Citation2008). Understanding solubility is crucial for studying the stability and dissolution of these drugs, as it affects their absorption and distribution in the body. Platinum-based drugs are relatively stable under normal environmental conditions. However, they may undergo decomposition under specific conditions. For example, cisplatin can undergo hydrolysis in the absence of chloride ions, which reduces its anti-tumor activity. Platinum-based drugs exhibit unique absorption properties, which play a significant role in analytical chemistry and pharmacokinetic studies. These drugs can form stable complexes and display characteristic absorption peaks in the ultraviolet-visible (UV-Vis) spectrum (Deng et al., Citation2007; Gong et al., Citation2014). These absorption peaks can be used to determine drug concentrations and assess their metabolism and elimination in the body. Please note that these properties may vary for different platinum-based drugs. Understanding the physicochemical properties of platinum-based drugs is essential for drug development, quality control, and the design of treatment regimens.
DNA-damage response
The main mechanism of platinum-based drugs involves DNA damage signals which have been extensively reviewed in other excellent studies (Khoury et al., Citation2020; Yu et al., Citation2020; Tchounwou et al., Citation2021). Platinum-based drugs can enter cells by passive diffusion or via membrane transporters like copper transporter (CTR) and organic cation transporters (OCTs) (Zhang et al., Citation2006; Kalayda et al., Citation2012), which is a crucial step for DNA plastination (). Platinum atom binds covalently to DNA to form monovalent adducts, interstrand cross-link adducts and intrastrand cross-link adducts thereby inhibiting replication and unwinding to cause DNA breakage (). Once the DNA lesions accumulate or cannot be repaired by the DNA damage repair mechanisms, the DNA damage signals can induce apoptosis (). Caspases and p53 are two essential response elements during apoptosis, p53 transmits DNA damage signals to mitochondria and stimulates the production of Cytochrome c(Cyt C). This causes membrane oxidative damage, mitochondria malfunction, and cell apoptosis (). Moreover, members of mitogen-activated protein kinase (MAPK) subfamily (e.g. p38 MAPK, c-Jun N-terminal kinase (JNK)), tyrosine kinases (TK) and Cyclobutanedicarboxylate (C-Abl) also participate in DNA damage-induced apoptosis.
Figure 1. Mechanisms involved in DNA damage signals. The anti-tumor mechanism of the platinum-based drugs: (A) Platinum enters cells via passive transport or mediated by CTR1, OTCs or passive diffusion; (B)The platinum covalent with DNA and trigger DNA damage; (C)Pt-DNA adducts activate MMR to activate c-Abl, leading to activation of the MAPK subfamily members p38 MAPK and JNK to induce apoptosis; (D) DNA damage signals are recognized and transduced by ERK, ATM/ATR, and p53, p53 signaling leads to the up-regulation of pro-apoptotic proteins and the inhibition of anti-apoptotic proteins in mitochondria, and activates the caspase cascade to induce apoptosis; (E) The DNA adducts trigger the inhabitation of rRNA thus lead to ribosome synthetic stress; (F)The platinum-based drugs contribute to ER stress and lead to mitophagy; (G) Platinum drugs bind to the cell membrane exchange protein NHE, the death receptor ligands are bound, and apoptosis is induced through the oligomerization and recruitment of procaspase-8 and induce cell apoptosis; (H) Platinum-based drugs enhance the specific recognition ability of T-cells to cancers cell.

RNA-damage response
Besides DNA damage response, platinum-based drugs show other anti-cancer mechanisms. For example, oxaliplatin is a FAD approved platinum drug that has an obvious anti-cancer effect on colorectal cancer. It forms less DNA adducts than CDDP and carboplatin (Jin et al., Citation2021). Based on the multi-platform genetic approach, Peter M Bruno et al. found that oxaliplatin modified DNA inhibits rRNA and triggers ribosome biogenesis stress. Oxaliplatin regulates pre-rRNA similarly to actinomycin D, a known inducer of ribosome biogenesis stress (Bruno et al., Citation2017). This suggests that oxaliplatin’s cytotoxicity relies on ribosome biogenesis stress (). Monofunctional platinum complexes are another type of platinum-based drugs that have only one unstable ligand. They do not interact with DNA but affect endoplasmic reticulum (ER) function through the PERK-ATF4-CHOP pathway. This leads to mitochondria mitophagy and inhibits ovarian cancer cell proliferation (). During this process, protein interactions and signaling pathways play important roles instead of covalently binding with DNA (Guo et al., Citation2022). Covalent bonds between platinum cations and nucleosides form the basis for DNA adducts. However, RNA has a higher density of negative charges than linear DNA, which allows for more binding opportunities. Indeed, researches have shown that RNA adducts accumulate after CDDP treatment, especially in rRNA, and the RNA adducts are 4–20 times more abundant than DNA adducts in yeast cells (Hostetter et al., Citation2012). Some reports suggest that RNA adducts affect RNA pathways and cause cytotoxicity. For example, CDDP could inhibit the synthesis of GFP proteins from GFP-encoding mRNA (Becker et al., Citation2014). Platinum drugs could also reduce the silencing ability of siRNA by binding to the antisense strand (Hedman et al., Citation2011). However, the relationship between RNA platination and these effects is still under debate. Some scientists think that these results are accompanied by DNA damage responses. Therefore, more research is needed to clarify the role of RNA adducts (Theile, Citation2017).
Protein targets
Moreover, we should note that DNA/RNA is not the only target of platinum-based drugs. They also bind strongly to cysteine and methionine residues in proteins, which provide sulfur atoms as binding sites (Muggia et al., Citation2015). This binding behavior can cause different consequences. On one hand, some proteins can reduce platinum drugs and make them inactive, such as glutathione (GSH) and metallothioneins (MT) (Galluzzi et al., Citation2012). On the other hand, some proteins can mediate cell cytotoxicity when bound by platinum drugs. Zinc finger proteins (ZFPs) play important roles in cellular processes such as cell proliferation, apoptosis and DNA replication. CDDP can bind to the cysteine residues of ZFPs which cause zinc release and structural damage to them (Yuan et al., Citation2017), leading to cell cytotoxicity. Platinum drugs can also regulate protein activity indirectly by inhibiting sodium/hydrogen exchanger 1 (NHE1) on the plasma membrane and inducing its fluctuation, which activates the FAS death receptor and triggers the extrinsic apoptosis pathway in cancer cells (Rebillard et al., Citation2008)().
Immunogenic cell death
Moreover, some platinum-based drugs can modulate immune system activity and sensitize immune cells to exert anti-cancer effects. The immunogenic cell death (ICD) is a unique cell death pathway induced by immune cells in cancer cells. The ICD involves three main factors: (1) ER stress induces calreticulin (CRT) exposure as an engulfment signal for dendritic cells (DCs); (2) cancer cells release ATP as a recruitment signal for DCs and macrophages; (3) nucleus releases high-mobility group protein box-1 (HMGB-1) to enhance antigen presentation and cytokine secretion (Hato et al., Citation2014). Ke-Bin Huang et al. reported that a platinum (II) complex could trigger ER stress and CRT exposure, induce ATP and HMGB-1 secretion, and activate immune cells in vitro. This platinum (II) complex also showed anti-cancer immune response in vivo (Huang et al., Citation2019). Signal transducer and activator of transcription (STAT) signal pathway is associated with platinum-based drugs anti-cancer effects from a clinical study, as STAT6 negative patients had better prognosis than STAT6-positive patients, but platinum-based drugs treatment could reverse this outcome (Hato et al., Citation2012). The reason may be related to that the platinum-based drugs could inactive the STAT6 thus down regulate the program cell death ligand 2 (PD-L2) of antigen presentation cells (APCs) and cancer cells, finally enhancing the specific recognition ability of T-cells to cancers cells (Hato et al., Citation2014). Obviously, the ICD dependent pathways could be a promising field to work with, but the mechanisms are still not clear ().
Platinum/hydrogel combination systems
Platinum-based drugs are among the most effective cancer chemotherapeutic agents due to their unique anticancer mechanisms, but they also have serious side effects. To overcome these clinical challenges, it is essential to develop novel strategies for platinum-based drugs. One method is to design new platinum-based drugs, but most of them have failed because of excessive cytotoxicity or severe side effects. Another method is to improve the delivery systems of drugs to enhance their efficacy. Fortunately, recent studies have shown that drugs can be combined with well-designed carriers, such as nanolipids and nanoparticles, to form drug delivery systems (DDS) that can provide better anti-cancer effects and fewer adverse effects than free drugs. In recent years, hydrogels have emerged as one of the most promising loading systems for DDS development.
The platinum-based hydrogel drug delivery systems
Nanoparticle drug delivery systems (NDDSs) have been developed for nearly 40 years and have shown higher efficacy in breast cancer (Yang et al., Citation2020), pancreatic cancer (Xiangsheng et al., Citation2021), ovarian cancer (Wang et al., Citation2017), and other cancers. Several platinum-based NDDSs are currently in clinical trials. NDDSs can be classified into organic, inorganic, and biomimetic types. Organic platinum-based NDDSs use polymers, micelles, dendrimers, and nanotubes as raw materials. They are widely used for platinum-based drug delivery because of their modifiability, low toxicity, and biodegradability. Inorganic NDDSs have advantages such as enhanced stability, increased loading efficiency, and unique porous structure (Lin et al., Citation2016). Some inorganic NDDSs also have dual functions, such as gold nanoparticles for photothermal therapy and iron oxide nanoparticles for imaging (Zhou et al., Citation2012; Ketabat et al., Citation2019). Biomimetic nanoparticles are made from natural components such as exosomes (Li et al., Citation2020; Tomita et al., Citation2020). They have inherent biocompatibility as a key benefit. Nanolipids are liposome formulations of platinum-based drugs that can achieve higher drug concentration in cancer cells and better anti-cancer effects than free drugs (Parker et al., Citation2016). Some promising liposome carriers based on platinum include lipoplatin, aroplatin, and lipoxal. However, these nanotechnology-based carriers also have drawbacks such as possible toxicity, low internalization and access difficulty that limit their application in platinum-based drug delivery (El-Sayed et al., Citation2006; Ha et al., Citation2016).
Hydrogels were first defined as “water-swellable macromolecules” by Wichterle and Lim in 1960 based on the synthesis of poly-2-hydroxyethyl methacrylate (PHEMA) hydrogels. The drug delivery hydrogels are the third generation of hydrogels, which are supramolecular structures formed by physical or chemical interactions from stereocomplex components (Chirani et al., Citation2015). One of the major advantages of hydrogels over conventional drug delivery vehicles is their ability to mitigate toxicological risks and minimize immunological rejection. Traditional drug delivery systems often employ carriers that may contain toxic components or elicit immune responses when introduced into the body. In contrast, hydrogels are typically composed of biocompatible polymers, such as polyethylene glycol (PEG), polyvinyl alcohol (PVA), or natural polymers like hyaluronic acid or chitosan. These materials have been extensively studied and found to have minimal toxicity and low immunogenicity. Another reason for the reduced toxicity and immunological rejection of hydrogels lies in their high-water content. This characteristic allows them to mimic the native extracellular environment, which in turn minimizes their recognition as foreign entities by the immune system. On the other hand, conventional carriers often have different physicochemical properties that can trigger an immune response (Ha et al., Citation2016). Furthermore, hydrogels offer the advantage of tunable physical and chemical properties, allowing for controlled drug release. For example, Hydrogel can provide a stable drug delivery platform, protecting cisplatin from premature metabolism or degradation, thereby increasing its bioavailability. However, Ensuring the proper rate of cisplatin release can be challenging. If released too quickly, it may result in a high drug concentration and increased risk of adverse reactions, while release that is too slow may fail to achieve therapeutic efficacy. The release kinetics can be adjusted by altering the crosslinking density, composition, or design of the hydrogel. This enables the sustained and controlled delivery of drugs, minimizing potential toxicity associated with high drug concentrations or frequent dosing. This makes hydrogels an attractive option for various biomedical and pharmaceutical applications, including targeted drug delivery, wound healing, tissue engineering, and regenerative medicine.
Natural and natural-derived hydrogel systems
Hydrogels are promising drug delivery vehicles in general. In recent years, more research has focused on hydrogels for platinum-based drug delivery. The platinum/hydrogel combination system has shown better anti-cancer effects and fewer side effects than free drugs according to recent studies. The hydrogel drug delivery systems based on natural and nature-derived compounds are widely used because of their biocompatibility, biodegradability and safety. These compounds include polysaccharides, proteins, and polypeptides.
Polysaccharides
Chitosan (CS) is a natural polysaccharide derived from crustaceans. It has basic amino groups that make it pH sensitive. When the pH is 6.8–7.2, the CS solution forms a gel-like precipitate and the polyol is counter ionized. The addition of β-glycerophosphate (β-GP) to the CS solution gives it heat-sensitive properties, as β-GP regulates the physical contact between CS molecules. The pH and temperature sensitive hydrogels based on CS and β-GP were developed to deliver CDDP alone or in combination with other drugs (Moura et al., Citation2013; Peng et al., Citation2019). However, physical cross-linked hydrogels have difficulties in controlling drug release compared with chemical cross-linked hydrogels (Mahkam & Doostie, Citation2005). Glycol Chitosan (GC) () is a water-soluble chitosan derivative with biocompatibility, water solubility and degradability. It has many amino and hydroxyl groups that can conjugate with photo-curing functional groups under visible light, which does not damage cells like UV light (Hyun et al., Citation2018). This chemically cross-linked hydrogels have interconnected porous architectures and can control CDDP release more easily by modifying pore size (Yoon et al., Citation2019).
Figure 2. Structures of natural polysaccharides derivatives. (A) Chitosan derivatives with ethylene glycol branched chains; (B) The structure of nature alginate, (C) Hyaluronic acid-aldehyde, it can cross-link with hyaluronic acid-adipic dihydrazide; (D) HA-FA ester formed by esterification of hyaluronic acid and folic acid Figure; (E) Hydroxyethyl cellulose, a soluble cellulose derivative; (F) Carboxymethyl cellulose, a soluble cellulose derivative.

Alginate (AL) is a salt derivative of alginic acid, a biocompatible and soluble natural polysaccharide (). AL copolymer production depends on either M or G blocks, but only G blocks can form hydrogel. The formation of pH-sensitive hydrogel networks depends on the interaction of α-L-glucuronic acid groups (Cong et al., Citation2018). Moreover, the high amount of carboxyl structure in alginate can form hydrogen bonds with mucoproteins, enhancing its mucoadhesion properties (Lee & Mooney, Citation2012). In the drug delivery system, AL can stay in the mucosal membrane for a longer time, thus delaying drug absorption. AL hydrogel could co-deliver gold nanoparticles and CDDP to achieve triple combination therapy according to researchers (Keshavarz et al., Citation2018; Mirrahimi et al., Citation2019; Mirrahimi et al., Citation2020).
Hyaluronic acid (HA) is a component of the extracellular matrix. The main methods for synthesizing HA-based hydrogels for platinum-based drug delivery are introducing functional groups to HA to make activated HA derivatives or using cross-linking agents. HA-aldehyde (HA-CHO) and HA-adipic dihydrazide (HA-ADH) () are two HA derivatives that can be cross-linked into HA-based hydrogels in situ after injection. The polymer content affects the gelation rate and the drug release. Moreover, CDDP partly forms a coordinate bond with the carbonyl group of HA in the interaction of CDDP and HA (Emoto et al., Citation2014). These features of HA-based hydrogels lead to slow release of CDDP. Furthermore, HA has targeted property to cluster of differentiation-44 (CD44) receptor, which is overexpressed in many tumor cells. This makes HA-based hydrogels more selective drug delivery vehicles (Ghosh et al., Citation2012). Ovarian cancer (OC) cells also overexpress folic acid (FA) receptor-α besides CD44. Therefore, studies were done on a more selective hydrogel based on HA and FA for CDDP delivery to treat OC cells, including CDDP-resistant OC cells (Serini et al., Citation2021). They cross-linked to produce HA-FA ester (). The hydrophobic interactions between HA and cross-linked agents Polaxamer 407 contributed to gelation, while the electrostatic repulsions between the ionized carboxylic groups of HA and FA contributed to the swelling behavior, especially under acidic and mildly acidic conditions. The injection of CDDP through these HA-FA-based hydrogels inhibited the proliferation and migration of OC cells and regulated epithelial-mesenchymal transition (EMT)-related proteins.
Cellulose’s insolubility has led scientists to explore its soluble derivatives for making pH-sensitive hydrogels. Hydroxyethyl cellulose (HEC) and carboxymethyl cellulose (CMC) () are soluble cellulose derivatives that can form hydrogen bonds with polyol and cross-link hydrogels (Tang et al., Citation2012). The hydroxyl group in HEC can prevent the intramolecular crosslinking of CMC, so they are compatible in hydrogel formation (Mahdavinia et al., Citation2017). Montmorillonite (NaMMT) was added to HEC to increase the cross-link density and create stiffer CHAP composite hydrogels for CDDP delivery, which will be discussed in composite hydrogel (Kouser et al., Citation2018).
Polypeptides
Gelatin and other proteins are nontoxic and biodegradable substances. The CDDP release rate from gelatin hydrogels depends on the cross-linking degree; more cross-linking means slower drug release (Kanda et al., Citation2021). Gelatin hydrogel granules (GHG) were made by cross-linking gelatin solution with glutaraldehyde. The platinum-based drugs and gelatin side chains formed coordination bonds through carboxyl groups, which slowed down drug release. For gastric cancer with peritoneal metastases, GHG-CDDP injected into the peritoneum was a better option than free CDDP to reduce the initial burst (Yamashita et al., Citation2019). Polypeptides are short amino acid sequences that can assemble with negatively charged peptides through electrostatic and hydrogen bonding among peptide segments (Cui et al., Citation2010). H9e is a new peptide with high biocompatibility and safety, composed of the α-helix motif of spider flagellar silk protein and the calcium-binding motif of human muscle protein. It self-assembled into hydrogels under physiological conditions (Carter et al., Citation2021). The h9e hydrogels showed shear thinning properties and could switch between sol-gel states repeatedly, which helped hydrogel injection and drug delivery. CDDP was delivered through this peptide hydrogels by conjugating with bovine serum albumin (BAS). This combination induced hydrogel formation due to hydrophobic forces. Compared with the BSA-CDDP release rate in PBS, the drug release speed was lower through hydrogel administration. In vitro toxicity experiment showed that Hela cell viability increased with higher hydrogel concentration, indicating the controlled release of BAS-CDDP (Liang et al., Citation2017). One advantage of polypeptide hydrogel is that the gelation behavior can be changed by altering the secondary structure and amino acid content (Xu et al., Citation2020). For instance, peptides with some phenylalanine (Phe) structures tend to form gels more easily than other non-aromatic species under certain conditions (Wang et al., Citation2019). D-amino acids resist enzymatic digestion better than L-amino acids, so they are used for longer drug release (Wang et al., Citation2019). Based on this, a peptide (D-Phe-D-Phe-D-Glu-D-Tyr) was attached to an anti-inflammatory drug naproxen (Npx) and co-assembled with CDDP to make hydrogel nanoparticle fibers; CDDP acted as a coacervation inducer in this system (Wang et al., Citation2020). This hydrogel could deliver CDDP more effectively and cause more DNA damage and cell death. Amphiphilic peptide is a type of self-assemble peptide conjugate that forms under hydrophobic and hydrogen bonding forces. An amphiphilic peptide based on 3-mercaptopropionic acid-modified poly(L-cysteine)[P(Cys-SS-CH2CH2COOH)] was made by ring-opening reaction (ROP) and post-polymerization modification (PPM) route. It could self-assemble into hydrogel nanofibers when Ca2+ or CDDP was added. CDDP release from these amphiphilic peptide hydrogels was sustained, and half of the CDDP was released near the lining (Dong et al., Citation2021).
Synthetical copolymer hydrogel systems
Copolymer hydrogel systems for platinum drug delivery include block copolymer hydrogels and graft copolymer hydrogels, with block copolymer hydrogels being more common. Typical polymers are polyethylene glycol (PEG), poly(ε-caprolactone) (PCL), polyacrylic acid (PAA) and polyglutamic acid (PLG). Diblock and triblock-based hydrogels have better mechanical properties and adaptability, including temperature, light, and NIR responsiveness, than natural and natural-derived hydrogels. These features make them smarter and more promising carriers for platinum-based drugs.
Diblock copolymer poly(ethylene glycol)-poly(γ-ethyl-L-glutamate) (mPEG-b-PELG) () made by ring-opening can form thermal gels in situ. The network aggregated with temperature rise and the increase of intermolecular interactions (changes in β-sheets and α-helices) contributed to hydrogel formation (Cheng et al., Citation2012). This mPEG-b-PELG hydrogel had an open porous network structure with pore sizes of tens of microns, suggesting that it can load drugs effectively and control drug release (Yu et al., Citation2019). In another study, the mPEG-b-PELG diblock copolymer PBS solution could stay in solution state in the wide concentration range of 4 wt%-12wt%, indicating the tunable properties of this hydrogels. After subcutaneous injection of mPEG-b-PELG PBS solution into mice, this copolymer hydrogel turned into a hydrogel state after 20 minutes and degraded after 3 weeks (Wu et al., Citation2017). This hydrogel was typically used for CDDP delivery alone or together while CDDP showed slower release rate than other drugs, which was related to the higher solubility of CDDP in hydrogels. Similar to the diblock copolymer methoxyl poly (ethylene glycol)-poly (D,L-lactide-co-glycolide) (Bi(mPEG-PLA)) conjugated with platinum(IV) (), this conjugate copolymers self-assembled into hydrogels thermally in micelle formations and could release platinum(IV) for up to two months (Shen et al., Citation2015; Shen et al., Citation2017). In another supramolecular thermosensitive hydrogel based on diblock copolymers poly(ethylene glycol)-poly(N-phenylglycine) (PEG-PNPG) (), this diblock copolymer first interacted through hydrogen bonding and physical entanglement to form a sea urchin-like structure. Then, α-cyclodextrin (α-CD) intermolecular hydrogen bonding interactions led to crystallite aggregation and enhanced physical cross-linking of the new supramolecular hydrogels (PNPG-PEG/α-CD). Furthermore, PNPG acted as a medium for NIR absorption to achieve chemo-photothermal therapy. This thermosensitive hydrogel triggered CDDP release on demand and reduced off-target toxicity (Gil et al., Citation2017). The polymer can also be modified before block polymerization. By introducing the carboxyl side chain of L-glutamic acid to obtain a diblock copolymer methoxy-poly(ethylene glycol)-b-(poly(γ-ethyl-L-glutamic acid-co-L-glutamic acid) ((mPEG-b-P(ELG-co-LG)), the abundant carboxyl groups of L-glutamic acid increased the α-helix content in the copolymer, forming a thermal-responsive hydrogel with homogeneous porous structure. CDDP delivered by this polypeptide hydrogel showed better anti-tumor performance and lower tissue toxicity than other groups (Yu et al., Citation2017). The covalently cross-linked hydrogel made of norbornene-modified polyglutamic acid (PLG-Norb) and tetrazine-functionalized four-arm PEG (4aPEG-T) based on the Diels-Alder (iEDDA) click reaction had a wide range of mechanical properties. Moreover, the carboxyl groups from PLG-Norb complexed with CDDP to control their release and the SME showed that the hydrogel had a porous network structure to regulate drug release (Zhang et al., Citation2020).
Figure 3. The structure of eblock copolymers. (A) The copolymer mPEG-b-PELG was formed by the ring-opening reaction of copolymer poly(ethylene glycol) and poly(γ-ethyl-L-glutamate); (B) Bi(mPEG-PLA) is formed by polymerization of two poly (D,L-lactide-co-glycolide), and the hydroxyl group of poly (D,L-lactide-co-glycolide) can be conjugated with platinum(IV); (C) PEG-PNPG is polymerized from poly(ethylene glycol)-poly(N-phenylglycine), and PNPG can absorb NIR to achieve photothermal combination therapy.

Triblock copolymers also form hydrogels with better features, similar to diblock copolymers. As shown in , the triblock copolymer poly(ethylene glycol)-poly(-caprolactone)-poly(ethylene glycol) (PECE)() had injectable and degradable abilities, and PECE hydrogels could release drugs upon temperature change. This thermosensitive hydrogel could co-deliver suberoylanilide hydroxamic acid (SAHA) and CDDP (Li et al., Citation2012). Poly(D, L-lactide-co-glycolide)-poly(ethylene glycol)-poly(D, L-lactide-co-glycolide) (PLGA-PEG-PLGA) () triblock copolymer was also thermal responsive and showed amphiphilic properties with the hydrophilic segment PEG and the hydrophobic segment PLGA. CDDP was encapsulated in the pores of the Ti6Al4V implants, and the implanted 3D printed Ti6Al4V could improve the mechanical properties of the hydrogel. The resulting hydrogels could not only provide the anticancer effect of CDDP but also achieve the repair function of the implant to bone damage (Jing et al., Citation2021). Pluronic F-127 is a poly(ethylene oxide)-poly(propylene oxide)-poly(ethylene oxide) (PEO-PPO-PEO)-based heat-sensitive hydrogel () triblock copolymer was also a controllable drug delivery system for RES and CDDP combination delivery (Wen et al., Citation2020).
Figure 4. The structure of triblock copolymers. (A) This triblock copolymer PECE is consisted of poly(caprolactone) with two poly(ethylene glycol), It is thermally responsive to control drug release; (B) The Heat-sensitive and amphiphilic triblock hydrogel PLGA-PEG-PLGA is consisted of poly(D, L-lactide-co-glycolide) and poly(ethylene glycol), the poly(ethylene glycol) is the hydrophilic segment and the hydrophobic segment is poly(D, L-lactide-co-glycolide); (C) The poly(ethylene oxide) and poly(propylene oxide) polymerize hydrogel named Pluronic F-127, which is a heat sensitive hydrogel.

Graft copolymers can be synthesized more easily than block copolymers by grafting one or more monomers to a main polymer chain. However, graft copolymers cannot fully exhibit the properties of all polymers. One example of a grafted copolymer hydrogel for platinum-based drugs was composed of polyacrylic acid (PAA) and cellulose nanocrystals (CNCs). PAA has mucoadhesive properties due to its side chain carboxyl groups that can form hydrogen bonds with mucoproteins. CNCs are rod-shaped nanoparticles obtained by hydrolyzing natural cellulose acids. By attaching PAA to CNCs, a rigid bottlebrush polymer (HCBBP) can be created. This unique structure can provide enough space for CDDP loading and increase the mucoadhesion between CNC and mucosal proteins. Hydrogels with mucoadhesive properties can reduce drug systemic toxicity and avoid the reduced efficacy caused by liver first-pass effect. Moreover, under hydration, the chloride ion of CDDP can be replaced by the carboxylic acid of PAA or the hydroxyl group on CNCs, making it easier for PAA to form a CDDP-hydrogel network complex (Vakili et al., Citation2021).
Hybrid hydrogel systems
Different materials have their own advantages and disadvantages, so hybrid or composite hydrogels that are composed of different kinds of materials can balance their strengths and weaknesses and enhance the properties of hydrogels. For instance, CMC and HEC based hydrogels have poor mechanical properties that contribute to low drug delivery efficacy, but adding NaMMT results in superior mechanical strength hydrogel systems with porous and highly cross-linked structures as seen under scanning electron microscope (SEM) (Kouser et al., Citation2018). Another example is chemically cross-linking natural polymer chondroitin sulfateand and synthetic monomer acrylic acid (AA) to produce oral hydrogels (CSA) for oxiaplatin administration. The thermal stability of the hydrogels can be enhanced by mixing natural and synthetic materials (Barkat et al., Citation2020). For example, a combination of synthetic polyvinyl alcohol (PVA) and natural-derivative carboxymethyl chitosan (CMCh) showed improved qualities for oxiaplatin administration. The hydrogels exhibited excellent swelling properties and pH sensitivity based on PVA and CMCh, respectively (Ullah et al., Citation2019). Poly(N-isopropylacrylamide) (PNIPAAm) is a drug delivery material with unique thermo-gelatin properties, but its toxicity limits its application. To solve this problem, Cheng et al. combined natural derived polymer carboxymethyl chitosan with it to balance its toxicity (Cheng et al., Citation2015).
Nanocomposite hydrogels that incorporate nanomaterials into hydrogel matrix have more multi-functional properties than individual components for platinum-based drugs delivery. These properties include enhanced strength, increased loading capacity and higher cellular uptake. The nanomaterials usually consist of micelles (Yang et al., Citation2022), nanogels or microemulsions (Abdel-Bar et al., Citation2016). An AL-based bilayer reservoir system was developed to deliver CDDP through chelating ligand-metal coordination cross-linking reaction. This bilayer structure enabled the hydrogel to sustain the release of CDDP and maintain high concentration of CDDP in abdominal cavity (Yamaguchi et al., Citation2021). For abdominal metastatic cancer, preventing peritoneal adhesion is also essential after surgery. Nanocomposite hydrogels containing oxialipatin could protect injured tissue against peritoneal adhesion more effectively than pure hydrogels (Abuzar et al., Citation2019; Lee et al., Citation2019). The initial burst can also be eliminated by the double layer depot hydrogel systems such as PEG-PAEU hydrogels encapsulating CDDP loading chondroitin sulfate nanogels. The PDMP nanocomposite hydrogel composed of PTX-loading micelles and copolymer PECE could realize dual drug loading. The micelles could also enhance the strength of PDMP hydrogels. This nanocomposite system utilized the diffusion rate of PTX in the micelle networks and degradation of hydrogel matrix to achieve controlled dual drug release (Gil et al., Citation2017). These properties were also observed in other nanoparticle/hydrogel combinations. Moreover, this supramolecular hydrogel nanocomplex avoided the problems of nanoparticles’ fast diffusion into surrounding tissues (Wu et al., Citation2020). For nanoparticles, they entered cells through endocytosis after releasing from hydrogels and were cleaved by lysosomes, which enhanced cellular uptake and allowed the platinum drug to reach the cell nucleus and exert anti-cancer effect (Davoodi et al., Citation2016), as shown in .
Figure 5. Drug delivery approaches of nanocomposite hydrogel. Drug delivery approaches of nanocomposite hydrogel: Drug-loaded nanoparticles enter tumor cells through endocytosis, and after lysosome cleavage, platinum drugs are released and enters the nucleus to play an anti-tumor effect.

Studies indicate that hydrogels are promising vehicles for platinum-based drug delivery. The natural and natural-derived hydrogels are biocompatible and biodegradable, and they usually respond to endogenous stimuli under physiological conditions. The synthetic hydrogels have improved elasticity and rigidity but they may have drawbacks in terms of safety. Fortunately, the hybrid hydrogels can combine the advantages of natural and synthetic materials for more efficient platinum-based drug delivery. Furthermore, the nanocomposite hydrogels are noteworthy in hydrogel researches for their multi-functional roles in drug delivery. The features of hydrogel raw material are important in drug delivery as they can enhance efficiency with proper design rather than by chance. For instance, FA-based hydrogel delivery system is more suitable for targeting OC cells that overexpress FA receptor-α. The co-delivery of platinum-based drugs and hydrophobic anti-tumor drugs can also be achieved by creating amphiphilic copolymer hydrogels or nanocomposite hydrogels. Therefore, suitable hydrogels should be designed according to tumor types to maximize the anti-cancer efficiency of platinum-based drugs. The well-designed hydrogels are ideal vehicles for platinum-based drugs, and the hydrogel/platinum combinations usually show excellent anti-tumor efficiency compared to free drugs. The satisfactory results are related to three aspects: targeted delivery, controlled drug release and higher synergistic effect.
Advantages of the hydrogel release systems
Targeted delivery
With intravenous injection, free platinum-based drugs can circulate throughout the body and cause toxicity to healthy tissues. The platinum/hydrogel systems are locally administered due to their special structure and can achieve higher but limited drug concentration at the tumor sites, resulting in less adverse effects and better anti-tumor efficacy. The intraperitoneal injection, peritumoral injection and intratumoral injection are common local injection methods. Compared with free platinum-based drugs, the platinum/hydrogel can achieve better anti-cancer effect at the same dose under the same injection methods. This targeting effect is not limited to intratumoral injection but encompasses various application scenarios where the drug is accurately delivered to the desired location through functionalized hydrogel systems. For example, compared with intraperitoneal injection of AL/CDDP/Ca hydrogel and free CDDP, the former group maintained higher drug concentration in the peritoneal cavity. Histological analysis also showed that peritoneal tumor formation was significantly inhibited in hydrogel-treated mice. Consequently, the AL/CDDP/Ca hydrogel improved the overall survival of mice compared to the free CDDP. At the same time, this local administration strategy of hydrogels could also prevent tumor spread and efficiently inhibit tumor recurrence, which was of great value for the prognosis of patients in clinic (Yamaguchi et al., Citation2021). The similar superior effect of platinum/hydrogel combination was observed in gastric cancer treatment (Ohta et al., Citation2017). The beneficial effect of hydrogel through intraperitoneal delivery may result from the continuous exposure of tumor tissue to drugs and the subsequent penetration of drugs into tumor nodules. As for whether these mentioned hydrogels can facilitate the cellular uptake of platinum-based drugs, it depends on the design and functionalization of the hydrogel. Some hydrogels can be designed to promote cellular uptake through specific chemical structures or functional groups on the carrier. The promotion of cellular uptake can be achieved by altering the surface properties, charge, or structure of the hydrogel. For example, surface modifications of the hydrogel can introduce ligands that can specifically bind to receptors on target cells, enhancing the interaction between platinum-based drugs and the target cells, thereby improving cellular uptake efficiency (Serini et al., Citation2021). However, we should note that the intraperitoneal administration method may be more suitable for small tumor nodules (El-Kareh & Secomb, Citation2004). The same outcomes may also occur with intratumoral injection or peritumoral injection. Furthermore, due to the hydrogel’s ability for repair and regeneration, direct injection at the tumor site can accelerate the regeneration of injured tissue and prevent tumor recurrence. Kanda Y et al. studied the antitumor efficacy of hydrogel microspheres incorporating CDDP (GM-CDDP) in bone metastasis model. The results showed that GM-CDDP exhibited more remarkable antitumor effect than free CDDP. In addition, local administration of GM-CDDP resulted in the maximum amount of bone residue, reducing the damage to surrounding healthy tissue caused by free administration and preserving the integrity and mechanical strength of bone tissues as much as possible (Kanda et al., Citation2021). For peritumoral injection, a CDDP-loaded thermosensitive polypeptide hydrogel displayed more significant tumor inhibition than free CDDP group with the same dose (10 mg/kg) during weekly injection to MCF-7 cell transplanted tumor model, supporting the effectiveness of targeted delivery (Yu et al., Citation2017).
Besides injection, hydrogels can also be delivered in various ways to create customized therapeutic effects, which is something free drugs cannot achieve. Tran-portal vein embolization (TPVE) is considered as a standard method for the treatment of intermediate-stage hepatocellular carcinoma. After injecting through the main portal vein, the hydrogel immediately blocked the left portal vein after polymerization, and gradually dissolved and flowed to the liver later, causing severe damage to the left hepatic lobe ischemia (Otto et al., Citation2013). Zhang et al. developed a CDDP loading IPN hydrogel as embolic material by TPVE and exhibited the dual effects of restricting blood flow to tumor tissue and CDDP delivery. Controlled experiments in an orthotopic stem cell carcinoma (HCC) mouse model showed that CDDP/IPN combination system via TPVE could significantly inhibit the proliferation and angiogenesis of cancer cells, down regulate alpha-fetoprotein expression, which is a plasma-specific biomarker of HCC occurrence (Yang et al., Citation2021). The hydrogel can also be placed subcutaneously in the form of implants to deliver drug at the tumor sites. Jing et al. administered covalently bound CDDP and Ti6Al4V using a PLGA-PEG-PLGA thermosensitive hydrogel implant, implanted subcutaneously in vivo. The osteosarcoma tumor volume reduced two-thirds of systemic CDDP delivery system at 1.6 mg/mL, which implied the significant drug release efficiency of hydrogel systems. Although having some drawbacks such as low targeting, gastrointestinal instability, and low efficiency, oral administration is still a preferred way for the treatment of gastric and intestinal cancers (Jing et al., Citation2021). Oral delivery of hydrogel systems may overcome the poor penetration of lymph nodes and tumors caused by intravenous infusion of free drugs. In a mouse model of gastric cancer, oral administration of CDDP via peptide hydrogel showed an optimal therapeutic effect. The CDDP/Gel group had a longer survival time than the free CDDP and control groups (Qian et al., Citation2019).
Controlled and sustained drug release
Depending on the properties of raw materials, hydrogels can release platinum drugs through endogenous signals or exogenous physical stimuli. The most common stimuli-responsive platinum-loaded hydrogels are responsive to pH, temperature, light and NIR, as shown in . By changing the internal environment or applying external stimuli, hydrogels can control the release of platinum-based drugs at the target sites through sol-gel transition.
Table 1. The platinum-loaded hydrogels.
Platinum-based drugs can be encapsulated within hydrogels through physical interactions or covalent bonds, so they can be released gradually under changing interaction forces (Davoodi et al., Citation2016; Qian et al., Citation2019). Due to the sustained release, platinum-based drugs delivered by hydrogels have a longer duration in the body than free platinum drugs at the same dose. Regarding the long-term stability of platinum-based drugs in vivo, it is an important factor to ensure the activity and efficacy of the drugs during long-term loading and release. For researchers involved in the design and development of hydrogel release systems, assessing the long-term stability of platinum-based drugs in vivo is necessary. This involves studying the degradation rate of platinum-based drugs within the hydrogel and their stability during long-term loading and release processes. The long-term stability of platinum-based drugs delivery in vivo is influenced by various factors, including the in vivo environment (such as temperature, pH, etc.), properties of the hydrogel material, and characteristics of the drugs themselves. Therefore, researchers need to select suitable hydrogel materials and appropriately encapsulate and protect platinum-based drugs to ensure their stability during loading and release. Additionally, the stability of platinum-based drugs in the hydrogel environment can be enhanced through chemical modifications or other means. This can help reduce the degradation and decomposition of drugs during long-term loading and release, thereby ensuring their long-term activity. Initial burst of platinum-based drugs is a challenge in sustained delivery systems as it may lead to high blood concentration of drugs. Nanocomposite hydrogel systems can achieve a double-layer encapsulation effect that can reduce this problem. Davoodi et al. encapsulated cisplatin in microspheres and prepared hydrogels as an additional barrier to control the initial burst of cisplatin to 40% and achieve a more sustained release (Davoodi et al., Citation2016). Moreover, because of the gradual release function of hydrogel, tissues can tolerate higher concentrations of platinum without damaging human tissues. For example, cellulose nanocrystal-grafted-polyacrylic acid cisplatin (CNC-g-PAA CDDP) hydrogel had three times greater IC50 than free CDDP. This suggested that hydrogels delivery increased the restricted concentration of platinum-based drugs.
Higher combination effect
Hydrogels can also deliver platinum drugs in combination with other anti-cancer drugs more effectively than other vehicles. The reported drugs include combretastatin A4 disodium phosphate (CA4P) (Yu et al., Citation2019), interleukin-15 (IL-15) (Wu et al., Citation2017), suberoylanilide hydroxamic acid (SAHA) (Li et al., Citation2012), resveratrol (RES) (Wen et al., Citation2020), irinotecan (Irino) (Davoodi et al., Citation2016), paclitaxel(PTX) (Davoodi et al., Citation2017; Xu et al., Citation2017; Xu et al., Citation2018), and Doxorubicin(DOX) (Cheng et al., Citation2017; Yoon et al., Citation2019; Chen et al., Citation2020; Anirudhan et al., Citation2022). The combination index (CI) can quantify the synergy of two drugs in combination. The lower the CI value, the greater the synergy. A strong synergy is indicated by 0.2 ≤ CI < 0.4. For example, the CI value of CDDP/irino combination was 0.3 when CDDP and irino were simply mixed, but it was 0.1 when they were delivered by CDDP/Pept-AlgNP/Irino composite hydrogel. This suggests that the hydrogel carrier can enhance the synergistic effect of the loaded drugs (Wu et al., Citation2020). Another study showed a similar result with CDDP/Peptide@NP/Irino composite hydrogel, which had a CI value of 0.17 and an anti-tumor effect on A549 cells comparable to that of CDDP/irino combination (Wu et al., Citation2020). These studies demonstrate that hydrogels can improve the combination effect of platinum-based drugs with other anti-cancer drugs. This may be because hydrogels release the combination drugs at different rates depending on their solubility and loading methods, which are favorable for their synergy.
Hydrogels can also enhance the combination effect of different therapy approaches. Since platinum-based drugs can increase radio-sensitivity, chemoradiation therapy is more effective than chemotherapy alone, but it also causes more damage to healthy tissue. Hydrogel can solve this problem by delivering gold nanoparticles and CDDP in an AL based nanocomposite hydrogel for triple therapy of radiation, photothermal and chemotherapy to tumors. This nanocomposite hydrogel showed the best tumor reduction and inhibition both macroscopically and microscopically under 523 nm laser irradiation. It also induced massive cell necrosis and apoptosis with 4.4 times more ROS generation than the untreated group. Moreover, it prevented tumor recurrence and systemic toxicity in mice (Alamzadeh et al., Citation2020). Therefore, hydrogel system is a promising platform for improving the combination efficiency of platinum drugs with other therapy approaches for cancer treatment. Besides the advantages of hydrogels for platinum-based drug delivery, hydrogels are also preferred as a drug delivery system because of their degradability, nontoxicity and tissue biocompatibility. They usually degrade within weeks after injection, which means they are safe enough. All these factors contribute to higher anti-tumor efficacy of platinum drugs, mainly manifested in inducing cancer cell apoptosis, inhibiting cancer cell proliferation and migration, enhancing immune system activity and promoting survival rate, as shown in .
Figure 6. How platinum/hydrogel complexes outperform free platinum drugs. With the aim of enhancing anti-tumor efficacy, platinum-based drugs are delivered through hydrogels can achieve better outcomes than free drugs. The satisfied results are related to three aspects, target administration, controlled drug release and higher combination effect. Firstly, on account of the unique administration ways and unique tumor microenvironment, the hydrogel would release drugs at the tumor site more than free drug administration; secondly, the hydrogels are stimuli response so that achieving controlled and sustained drugs release under environmental or external stimuli; lastly, when both or more drugs are delivered through hydrogels, they may achieve better combination effect instead of causing toxicity and drug resistance.

Conclusion
Platinum-based drugs are a type of chemotherapy with a long history, wide use and significant anti-tumor efficacy. However, their clinical application is limited by severe systemic toxicity and drug resistance. Developing new platinum-based drugs is also challenging from experiment to clinic. Fortunately, drug delivery devices can overcome this problem. Among them, hydrogels are a reliable platform for targeting tumor sites and reducing toxicity with their ability to change from sol state to gel state and control drug release by responding to stimuli in the internal and external environment. In this article, we review the platinum/hydrogel combination systems for improved anti-cancer efficacy. Based on the unique features of hydrogels, platinum/hydrogel combination systems have advantages over free drug administration in terms of targeted delivery, sustained drug release and higher combination effects.
Platinum-based drugs have shown significant potential in cancer treatment due to their ability to inhibit tumor growth and progression. By incorporating these drugs into hydrogels, we can achieve controlled and sustained drug release, which can enhance therapeutic efficacy and reduce side effects. This combination has several potential clinical applications worth discussing. Firstly, the controlled release of platinum-based drugs from hydrogels can provide prolonged exposure to the tumor site, ensuring sustained drug concentrations and maximizing their cytotoxic effects. This can potentially improve treatment outcomes and enhance patient compliance. The stimuli-responsive and targeted features of hydrogels depend on their raw materials, so materials with stimuli-responsive function or tumor cell marker affinity are sought and created. Besides pH and temperature, endogenous signals such as ROS from redox reactions and enzymes can also trigger drug release from hydrogels, such as DNA hydrogels responsive to nucleases and fatty acids hydrogels responsive to matrix metalloproteinases (Joshi et al., Citation2018; Obuobi et al., Citation2019). Secondly, the use of hydrogels as drug delivery systems can overcome the limitations of conventional administration routes. For example, localized delivery of platinum-based drugs using hydrogels can minimize systemic toxicity and improve drug distribution within the tumor, increasing treatment efficacy while reducing adverse effects. The challenge in controlling drug release by endogenous signal activation is the complex body environment. Most tumor models for platinum drug loaded hydrogels lack tumor-specific microenvironment. Therefore, newer spontaneous or induced tumor animal models are needed to reflect the sustained release effect of hydrogel drug delivery systems in different tumor microenvironments more accurately, in order to optimize the use of human anti-cancer drugs. Furthermore, the versatility of hydrogel formulations allows for easy customization and modification to suit specific clinical needs. For instance, hydrogels can be engineered to respond to specific stimuli, such as pH or temperature, enabling triggered drug release at the desired site and time. External physical stimuli such as light, magnetism, electricity and ultrasound can also be used to control drug release in hydrogels. For instance, magnetic supramolecular hydrogels containing Fe3O4 nanoparticles could regulate the release of DOX and PTX through the magnetocaloric effect (Wu et al., Citation2018). Calcium-crosslinked alginate hydrogel technology could deliver 5-FU and Irino in response to remote ultrasonic stimulation (Emi et al., Citation2019). For external physical stimuli, the key to effectively control the drug release is to find appropriate intensity, frequency and duration to avoid damage to normal tissues and hydrogel matrix, so as to achieve the desired therapeutic effect.
It is important to highlight that the combination of platinum-based drugs and hydrogels in clinical applications is still largely in its early stages, with ongoing research and development. However, the promising preclinical results and the unique advantages of this combination make it a potential candidate for further exploration and translation into clinical settings. In conclusion, this review include a prospect for the clinical applications of platinum-based drugs and hydrogel combination, highlighting their potential in improving cancer treatment outcomes and discussing the opportunities and challenges for their future use in a clinical setting.
Disclosure statement
The authors report there are no competing interests to declare.
Data availability statement
There is no data was used for this manuscript.
Additional information
Funding
References
- Abdel-Bar HM, Abdel-Reheem AY, Osman R, et al. (2014). Defining cisplatin incorporation properties in thermosensitive injectable biodegradable hydrogel for sustained delivery and enhanced cytotoxicity. Int J Pharm 477:1–18. doi:10.1016/j.ijpharm.2014.11.005.
- Abdel-Bar HM, Osman R, Abdel-Reheem AY, et al. (2016). Tunable biodegradable nanocomposite hydrogel for improved cisplatin efficacy on HCT-116 colorectal cancer cells and decreased toxicity in rats. Biomacromolecules 17:407–14. doi:10.1021/acs.biomac.5b01206.
- Abuzar SM, Ahn J-H, Park KS, et al. (2019). Pharmacokinetic profile and anti-adhesive effect of oxaliplatin-PLGA microparticle-loaded hydrogels in rats for colorectal cancer treatment. Pharmaceutics 11:392. doi:10.3390/pharmaceutics11080392.
- Alamzadeh Z, Beik J, Mirrahimi M, et al. (2020). Gold nanoparticles promote a multimodal synergistic cancer therapy strategy by co-delivery of thermo-chemo-radio therapy. Eur J Pharm Sci 145:105235. doi:10.1016/j.ejps.2020.105235.
- Anirudhan TS, Mohan M, Rajeev MR. (2022). Modified chitosan-hyaluronic acid based hydrogel for the pH-responsive Co-delivery of cisplatin and doxorubicin. Int J Biol Macromol 201:378–88. doi:10.1016/j.ijbiomac.2022.01.022.
- Barkat K, Ahmad M, Minhas MU, et al. (2020). Chondroitin sulfate-based smart hydrogels for targeted delivery of oxaliplatin in colorectal cancer: preparation, characterization and toxicity evaluation. Polym Bull 77:6271–97. doi:10.1007/s00289-019-03062-w.
- Becker JP, Weiss J, Theile D. (2014). Cisplatin, oxaliplatin, and carboplatin unequally inhibit in vitro mRNA translation. Toxicol Lett 225:43–7. doi:10.1016/j.toxlet.2013.11.015.
- Bruno PM, Liu Y, Park GY, et al. (2017). A subset of platinum-containing chemotherapeutic agents kills cells by inducing ribosome biogenesis stress. Nat Med 23:461–71. doi:10.1038/nm.4291.
- Carter T, Qi G, Wang W, et al. (2021). Self-assembling peptide solution accelerates hemostasis. Adv Wound Care (New Rochelle) 10:191–203. doi:10.1089/wound.2019.1109.
- Chen T-Y, Tsai M-J, Chang L-C, et al. (2020). Co-delivery of cisplatin and gemcitabine via viscous nanoemulsion for potential synergistic intravesical chemotherapy. Pharmaceutics 12:949. doi:10.3390/pharmaceutics12100949.
- Cheng C, Xia D, Zhang X, et al. (2015). Biocompatible poly(N-isopropylacrylamide)-g-carboxymethyl chitosan hydrogels as carriers for sustained release of cisplatin. J Mater Sci 50:4914–25. doi:10.1007/s10853-015-9036-7.
- Cheng C, Zhang X, Meng Y, et al. (2017). Development of a dual drug-loaded hydrogel delivery system for enhanced cancer therapy: in situ formation, degradation and synergistic antitumor efficiency. J Mater Chem B 5:8487–97. doi:10.1039/c7tb02173a.
- Cheng Y, He C, Xiao C, et al. (2012). Decisive role of hydrophobic side groups of polypeptides in thermosensitive gelation. Biomacromolecules 13:2053–9. doi:10.1021/bm3004308.
- Chirani N, Yahia LH, Gritsch L, et al. (2015). History and applications of hydrogels. J Biomed Sci 04:13–23. doi:10.4172/2254-609X.100013.
- Cong Z, Shi Y, Wang Y, et al. (2018). A novel controlled drug delivery system based on alginate hydrogel/chitosan micelle composites. Int J Biol Macromol 107:855–64. doi:10.1016/j.ijbiomac.2017.09.065.
- Cui H, Webber MJ, Stupp SI. (2010). Self-assembly of peptide amphiphiles: from molecules to nanostructures to biomaterials. Biopolymers 94:1–18. doi:10.1002/bip.21328.
- Davoodi P, Ng WC, Srinivasan MP, et al. (2017). Codelivery of anti-cancer agents via double-walled polymeric microparticles/injectable hydrogel: a promising approach for treatment of triple negative breast cancer. Biotechnol Bioeng 114:2931–46. doi:10.1002/bit.26406.
- Davoodi P, Ng WC, Yan WC, et al. (2016). Double-walled microparticles-embedded self-cross-linked, injectable, and antibacterial hydrogel for controlled and sustained release of chemotherapeutic agents. ACS Appl Mater Interf 8:22785–800. doi:10.1021/acsami.6b03041.
- Deng J-Y, Liu Y, Hu Z-Y, et al. (2007). Synthesis and photophysical and electrochemical properties of new cyclometalated platinum complex containing oxadiazole ligand. J Cent South Univ Technol 14:344–7. doi:10.1007/s11771-007-0068-2.
- Dilruba S, Kalayda GV. (2016). Platinum-based drugs: past, present and future. Cancer Chemother Pharmacol 77:1103–24. doi:10.1007/s00280-016-2976-z.
- Dobrucka R, Romaniuk-Drapala A, Kaczmarek M. (2019). Evaluation of biological synthesized platinum nanoparticles using Ononidis radix extract on the cell lung carcinoma A549. Biomed Microdevices 21:75. doi:10.1007/s10544-019-0424-7.
- Dong L, Chen H, Liu T, et al. (2021). Poly(l-cysteine) peptide amphiphile derivatives containing disulfide bonds: synthesis, self-assembly-induced β-sheet nanostructures, pH/reduction dual response, and drug release. Biomacromolecules 22:5374–81. doi:10.1021/acs.biomac.1c01324.
- El-Kareh AW, Secomb TW. (2004). A theoretical model for intraperitoneal delivery of cisplatin and the effect of hyperthermia on drug penetration distance. Neoplasia 6:117–27. doi:10.1593/neo.03205.
- El-Sayed IH, Huang X, El-Sayed MA. (2006). Selective laser photo-thermal therapy of epithelial carcinoma using anti-EGFR antibody conjugated gold nanoparticles. Cancer Lett 239:129–35. doi:10.1016/j.canlet.2005.07.035.
- El-Shafie S, Fahmy SA, Ziko L, et al. (2020). Encapsulation of nedaplatin in novel PEGylated liposomes increases its cytotoxicity and genotoxicity against A549 and U2OS human cancer cells. Pharmaceutics 12:863. doi:10.3390/pharmaceutics12090863.
- Emi T, Michaud K, Orton E, et al. (2019). Ultrasonic generation of pulsatile and sequential therapeutic delivery profiles from calcium-crosslinked alginate hydrogels. Molecules 24:1048. doi:10.3390/molecules24061048.
- Emoto S, Yamaguchi H, Kamei T, et al. (2014). Intraperitoneal administration of cisplatin via an in situ cross-linkable hyaluronic acid-based hydrogel for peritoneal dissemination of gastric cancer. Surg Today 44:919–26. doi:10.1007/s00595-013-0674-6.
- Galluzzi L, Senovilla L, Vitale I, et al. (2012). Molecular mechanisms of cisplatin resistance. Oncogene 31:1869–83. doi:10.1038/onc.2011.384.
- Ghosh S. (2019). Cisplatin: the first metal based anticancer drug. Bioorg Chem 88:102925. doi:10.1016/j.bioorg.2019.102925.
- Ghosh SC, Alpay SN, Klostergaard J. (2012). CD44: a validated target for improved delivery of cancer therapeutics. Expert Opin Ther Targets 16:635–50. doi:10.1517/14728222.2012.687374.
- Gil MS, Thambi T, Phan VHG, et al. (2017). Injectable hydrogel-incorporated cancer cell-specific cisplatin releasing nanogels for targeted drug delivery. J Mater Chem B 5:7140–52. doi:10.1039/c7tb00873b.
- Gong X, Wei H, Luo K-J, et al. (2014). UV-Vis spectrum and the third-order nonlinear optical properties of the chiral camphor-derived beta-diketonate platinum complexes. Chin J Struct Chem 33:422–8.
- Guo Y, Jin S, Yuan H, et al. (2022). DNA-unresponsive platinum(II) complex induces ERS-mediated mitophagy in cancer cells. J Med Chem 65:520–30. doi:10.1021/acs.jmedchem.1c01690.
- Ha D, Yang N, Nadithe V. (2016). Exosomes as therapeutic drug carriers and delivery vehicles across biological membranes: current perspectives and future challenges. Acta Pharm Sin B 6:287–96. doi:10.1016/j.apsb.2016.02.001.
- Hato SV, Andrea K, de Vries IJM, et al. (2014). Molecular pathways: the immunogenic effects of platinum-based chemotherapeutics. Clin Cancer Res 20:2831–7. doi:10.1158/1078-0432.ccr-13-3141.
- Hato SV, de Vries IJM, Lesterhuis WJ. (2012). STATing the importance of immune modulation by platinum chemotherapeutics. Oncoimmunology 1:234–6. doi:10.4161/onci.1.2.18126.
- Hedman HK, Kirpekar F, Elmroth SKC. (2011). Platinum interference with siRNA non-seed regions fine-tunes silencing capacity. J Am Chem Soc 133:11977–84. doi:10.1021/ja111082e.
- Hostetter AA, Osborn MF, DeRose VJ. (2012). RNA-Pt adducts following cisplatin treatment of Saccharomyces cerevisiae. ACS Chem Biol 7:218–25. doi:10.1021/cb200279p.
- Huang K-B, Wang F-Y, Feng H-W, et al. (2019). An aminophosphonate ester ligand-containing platinum(II) complex induces potent immunogenic cell death in vitro and elicits effective anti-tumour immune responses in vivo. Chem Commun (Camb) 55:13066–9. doi:10.1039/c9cc06563f.
- Hyun H, Park MH, Lim W, et al. (2018). Injectable visible light-cured glycol chitosan hydrogels with controlled release of anticancer drugs for local cancer therapy in vivo: a feasible study. Artif Cells Nanomed Biotechnol 46:874–82. doi:10.1080/21691401.2018.1470529.
- Jin S, Guo Y, Guo Z, et al. (2021). Monofunctional platinum(II) anticancer agents. Pharmaceuticals (Basel) 14:133. doi:10.3390/ph14020133.
- Jing Z, Ni R, Wang J, et al. (2021). Practical strategy to construct anti-osteosarcoma bone substitutes by loading cisplatin into 3D-printed titanium alloy implants using a thermosensitive hydrogel. Bioact Mater 6:4542–57. doi:10.1016/j.bioactmat.2021.05.007.
- Joshi N, Yan J, Levy S, et al. (2018). Towards an arthritis flare-responsive drug delivery system. Nat Commun 9:1275. doi:10.1038/s41467-018-03691-1.
- Kalayda GV, Wagner CH, Jaehde U. (2012). Relevance of copper transporter 1 for cisplatin resistance in human ovarian carcinoma cells. J Inorg Biochem 116:1–10. doi:10.1016/j.jinorgbio.2012.07.010.
- Kanda Y, Kakutani K, Yurube T, et al. (2021). A novel topical treatment for bone metastases using a gelatin hydrogel incorporating cisplatin as a sustained release system. J Orthop Res 39:525–35. doi:10.1002/jor.24874.
- Keshavarz M, Moloudi K, Paydar R, et al. (2018). Alginate hydrogel co-loaded with cisplatin and gold nanoparticles for computed tomography image-guided chemotherapy. J Biomater Appl 33:161–9. doi:10.1177/0885328218782355.
- Ketabat F, Pundir M, Mohabatpour F, et al. (2019). Controlled drug delivery systems for oral cancer treatment-current status and future perspectives. Pharmaceutics 11:302. doi:10.3390/pharmaceutics11070302.
- Khoury A, Deo KM, Aldrich-Wright JR. (2020). Recent advances in platinum-based chemotherapeutics that exhibit inhibitory and targeted mechanisms of action. J Inorg Biochem 207:111070. doi:10.1016/j.jinorgbio.2020.111070.
- Kouser R, Vashist A, Zafaryab M, et al. (2018). Na-montmorillonite-dispersed sustainable polymer nanocomposite hydrogel films for anticancer drug delivery. ACS Omega 3:15809–20. doi:10.1021/acsomega.8b01691.
- Lee JE, Abuzar SM, Seo Y, et al. (2019). Oxaliplatin-loaded chemically cross-linked hydrogels for prevention of postoperative abdominal adhesion and colorectal cancer therapy. Int J Pharm 565:50–8. doi:10.1016/j.ijpharm.2019.04.065.
- Lee KY, Mooney DJ. (2012). Alginate: properties and biomedical applications. Prog Polym Sci 37:106–26. doi:10.1016/j.progpolymsci.2011.06.003.
- Li J, Gong C, Feng X, et al. (2012). Biodegradable thermosensitive hydrogel for SAHA and DDP delivery: therapeutic effects on oral squamous cell carcinoma xenografts. Plos One 7:e33860. doi:10.1371/journal.pone.0033860.
- Li J, Mooney DJ. (2016). Designing hydrogels for controlled drug delivery. Nat Rev Mater 1:16071. doi:10.1038/natrevmats.2016.71.
- Li M, Li S, Zhou H, et al. (2020). Chemotaxis-driven delivery of nano-pathogenoids for complete eradication of tumors post-phototherapy. Nat Commun 11:1126. doi:10.1038/s41467-020-14963-0.
- Liang H-KT, Lai X-S, Wei M-F, et al. (2018). Intratumoral injection of thermogelling and sustained-release carboplatin-loaded hydrogel simplifies the administration and remains the synergistic effect with radiotherapy for mice gliomas. Biomaterials 151:38–52. doi:10.1016/j.biomaterials.2017.10.015.
- Liang J, Liu G, Wang J, et al. (2017). Controlled release of BSA-linked cisplatin through a PepGel self-assembling peptide nanofiber hydrogel scaffold. Amino Acids 49:2015–21. doi:10.1007/s00726-017-2444-z.
- Lin G, Mi P, Chu C, et al. (2016). Inorganic nanocarriers overcoming multidrug resistance for cancer theranostics. Adv Sci (Weinh) 3:1600134. doi:10.1002/advs.201600134.
- Loghmani MH, Shojaie AF, Hosseini SA. (2021). Glutathione-responsive hydrogel and molecularly imprinted polymer nanospheres: new aspect on cisplatin delivery. J Ind Eng Chem 96:98–108. doi:10.1016/j.jiec.2020.12.018.
- Lu QB. (2007). Molecular reaction mechanisms of combination treatments of low-dose cisplatin with radiotherapy and photodynamic therapy. J Med Chem 50:2601–4. doi:10.1021/jm061416b.
- Mahdavinia GR, Afzali A, Etemadi H, et al. (2017). Magnetic/pH-sensitive nanocomposite hydrogel based carboxymethyl cellulose –g-polyacrylamide/montmorillonite for colon targeted drug delivery. Nanomed Res J 2:111–22. doi:10.22034/nmrj.2017.58964.1058.
- Mahkam M, Doostie L. (2005). The relation between swelling properties and cross-linking of hydrogels designed for colon-specific drug delivery. Drug Deliv 12:343–7. doi:10.1080/10717540590952627.
- Mirrahimi M, Beik J, Mirrahimi M, et al. (2020). Triple combination of heat, drug and radiation using alginate hydrogel co-loaded with gold nanoparticles and cisplatin for locally synergistic cancer therapy. Int J Biol Macromol 158:617–26. doi:10.1016/j.ijbiomac.2020.04.272.
- Mirrahimi M, Khateri M, Beik J, et al. (2019). Enhancement of chemoradiation by co-incorporation of gold nanoparticles and cisplatin into alginate hydrogel. J Biomed Mater Res B Appl Biomater 107:2658–63. doi:10.1002/jbm.b.34356.
- Mitsushima S, Koizumi Y, Uzuka S, et al. (2008). Dissolution of platinum in acidic media. Electrochim Acta 54:455–60. doi:10.1016/j.electacta.2008.07.052.
- Moura MJ, Gil MH, Figueiredo MM. (2013). Delivery of cisplatin from thermosensitive co-cross-linked chitosan hydrogels. Eur Polym J 49:2504–10. doi:10.1016/j.eurpolymj.2013.02.032.
- Muggia FM, Bonetti A, Hoeschele JD, et al. (2015). Platinum antitumor complexes: 50 years since Barnett Rosenberg’s discovery. J Clin Oncol 33:4219–26. + doi:10.1200/jco.2015.60.7481.
- Němec T, Šonský J, Gruber J, et al. (2020). Platinum and platinum oxide nanoparticles generated by unipolar spark discharge. J Aerosol Sci 141:105502. doi:10.1016/j.jaerosci.2019.105502.
- Obuobi S, Tay HK, Tram NDT, et al. (2019). Facile and efficient encapsulation of antimicrobial peptides via crosslinked DNA nanostructures and their application in wound therapy. J Control Release 313:120–30. doi:10.1016/j.jconrel.2019.10.013.
- Ohta S, Hiramoto S, Amano Y, et al. (2017). Intraperitoneal delivery of cisplatin via a hyaluronan-based nanogel/in situ cross-linkable hydrogel hybrid system for peritoneal dissemination of gastric cancer. Mol Pharm 14:3105–13. doi:10.1021/acs.molpharmaceut.7b00349.
- Otto G, Schuchmann M, Hoppe-Lotichius M, et al. (2013). How to decide about liver transplantation in patients with hepatocellular carcinoma: size and number of lesions or response to TACE? J Hepatol 60:464–5. doi:10.1016/j.jhep.2013.04.006.
- Parker JP, Ude Z, Marmion CJ. (2016). Exploiting developments in nanotechnology for the preferential delivery of platinum-based anticancer agents to tumours: targeting some of the hallmarks of cancer. Metallomics 8:43–60. doi:10.1039/c5mt00181a.
- Peng H, Huang Q, Yue H, et al. (2019). The antitumor effect of cisplatin-loaded thermosensitive chitosan hydrogel combined with radiotherapy on nasopharyngeal carcinoma. Int J Pharm 556:97–105. doi:10.1016/j.ijpharm.2018.11.068.
- Qian K, Qian H, Cai J, et al. (2019). Evaluation of cisplatin-hydrogel for improving localized antitumor efficacy in gastric cancer. Pathol Res Pract 215:755–60. doi:10.1016/j.prp.2019.01.005.
- Rebillard A, Lagadic-Gossmann D, Dimanche-Boitrel M-T. (2008). Cisplatin cytotoxicity: DNA and plasma membrane targets. Curr Med Chem 15:2656–63. doi:10.2174/092986708786242903.
- Ren Y, Li X, Han B, et al. (2019). Improved anti-colorectal carcinomatosis effect of tannic acid co-loaded with oxaliplatin in nanoparticles encapsulated in thermosensitive hydrogel. Eur J Pharm Sci 128:279–89. doi:10.1016/j.ejps.2018.12.007.
- Rosenberg B, Van Camp L, Krigas T. (1965). Inhibition of cell division in Escherichia coli by electrolysis products from a platinum electrode. Nature 205:698–9. doi:10.1038/205698a0.
- Rosenberg B, Vancamp L, Trosko JE, et al. (1969). Platinum compounds-a new class of potent antitumour agents. Nature 222:385–6. + doi:10.1038/222385a0.
- Serini S, Cassano R, Bruni M, et al. (2021). Characterization of a hyaluronic acid and folic acid-based hydrogel for cisplatin delivery: antineoplastic effect in human ovarian cancer cells in vitro. Int J Pharm 606:120899. doi:10.1016/j.ijpharm.2021.120899.
- Shen W, Chen X, Luan J, et al. (2017). Sustained codelivery of cisplatin and paclitaxel via an injectable prodrug hydrogel for ovarian cancer treatment. ACS Appl Mater Interf 9:40031–46. doi:10.1021/acsami.7b11998.
- Shen W, Luan J, Cao L, et al. (2015). Thermogelling polymer-platinum(IV) conjugates for long-term delivery of cisplatin. Biomacromolecules 16:105–15. doi:10.1021/bm501220a.
- Tang XZ, Kumar P, Alavi S, et al. (2012). Recent advances in biopolymers and biopolymer-based nanocomposites for food packaging materials. Crit Rev Food Sci Nutr 52:426–42. doi:10.1080/10408398.2010.500508.
- Tchounwou PB, Dasari S, Noubissi FK, et al. (2021). Advances in our understanding of the molecular mechanisms of action of cisplatin in cancer therapy. J Exp Pharmacol 13:303–28. doi:10.2147/jep.s267383.
- Thakur S, Singh H, Singh A, et al. (2020). Thermosensitive injectable hydrogel containing carboplatin loaded nanoparticles: a dual approach for sustained and localized delivery with improved safety and therapeutic efficacy. J Drug Delivery Sci Technol 58:101817. doi:10.1016/j.jddst.2020.101817.
- Theile D. (2017). Under-reported aspects of platinum drug pharmacology. Molecules 22:382. doi:10.3390/molecules22030382.
- Tomita R, Sasabe E, Tomomura A, et al. (2020). Macrophage-derived exosomes attenuate the susceptibility of oral squamous cell carcinoma cells to chemotherapeutic drugs through the AKT/GSK-3 beta pathway. Oncol Rep 44:1905–16. doi:10.3892/or.2020.7748.
- Ullah K, Sohail M, Murtaza G, et al. (2019). Natural and synthetic materials based CMCh/PVA hydrogels for oxaliplatin delivery: fabrication, characterization, in-vitro and in-vivo safety profiling. Int J Biol Macromol 122:538–48. doi:10.1016/j.ijbiomac.2018.10.203.
- Vakili MR, Mohammed-Saeid W, Aljasser A, et al. (2021). Development of mucoadhesive hydrogels based on polyacrylic acid grafted cellulose nanocrystals for local cisplatin delivery. Carbohydr Polym 255:117332. doi:10.1016/j.carbpol.2020.117332.
- Wang Q, Hou X, Gao J, et al. (2020). A coassembled peptide hydrogel boosts the radiosensitization of cisplatin. Chem Commun (Camb) 56:13017–20. doi:10.1039/d0cc05184e.
- Wang Q, Jiang N, Fu B, et al. (2019). Self-assembling peptide-based nanodrug delivery systems. Biomater Sci 7:4888–911. doi:10.1039/c9bm01212e.
- Wang X, Wang J, Wu W, et al. (2016). Vaginal delivery of carboplatin-loaded thermosensitive hydrogel to prevent local cervical cancer recurrence in mice. Drug Deliv 23:3544–51. doi:10.1080/10717544.2016.1205158.
- Wang Y, Wang L, Chen G, et al. (2017). Carboplatin-complexed and cRGD-conjugated unimolecular nanoparticles for targeted ovarian cancer therapy. Macromol Biosci 17:10.1002. doi:10.1002/mabi.201600292.
- Wen Q, Zhang Y, Luo J, et al. (2020). Therapeutic efficacy of thermosensitive pluronic hydrogel for codelivery of resveratrol microspheres and cisplatin in the treatment of liver cancer ascites. Int J Pharm 582:119334. doi:10.1016/j.ijpharm.2020.119334.
- Wu C, Liu J, Zhai Z, et al. (2020). Double-crosslinked nanocomposite hydrogels for temporal control of drug dosing in combination therapy. Acta Biomater 106:278–88. doi:10.1016/j.actbio.2020.02.021.
- Wu C, Wang C, Sun L, et al. (2020). PLGA nanoparticle-reinforced supramolecular peptide hydrogels for local delivery of multiple drugs with enhanced synergism. Soft Matter 16:10528–36. doi:10.1039/d0sm01152e.
- Wu H, Song L, Chen L, et al. (2018). Injectable magnetic supramolecular hydrogel with magnetocaloric liquid-conformal property prevents post-operative recurrence in a breast cancer model. Acta Biomater 74:302–11. doi:10.1016/j.actbio.2018.04.052.
- Wu X, Wu Y, Ye H, et al. (2017). Interleukin-15 and cisplatin co-encapsulated thermosensitive polypeptide hydrogels for combined immuno-chemotherapy. J Control Release 255:81–93. doi:10.1016/j.jconrel.2017.04.011.
- Xiangsheng L, Jinhong J, Chong Hyun C, et al. (2021). Development of facile and versatile platinum drug delivering silicasome nanocarriers for efficient pancreatic cancer chemo-immunotherapy. Small 17:e2005993. doi:10.1002/smll.202005993.
- Xiao S, Wang Y, Ma W, et al. (2022). Intraperitoneal administration of thermosensitive hydrogel Co-loaded with norcantharidin nanoparticles and oxaliplatin inhibits malignant ascites of hepatocellular carcinoma. Drug Deliv 29:2713–22. doi:10.1080/10717544.2022.2111480.
- Xu S, Du X, Feng G, et al. (2018). Efficient inhibition of cervical cancer by dual drugs loaded in biodegradable thermosensitive hydrogel composites. Oncotarget 9:282–92. doi:10.18632/oncotarget.22965.
- Xu S, Tang YY, Yu YX, et al. (2017). Novel composite drug delivery system as a novel radio sensitizer for the local treatment of cervical carcinoma. Drug Deliv 24:1139–47. doi:10.1080/10717544.2017.1362676.
- Xu T, Liang C, Zheng D, et al. (2020). Nuclear delivery of dual anticancer drug-based nanomedicine constructed by cisplatinum-induced peptide self-assembly. Nanoscale 12:15275–82. doi:10.1039/d0nr00143k.
- Yamaguchi K, Hiraike O, Iwaki H, et al. (2021). Intraperitoneal administration of a cisplatin-loaded nanogel through a hybrid system containing an alginic acid-based nanogel and an in situ cross-linkable hydrogel for peritoneal dissemination of ovarian cancer. Mol Pharm 18:4090–8. doi:10.1021/acs.molpharmaceut.1c00514.
- Yamashita K, Tsunoda S, Gunji S, et al. (2019). Intraperitoneal chemotherapy for peritoneal metastases using sustained release formula of cisplatin-incorporated gelatin hydrogel granules. Surg Today 49:785–94. doi:10.1007/s00595-019-01792-y.
- Yang C-X, Xing L, Chang X, et al. (2020). Synergistic platinum(II) prodrug nanoparticles for enhanced breast cancer therapy. Mol Pharm 17:1300–9. doi:10.1021/acs.molpharmaceut.9b01318.
- Yang X, Yeung W-HO, Tan KV, et al. (2021). Development of cisplatin-loaded hydrogels for trans-portal vein chemoembolization in an orthotopic liver cancer mouse model. Drug Deliv 28:520–9. doi:10.1080/10717544.2021.1895908.
- Yang X, Zhang L, Zheng L, et al. (2022). An in situ spontaneously forming micelle-hydrogel system with programmable release for the sequential therapy of anaplastic thyroid cancer. J Mater Chem B 10:1236–49. doi:10.1039/d1tb01904j.
- Yoon SJ, Moon YJ, Chun HJ, et al. (2019). Doxorubicin center dot hydrochloride/cisplatin-loaded hydrogel/nanosized (2-hydroxypropyl)-beta-cyclodextrin local drug-delivery system for osteosarcoma treatment in vivo. Nanomaterials 9:1652. doi:10.3390/nano9121652.
- Yu C, Wang Z, Sun Z, et al. (2020). Platinum-based combination therapy: molecular rationale, current clinical uses, and future perspectives. J Med Chem 63:13397–412. doi:10.1021/acs.jmedchem.0c00950.
- Yu S, Wei S, Liu L, et al. (2019). Enhanced local cancer therapy using a CA4P and CDDP co-loaded polypeptide gel depot. Biomater Sci 7:860–6. doi:10.1039/c8bm01442f.
- Yu S, Zhang D, He C, et al. (2017). Injectable thermosensitive polypeptide-based CDDP-complexed hydrogel for improving localized antitumor efficacy. Biomacromolecules 18:4341–8. doi:10.1021/acs.biomac.7b01374.
- Yuan S, Ding X, Cui Y, et al. (2017). Cisplatin preferentially binds to zinc finger proteins containing C3H1 or C4 Motifs. Eur J Inorg Chem 2017:1778–84. doi:10.1002/ejic.201601140.
- Zhang S, Lovejoy KS, Shima JE, et al. (2006). Organic cation transporters are determinants of oxaliplatin cytotoxicity. Cancer Res 66:8847–57. doi:10.1158/0008-5472.can-06-0769.
- Zhang Z, He C, Chen X. (2020). Injectable click polypeptide hydrogels via tetrazine-norbornene chemistry for localized cisplatin release. Polymers (Basel) 12:884. doi:10.3390/polym12040884.
- Zhou Z, Huang D, Bao J, et al. (2012). A synergistically enhanced T-1-T-2 dual-modal contrast agent. Adv Mater 24:6223–8. doi:10.1002/adma.201203169.