Abstract
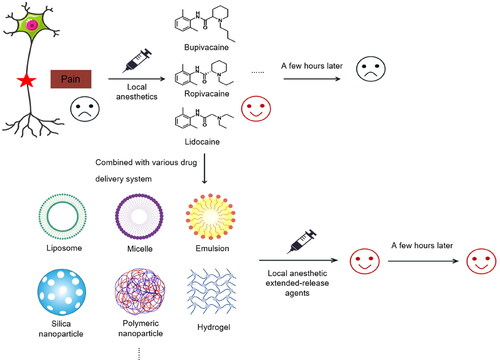
Pain management remains among the most common and largely unmet clinical problems today. Local anesthetics play an indispensable role in pain management. The main limitation of traditional local anesthetics is the limited duration of a single injection. To address this problem, catheters are often placed or combined with other drugs in clinical practice to increase the time that local anesthetics act. However, this method does not meet the needs of clinical analgesics. Therefore, many researchers have worked to develop local anesthetic extended-release types that can be administered in a single dose. In recent years, drug extended-release systems have emerged dramatically due to their long duration and efficacy, providing more possibilities for the application of local anesthetics. This paper summarizes the types of local anesthetic drug delivery systems and their clinical applications, discusses them in the context of relevant studies on local anesthetics, and provides a summary and outlook on the development of local anesthetic extended-release agents.
1. Introduction
With an aging population and emergence of fast-paced lifestyles in recent years in recent years, pain can be caused by a variety of factors such as physical stress, emotional highs and lows, and social and cultural pressures; thus, the need for pain management is increasing (Mills et al., Citation2019). Rational medication use is essential for pain management. According to the three-step approach to pain management, opioid analgesics are the most effective drugs, but due to their potentially addictive nature, opioid analgesics are commonly abused and involve, many serious complications, causing serious public health and social problems (Stayner & Copenhaver, Citation2012; Dowell et al., Citation2016; Andreu & Arruebo, Citation2018). To improve the reliability and safety of pain treatment, a variety of nonopioid drugs as well as new analgesic techniques are gradually being used in clinical practice. Local anesthetics exhibit several advantages, as they have diverse forms of application, widespread clinical applications, relatively few complications, and low impacts on the physiological function of the patient; thus, local anesthetics occupy an important position in pain treatment and are undoubtedly among the first choices for development and utilization (Barletta & Reed, Citation2019).
However, the current clinical management of pain based on local anesthetics, whether it is nerve blocks or catheter placement, is greatly limited by the short duration of action generated by a single injection. Moreover, it can be accompanied by many complications, such as catheter displacement, obstruction, local hematoma, nerve injury, infection, and local anesthetic toxicity (Jeng et al., Citation2010). Therefore, a sustained-release dosage form that can release local anesthetics for a long period is urgently needed to solve these problems in the clinical setting.
With the continuous development of material science and technology, several types of drug delivery carriers have been developed, including liposomes, microemulsions, polymer-based carriers, metallic particles, and others (Joudeh & Linke, Citation2022), all of which have been investigated in the context of local anesthetic agents. In this paper, we will initially summarize various local anesthetic drug delivery carriers and analyze their advantages and disadvantages, as well as discuss them in the context of relevant studies on local anesthetics. Furthermore, a summary and outlook on the clinical applications and developments related to local anesthetic retardants will be provided.
2. Lipid-based local anesthetic drug delivery system
2.1. Liposomes
Liposomes are spherical vesicles with a hydrophilic nucleus surrounded by a phospholipid bilayer, which can be loaded with both hydrophilic and hydrophobic substances (Euliss et al., Citation2006). As first described by Bangham in 1965, liposome were the first nano drug delivery system to transition from conceptual to clinical application and are highly accepted in the clinic (Allen & Cullis, Citation2013; Beiranvand et al., Citation2016). Liposomes are biodegradable, soluble, nonimmunogenic, and inexpensive; exhibit low toxicity; and have proven to be an effective carrier for a variety of drugs, such as antineoplastic drugs, insulin, amphotericin B, methotrexate, and local anesthetics, etc. (Chang & Yeh, Citation2012; Beiranvand et al., Citation2016; de Araújo et al., Citation2019).
Bupivacaine liposome suspension for injection (trade name Exparel™) is a long-acting local anesthetic formed by the DepoFoam technology, which consists of multivesicular liposomes (DepoFoam particles). A single division of the outer membrane of the DepoFoam particles releases only a portion of the bupivacaine within them, so this multivesicular nature also provides for the sustained release of bupivacaine () (Ye et al., Citation2000; Lu B et al., Citation2021). Bupivacaine liposome suspension for injection was approved for marketing by the FDA in 2011, making it the first local anesthetic extended-release formulation approved for clinical use. The FDA approved Exparel™ primarily for postoperative local infiltration anesthesia, cystectomy, and hemorrhoidectomy and later approved the intermuscular sulcus approach for brachial plexus block (Kaye, Armstead-Williams, et al., Citation2020; Kaye, Novitch, et al., Citation2020). In addition, it has applications related to knee and hip replacements, hysterectomies, third molar extractions and cesarean sections, mastectomies, open-heart surgeries, and other types of surgery (Hutchins et al., Citation2015; Jacob et al., Citation2017; Lieblich & Danesi, Citation2017; Chiu et al., Citation2018; Prabhu et al., Citation2018; Lee CY et al., Citation2019).
Figure 1. Flow chart of DepoFoam technology and structural characteristics of bupivacaine multivesicular liposomes. Reprinted with permission from Lu B et al. (Citation2021), copyright© 2021 Elsevier B.V.

However, the results obtained by a series of recent clinical studies with bupivacaine liposomes raised concerns regarding their efficacy and value. The results obtained by many different clinical studies have shown no significant differences with standard bupivacaine preparations (Ottoboni et al., Citation2020). In addition, bupivacaine liposomes do not reduce postoperative opioid use in some procedures, and their role in improving postoperative pain and accelerating functional recovery is unclear (Cloyd et al., Citation2018). Due to the strong mobility, poor stability, and low drug loading capacity of liposomes, several problems remained to be solved; for example, the drug encapsulation rates should be increased and drug leakage should be reduced (Rideau et al., Citation2018).
2.2. Solid lipid nanoparticles
Solid lipid nanoparticles (SLNs) are a solid drug delivery systems consisting of solid lipids and surfactants with an average diameter of 50–1000 nm, which are solid at room temperature and can bind lipophilic or hydrophilic drugs. To overcome the problems caused by poor stability of physicochemical properties and low encapsulation efficiency of hydrophobic drugs, SLNs were created as a substitute for traditional carriers (liposomes, emulsions, particles, etc.) (Wang J et al., Citation2012; Andreu & Arruebo, Citation2018). Therefore, SLNs are particularly suitable for encapsulating hydrophobic drugs. SLNs have the advantages of good stability, resistance to chemical degradation, controlled release, targeting capabilities, biocompatibility and biodegradability, low toxicity, and sterilization; furthermore, SLNs can be easily mass produced (Wang J et al., Citation2012; Rajpoot, Citation2019).
Pathak, P. et al. used ultrasonic dispersion to prepare lidocaine SLNs and lidocaine nanostructured lipid carriers (NLCs) hydrogels for application on the skin surface of guinea pigs. The duration of anesthesia was increased 5-fold and 6-fold, respectively, compared with that of lidocaine gel alone (Pathak & Nagarsenker, Citation2009). Leng, F. et al. used different lipids, such as monostearin (MS), glyceryl palmitostearate (GP), and stearic acid (SA), to prepare lidocaine-loaded SLN for epidural anesthesia. The duration of epidural block was prolonged for different times with each lipid class (Leng et al., Citation2012). However, SLNs also exhibits some limitations, as the drug migration rate in the solid state being reduced compared to the oil state; in addition, the drug loading capacity and drug efflux are insufficient during production and storage (Wang J et al., Citation2012).
2.3. Nanostructured lipid carriers
Nanostructured lipid carriers (NLCs) are the second generation of lipid nanoparticles. Compared with SLNs, NLCs add liquid lipids and contain a mixture of liquid and solid lipids; as a result, when the temperature drops, the drug is unlikely to precipitate out of the solid lipids. thus, the limitations of SLNs are overcome, drug loading is increased, drug efflux is reduced, and the drug release are properties; in addition, the risk of drug loss during the curing step in the synthesis process and storage is reduced () (Wang J et al., Citation2012; Rajpoot, Citation2019; de Souza Guedes et al., Citation2021).
Figure 2. SLN and NLC structure model diagram. Reprinted with permission from de Souza Guedes et al. (Citation2021), copyright© 2021 MDPI.

Some investigators prepared SLNs and NLCs coloaded with lidocaine and proparacaine, and the results showed that the NLCs system induced more significant anesthesia in comparison, but the permeation efficiency of SLNs was superior to that of NLCs (You et al., Citation2017). Liu and Zhao used SLNs and NLCs loaded with tetracaine and similarly found that NLCs excelled in terms of analgesic duration and analgesic effect, while SLNs exhibited the best in vitro penetration efficiency (Liu & Zhao, Citation2019). Overall, both SLNs and NLCs have their advantages, and both carriers are promising dual delivery systems for surface anesthesia and analgesia. In addition, lidocaine-loaded NLCs developed by Suo, Meng et al. were noticeably more effective during skin surface anesthesia (10 h) compared to commercially available ointments (4 h), and the effect of sciatic nerve block was also significantly prolonged (Suo et al., Citation2020).
2.4. Lipid nanocapsules
Lipid nanocapsules (LNCs) are a type of nanocarrier prepared by the phase-inversion temperature (PIT) method. It consists of an oily lipid nucleus surrounded by a nonionic surfactant layer. The larger the number of surfactants, the smaller the size of the LNCs and the larger the contact area compared to SLNs and NLCs (Cordeiro Lima Fernandes et al., Citation2021). This results in very small particles (<100 nm) (de Araújo et al., Citation2019). The LNCs is highly stable and has an efficient drug-loading capacity with a much higher encapsulation rate than that of liposomes (Huynh et al., Citation2009). Organic solvents are not needed during the preparation of LNCs and the potential for toxicity after skin application is eliminated. In addition, LNCs has superiority in contact with the stratum corneum, which makes it more suitable for dermal administration (Zhai et al., Citation2014).
Zhai Y et al. prepared ropivacaine-loaded LNCs for in vitro transdermal administration to the dorsum of mice. The results showed that the cumulative penetration of ropivacaine-loaded LNCs in isolated mouse skin was more than twice that of propylene glycol ropivacaine (control), and the ropivacaine content in the dermis was increased compared with that in the control group. The interaction of LNCs with the skin altered the morphology of the stratum corneum, thereby enhancing skin penetration and increasing the surface anesthetic effects of local anesthetics (Zhai et al., Citation2014). In other studies, lipid nanocapsules loaded with lidocaine and prilocaine were mixed with carbopol gel, which have a 4-fold increase in anesthesia time compared to that of gels preparations prepared without LNCs and does not cause skin damage and can be used as a preanesthetic for mouth (Cordeiro Lima Fernandes et al., Citation2021).
2.5. Microemulsions
Microemulsions (MEs) consist of an oil phase, water phase, surfactant, and cosurfactant, and can be divided into water-in-oil (w/o) type, water-in-oil (o/w) type, and two-phase continuous type according to the structure (Karasulu, Citation2008; Gradzielski et al., Citation2021). MEs systems exhibit several advantages, including a strong solubilizing power, which can dissolve various drugs with different polarities and improve the solubility and bioavailability of drugs; thermodynamic stability, which enhances the stability of drugs; optical clarity, which is easy to prepare; strong permeability, high levels of diffusion, and a high absorption rate, and the ability to easily penetration into the keratin layer (Vadlamudi et al., Citation2014; Daryab et al., Citation2022). Therefore, MEs are a good vehicle for drug delivery.
The ratio of MEs components and the physicochemical properties of the local anesthetic are considered crucial to obtain a prolonged duration of action of the local anesthetic. MEs drug delivery systems are feasible for multiple modes of administration (injection, oral, transdermal, etc.) and are commonly used for transdermal administration, including lidocaine, benzocaine, bupivacaine, procaine, and many other types of local anesthetics have been studied. In addition, ropivacaine is not usually used for transdermal administration (Sapra et al., Citation2014; Lu I-J et al., Citation2019). Zhao L et al. prepared MEs of ropivacaine and ME gels of ropivacaine for transdermal administration, both of which showed significant analgesic activity () (Zhao L et al., Citation2014).
Figure 3. Ropivacaine microemulsions and microemulsion gels: a scheme for preparation, optimization, and evaluation steps. Reprinted with permission from Zhao L et al. (Citation2014), copyright© 2014 Elsevier B.V.

However, MEs involve many shortcomings as drug carriers. For example, some of the surfactants necessary for MEs are toxic, so the choice of ingredients is somewhat limited. In addition, MEs are sensitive to changes in temperature and salinity, which may lead to structural damage to MEs (Callender et al., Citation2017). Therefore, much potential remains for the development of MEs as drug delivery carriers.
3. Polymer-based local anesthetic drug delivery system
3.1. Natural polymer local anesthetic drug delivery systems
3.1.1. Cellulose
Cellulose is one of the most widely distributed and abundant biopolymers in nature and exhibits a variety of properties, such as renewability, biocompatibility, biodegradability, and nontoxicity (Karimian et al., Citation2019). Cellulose is widely found in plants, animals, and bacteria as a natural material. Cellulose nanoparticles (CNs) are ideal materials for building new biopolymers with properties such as hydrophilicity, high tensile strength, high stiffness, large specific surface area, large aspect, and low coefficient of thermal expansion (Moon et al., Citation2011).
Some researchers have prepared fish-scale nanocellulose composite microneedles (MNs) loaded with lidocaine that can successfully penetrate the stratum corneum, and absorb moisture to separate the drug polymer matrix, thereby releasing the drug from the polymer material. This method not only replaces traditional transdermal drug delivery but also increases the permeability of the skin, thus improving the bioavailability of local anesthetics () (Medhi et al., Citation2017; Lee B-M et al., Citation2020). Hoare et al. used a rheological polymer blend of hyaluronic acid (HA) and hydroxypropylmethyl cellulose (HPMC) as a carrier for bupivacaine to perform sciatic nerve blocks in rats. The duration of the sensory nerve block could be prolonged approximately threefold. This mixture was not cytotoxic and caused only a mild short-term inflammatory response at the injection site (Hoare et al., Citation2010). Georgios K. et al. prepared hydroxypropyl methylcellulose-based film and developed an oral mucosal drug delivery system that enables codelivery of local anesthetics and nonsteroidal anti-inflammatory drugs (NSAIDs) to the oral mucosa for the treatment of oral diseases (Eleftheriadis et al., Citation2020).
Figure 4. Lidocaine cellulose composite microneedle preparation flow chart. (A) Microneedle manufacturing schematic. (B) Transmission electron microscopy (TEM) showing cellulose microfibrils. (C) Lidocaine fish scale biopolymer nanocellulose MNs arrays. Reprinted with permission from Medhi et al. (Citation2017), copyright© 2017 American Association of pharmaceutical scientists.

3.1.2. Chitosan
Chitosan (CH) is the second most abundant natural cationic polysaccharide after cellulose. CH is deacetylated from chitin and is widely used for drug delivery due to its nontoxicity, biodegradability, biocompatibility, and antibacterial, hemostatic, antibacterial and anticancer properties (Ali & Ahmed, Citation2018). Thus far, chitosan has been studied as a carrier for local anesthetics in various routes of administration, including oral, transdermal, injectable, and transmucosal drug delivery (Kp & R, Citation2021; Deng et al., Citation2022; Hou et al., Citation2022).
Zhang Y et al. prepared chitosan (CH)-coated poly(ε-caprolactone) (PCL) nanoparticles to co-deliver ropivacaine and dexamethasone complexes. The results obtained by in vivo and in vitro experiments after transdermal administration in mice showed that CH-PCL nanoparticles can act as effective drug carriers to prolong and enhance the anesthetic effect of ropivacaine (Zhang et al., Citation2017). In other experiments, Ma RR et al. prepared a dressing containing lidocaine using chitosan, porous gelatin, and calcium alginate. This dressing provides a rapid release of 90.5% lidocaine in 18 min, after which the remaining lidocaine is released slowly, resulting in better pain control (Ma R-R et al., Citation2022). Harrison et al. prepared chitosan membranes loaded with cis-2-decenoic acid (C2DA) and bupivacaine as biofilms that provided pain relief while preventing infection (Harrison et al., Citation2021). Hanif, S. et al. prepared a chitosan adhesive that performs oral mucosal adhesion to deliver iodinated tebuconazole and lidocaine hydrochloride; this adhesive is used to treat oral diseases, such as sore throat, using the characteristics of chitosan like its mucosal adhesion and its anti-microbial activity (Hanif et al., Citation2021).
3.1.3. Cyclodextrins
Cyclodextrins (CDs) are nontoxic cyclic oligosaccharides formed from α-1,4-bonded D-glucopyranose units. They are obtained by the degradation of straight-chain starch by cyclodextrin glucosyltransferase. Common natural CDs are classified as α-CD, β-CD, and γ-CD depending on the number of glucose subunits, and consist of 6,7, and 8 glucose subunits, respectively (Hammoud et al., Citation2019). CDs have a hydrophilic outer shell and hydrophobic inner lumen, which can improve drug solubility, increase drug stability, enhance drug absorption, improve drug bioavailability, mask drug odor, control drug release, reduce gastrointestinal irritation, reduce drug toxicity, etc. (Zhang & Ma, Citation2013).
One investigator used a cyclodextrin-based drug delivery system to prepare levobupivacaine complexes containing maltosyl-β-cyclodextrin (G2-β-CD). The results showed that the complexes of levobupivacaine with G2-β-CD showed significantly prolonged anesthetic effects in intrathecal and sciatic nerve blocks in rats (Karashima et al., Citation2007). Additionally, some studies have reported an almost twofold increase in the duration of epidural anesthesia with bupivacaine in combination with hydroxypropyl-β-cyclodextrin in rabbits compared with bupivacaine (Fréville et al., Citation1996). Similar results have been reported with sciatic nerve blocks. In sheep, it was also confirmed that bupivacaine cyclodextrin epidural anesthesia was more effective than bupivacaine (Estebe et al., Citation2002). Lidocaine (LDC)-loaded sulfobutyl ether β-cyclodextrin (SCD)/hyaluronic acid (HA) hydrogels were prepared by Zhou et al. compared to free lidocaine, SCD/HA-LDC showed significantly prolonged analgesic effects and lower cytotoxicity (Zhou et al., Citation2022).
3.1.4. Hyaluronic acid
Hyaluronic acid (HA) is a naturally occurring negatively charged mucopolysaccharide with a variety of properties, including biocompatibility, biodegradability, water absorption, water retention, and high viscosity. HA is mainly found in the dermis of the skin, synovial fluid, heart valves, vitreous humor of eyeglasses, cartilage, and extracellular matrix. Gelatin is naturally degraded in the body by an enzymatic mechanism (Schanté et al., Citation2011).
Yang et al. (Citation2020) prepared lidocaine dissolving microneedles (Li-DMN) using hyaluronic acid as a backbone. A minimally invasive method was used to deliver lidocaine to the skin. Based on in vivo experiments, the onset of Li-DMN action is rapid, and can be achieved within 10 min. In addition, the effects last for a long time, the effects does not cause various adverse skin reactions, and the drug exhibits high clinical safety (). Qiao et al. (Citation2022) combined hydroxypropyl chitin thermo-sensitive hydrogel (HPCH) and HA in combination to construct a ropivacaine sustained release system. It takes advantage of the negatively charged property of HA, which shows mutual attraction with positively charged ropivacaine, thus increasing the drug delivery rate. The duration and effect of sciatic nerve block in rats were significantly better than that of ropivacaine hydrochloride, and less cytotoxicity was observed.
Figure 5. Flow chart of HA-loaded lidocaine for the preparation of dissolving MNs. Reprinted with permission from Yang et al. (Citation2020), copyright© 2020 MDPI.

3.2. Synthetic polymer local anesthetic delivery system
3.2.1. Polylactic acid
Polylactic acid (PLA) is formed by the polymerization of lactic acid (LA), which is hydrolytically degraded and then converted into LA monomer, which is eventually decomposed into CO2 and H2O without the presence of enzymes. PLA is now commonly used in biomedicine because of its environmental nontoxicity, biocompatibility, biodegradability, mechanical strength, and processability (Singhvi et al., Citation2019).
Some researchers have prepared nanocapsules loaded with benzocaine using three polymers, PLA, polylactic-co-glycolic acid (PLGA), and polycaprolactone (PLC), and compared their anesthetic activity. The results showed that compared to free benzocaine, benzocaine formulations using polymeric nanocapsules had a slower release rate, and the PLA system exhibited the slowest release rate. PLA nanoparticle encapsulated benzocaine reduced the release rate, prolonged the duration of the analgesic effect, and increased the intensity of the analgesic effect. Therefore, PLA can be used as a carrier for local anesthetics to achieve slower in situ drug delivery (De Melo et al., Citation2012).
3.2.2. Poly lactic-co-glycolic acid
Poly lactic-co-glycolic acid (PLGA) is a polymerization of lactic acid (LA) and glycolic acid (GA). PLGA is hydrolyzed in vivo by ester bonding to produce the corresponding monomeric acid, lactic acid, and glycolic acid, and then converted to CO2 and H2O after the tricarboxylic acid cycle; thus, PLGA is nontoxic, biocompatible, and degradable (Harada et al., Citation2020).
Bragagni et al. prepared proparacaine-loaded PLGA particles using a double emulsion method and administered them to rats by subcutaneous injection. In vivo experiments revealed a 1.6-fold and 2-fold increase in anesthetic effect and anesthetic time, respectively, with this treatment compared to an equivalent amount of proparacaine hydrochloride in aqueous solution () (Bragagni et al., Citation2018). Wang C et al. prepared PLGA microspheres containing ropivacaine and betamethasone (RPC/BTM-PLGA-MS). In vitro release by this system can last up to 16 days. In addition, in vivo sciatic nerve block showed that PLGA microspheres (RPC/BTM-PLGA-MS) co-coated with local anesthetics and hormones exhibited a better anesthetic effect and duration compared to that of free ropivacaine and ropivacaine-only PLGA microspheres (RPC-PLGA-MS) (Wang C et al., Citation2022).
Figure 6. Flow chart for the preparation of PLGA particles loaded with proparacaine. Reprinted with permission from Bragagni et al. (Citation2018), copyright © 2018 Elsevier B.V.

3.2.3. Polycaprolactone
Polycaprolactone (PCL) is a polymer formed from ε-caprolactone in the presence of a catalyst that is widely compatible and biodegradable; and can sustain prolonged drug release. It has been approved by the FDA as a suture material, drug delivery device, and adhesion barrier (Wang B et al., Citation2019). In the literature report, lidocaine (LDC) sustained release systems were prepared using PCL nanospheres (NS). The results showed that utilizing the NS-LDC formulation reduced the toxicity of lidocaine compared with free lidocaine and significantly increased the intensity of analgesia and the duration from 240 to 420 min (Ramos Campos et al., Citation2013). In another study, Zhao W et al. embedded bupivacaine in PCL nancapsules, which exhibited high physicochemical stability and remained stable for up to 120 days. Compared to free bupivacaine, its anesthetic duration was up to two times more effective, and no significant cytotoxicity was observed (Zhao W et al., Citation2022).
4. Inorganic local anesthetic drug delivery system
4.1. Sliver nanomaterials
Silver nanomaterials have many advantages as a drug carriers, including high density surface ligand attachment, adjustable size, and shape, protection of the attached therapeutic agent from degradation, the potential for improved timed/controlled intracellular drug delivery, and transmembrane delivery without irritant transfection agents (Qureshi, Citation2013; Ivanova et al., Citation2019). In addition, they have antibacterial, anti-inflammatory, and antitumor effects. Therefore, silver nanoparticles (AgNPs) are increasingly studied in the medical field (Noronha et al., Citation2017).
Some investigators have prepared lidocaine-ibuprofen ionic liquid stabilized silver nanoparticles (IL-AgNPs), and injecting it into the hind paw and tail of hairless rats for thermal and tactile experiments. The results showed that the onset of action of AgNPs was significantly faster than that of the control group. In addition, AgNPs generated a stronger and faster local anesthetic effect (Jiang et al., Citation2018). Researchers have also explored the interaction of AgNPs and gold nanoparticles (AuNPs) with procaine, dibucaine or tetracaine. Their interactions generate the synergistic effects exhibited by the components and lead to an increase in biological and medical effects induced by local anesthetics. However, none of the studies included had relevant efficacy data (Mocanu et al., Citation2012, Citation2013).
4.2. Gold nanomaterials
Gold nanoparticles have great potential as drug carriers due to their unique optical properties, biocompatibility, stability, hydrophilicity, non-immunogenicity, and low toxicity. Currently, gold nanoparticles are more extensively studied in cancer treatment and related to local anesthetics (Abu-Dief et al., Citation2021; Yafout et al., Citation2021).
Prieto, M et al. developed a triggerable drug delivery system of poly(oligo(ethylene glycol) methyl ether methacrylate (POEGMA) NG) and hollow gold nanoparticles (HGNPs) coloaded with bupivacaine. In vivo sciatic nerve block experiments have shown that it can block nevers for more than 6 h and can be reactivated multiple times by irradiation. The effect is significantly better than the 2-h block produced by free bupivacaine (Prieto et al., Citation2022). In addition, many researchers have prepared hollow gold nanoparticles (HGNPs), and gold nanorods (GNRs) and wrapped them in organic shells to form hybrid systems. The control of local anesthetic release was achieved by using their properties that can make the organic shell disintegrate under near-infrared light heating conditions. () (Zhan et al., Citation2016; Alejo et al., Citation2018)
Figure 7. Flow chart for preparation of hollow gold nanoparticles for the local anesthetic delivery system. Reprinted with permission from Alejo et al. (Citation2018), copyright © 2018 Elsevier Inc.

4.3. Magnetic iron oxide nanomaterials
Magnetic iron oxide nanoparticles can be targeted for drug delivery by acting in the presence of an external magnetic field due to their inherent magnetic properties (Vangijzegem et al., Citation2019). In addition to these remarkable magnetic properties, their biocompatibility, stability, nontoxicity and low-cost production have led to their extensive research (Turrina et al., Citation2021).
Mantha et al. (Citation2014) used magnetite (Fe3O4) compounded with ropivacaine to form ropivacaine-associated magnetic nanoparticles (MNP/Ropiv) and investigated the feasibility of MNP/Ropiv to produce block by intravenous injection at the ankle joint of rats and by utilizing magnet attraction. The results showed that MNP/Ropiv significantly increased the thermal pain threshold in rats. The absolute concentration of ropivacaine in the ankle tissue and the ratio of ankle tissue to plasma ropivacaine concentration were higher in the 30-min ankle joint using the magnet compared to the MNP/Ropiv group without the magnet. In addition, the combination of magnetic nanoparticles with ropivacaine was found to increase the clinically safe dose of ropivacaine intravenously by more than 14-fold (). Su et al. (Citation2021) prepared a novel ropivacaine nanocomposite hydrogel drug based on magnetic iron oxide, N-isopropylacrylamide-methacrylic acid/ropivacaine magnetic nanoparticles (NIP-MAA/Rop MNPs). This composite hydrogel can improve the stability and half-life and controllability of magnetic nanoparticles while retaining the magnetic properties of iron oxide to avoid neuro- and cardiotoxicity caused by excessive drug diffusion. These studies provide new opportunities for the application of clinical ropivacaine nerve block.
Figure 8. Preparation of a magnetic nanoparticle sustained release system for ropivacaine. Reprinted with permission from Mantha et al. (Citation2014), copyright © 2014 International anesthesia research society.

4.4. Carbon nanomaterials
Carbon nanomaterials (including graphene, carbon nanotubes, nanodiamond, etc.) exhibit several beneficial properties, as they can easily cross cell membranes and exhibit a large specific surface area, high electrical and thermal conductivity, unique optical properties and mechanical properties. Therefore, these materials have received increasing attention in recent years (Kakran & Li, Citation2012; Diez-Pascual, Citation2021).
Reem Al Homsi et al. combined graphene oxide (GO) with chitosan (CS)/β-glycerophosphate (GP) thermosensitive hydrogels to encapsulate bupivacaine, enhancing the mechanical properties while improving the controllability of bupivacaine release. The results of in vivo studies showed that the composite hydrogel loaded with bupivacaine had a significantly longer local anesthesia time compared to the bupivacaine hydrochloride solution, along with a 6.5-fold increase in local anesthetic effect () (Al Homsi et al., Citation2022).
Figure 9. Graphene oxide/chitosan-based nanocomposite hydrogels loaded with bupivacaine. Reprinted with permission from Al Homsi et al. (Citation2022), copyright © 2022 Elsevier B.V.

4.5. Silicon nanomaterials
Among silica nanoparticles, mesoporous silica nanoparticles (MSNs) are often chosen by researchers as drug delivery carriers because of their unique advantages such as a large specific surface area and pore volume (Wang Y et al., Citation2015). Wang H et al. prepared MSNs and mesoporous silica-coated gold nanorods (GNR@MSNs) loaded with ropivacaine by the vacuum aspiration method. Both exhibited an extended release mode in vitro, while the latter gold nanorods (GNRs) with the property of converting near-infrared (NIR) light into heat also exhibited a controlled release mode under NIR irradiation. The results showed that ropivacaine-loaded MSNs and GNR@MSNs initially induced sensory blockade that lasted 6 h in mice, which was almost 2–3 times higher than that of free ropivacaine solution. Both particles exhibited good biocompatibility, achieved high loading, and greatly reduced carrier-related toxicity and immunoreactivity () (Wang H et al., Citation2021). In addition, other researchers prepared complexes of MSNs encapsulated with lidocaine and showed that the skin release rate was faster than that of lidocaine alone (Nafisi et al., Citation2018).
Figure 10. Mesoporous silica drug delivery system for local anesthetics. Reprinted with permission from Wang H et al. (Citation2021), copyright © 2021 acta Materialia Inc. Published by Elsevier Ltd.

5. Local anesthetic compound delivery system
5.1. Inorganic and polymer composite drug delivery systems
Inorganic and polymer composite drug delivery systems are increasingly emerging. Qi et al. used glycosylated chitosan (GCS)-encapsulated mesoporous silica nanoparticles (GCS-MONPs) loaded with ropivacaine, which could achieve controlled release of the drug under ultrasound stimulation to prolong the duration of analgesia and improve the analgesic effect (Qi et al., Citation2021). Li et al. prepared phototriggered lidocaine microneedles using iron oxide nanoparticles (Fe3O4NPs) coated with polydopamine (PDA), polyvinylpyrrolidone (PVP) and polycaprolactone (PCL) to achieve painless and effective transdermal drug delivery by programmed release of lidocaine (Li Y et al., Citation2022). In additional studies, dual-network microgels carrying ropivacaine were prepared using polymers, such as polyethylene glycol (PEG), with graphene oxide (GO). Anesthesia induced by an initial burst release triggered by body temperature and a subsequent controlled release induced by NIR radiation can be achieved (Pang et al., Citation2020).
5.2. Lipid-based and polymer drug delivery systems
As mentioned earlier, both lipids and polymers exhibit advantages and disadvantages as common drug delivery systems. To overcome the disadvantages of lipid and polymer nanoparticles, a variety of composite drug delivery carriers including lipid-polymer hybrid nanoparticles (LPNs) have been generated (Wakaskar, Citation2017).
LPN consists of two main components: a polymer core and a lipid shell. The polymer core is used to encapsulate the drug; the lipid shell imparts biocompatibility while acting as a barrier to reduce drug leakage (Hadinoto et al., Citation2013; Ma P et al., Citation2017). Pengju Ma et al. prepared LPN with PLGA, lecithin, and 1,2-distearoyl-snglycero-3-phosphoenthaolamine-N-[methoxy(polyethylene glycol)-2000(DSPE-PEG2000) to deliver bupivacaine. Its analgesic effect and duration were better than those of free bupivacaine and bupivacaine-loaded PLGA nanoparticles. It has enhanced stability and lower cytotoxicity in vitro (Ma P et al., Citation2017). Li M et al. prepared transactivated transcriptional activator-modified, levobupivacaine and dexmedetomidine codelivered LPN, which showed significant improvements in skin penetration, analgesic duration, and analgesic efficacy (Li M et al., Citation2020). In another experiment, ropivacaine lipid-polymer hybrid nanoparticles (RPV-LPNs), which were formed by wrapping ropivacaine (RPV) with poly-ε-caprolactone (PCL) as the polymer core and poly(ethylene glycol)-distearoylphosphatidylethanolamine (PEG-DSPE) as the lipid shell were significantly more effective than free ropivacaine anesthesia, with a rapid response within minutes, a long duration, and high penetration (Li A et al., Citation2019).
6. Clinical applications
Pain can be classified as nociceptive pain, inflammatory pain, neuropathic pain, cancer pain, etc., according to its mechanisms/etiology (Woessner, Citation2005; Abd-Elsayed & Deer, Citation2019). The clinical application of local anesthetics is very wide, and this paper will introduce the application of local anesthetic retardants in various types of pain and related research respectively.
6.1. Nociceptive pain
Nociceptive pain is pain caused by the activation of nociceptive receptors by stimuli such as mechanical, thermal, or chemical changes (Prescott & Ratté, Citation2017). Nociceptive pain has a variety of clinical manifestations and is usually acute. Injury is the most common cause, but it can also be caused by certain diseases. This may include broken bones, abrasions, burns, crush injuries, sprains, surgery, etc. (Baliki & Apkarian, Citation2015). Managing postoperative pain remains very challenging. Although several new analgesic drugs, techniques and methods have been applied clinically, the occurrence of postoperative pain is still very common. In today’s concept of ‘enhanced recovery after surgery’ (ERAS), there is an urgent need for more effective nonopioid medications to reduce postoperative pain, which is essential to reduce complications, shorten hospital stays, reduce medical costs, improve patient prognosis, and improve quality of life (Simpson et al., Citation2019).
HTX-011 (bupivacaine/meloxicam extended-release solution) was approved for marketing by the US FDA in May 2021, becoming the first FDA-approved extended-release dual-action local anesthetic to provide sustained analgesic capacity during the critical 72 h postoperatively providing an important option for reducing the need for opioids. Ottoboni, T. et al. (Citation2020) used a porcine lumbar incision to establish a postoperative pain model, and subcutaneous injection of HTX-011 around the incision yielded good analgesia within 72 h post-operation. In addition, Wang Z et al. (Citation2016) created a postoperative pain model through a rat hind paw incision and prepared ropivacaine nanoparticles for sciatic nerve block using polyethylene glycol copolylactic acid (PELA). The results of behavioral tests showed that ropivacaine nanoparticles inhibited the nociceptive pain induced by the incision for more than 3 days. All these studies have confirmed that local anesthesia sustained-release agents have a good analgesic effect in acute nociceptive pain caused by surgery, providing a new choice for clinical postoperative analgesia.
6.2. Neuropathic pain
The International Association for the Study of Pain (IASP) recently defined neuropathic pain as ‘pain caused by lesions or diseases of the somatosensory nervous system’ (Murnion, Citation2018) Common neuropathic diseases include painful diabetic neuropathy (PDN), postherpetic neuralgia (PHN), complex regional pain syndrome(CRPS), trigeminal neuralgia, human immunodeficiency virus (HIV) infection, leprosy, and phantom limb syndrome, among others (Colloca et al., Citation2017). With the aging of our population, neuropathic pain is gradually becoming among the major causes of pain in the elderly. The treatment of neuropathic pain is currently dominated by oral medication, and ultrasound-guided nerve block therapy based on local anesthetics occupies an important auxiliary position in clinical practice (Gilron et al., Citation2006; Shankarappa et al., Citation2012).
The sciatic nerve chronic compression injury (CCI) model is a classic neuropathic pain model. A researcher used electrostatic spinning technology to prepare a PLGA electrostatic spinning film containing bupivacaine hydrochloride and a PLGA-PEG-PLGA temperature-sensitive gel wrapped around the spinning film to prepare an extended -release system containing bupivacaine hydrochloride. A rat CCI model was used to observe the analgesic effects caused by these two drug delivery systems in vivo. The results showed that both drug delivery systems released drug in vivo for up to 14 days (Sun et al., Citation2022). This offers the possibility of applying local anesthetic extended-release formulations to treat neuropathic pain. If successfully applied to the clinical consultation and treatment process, it will bring great convenience to patients with neuropathic pain.
6.3. Inflammatory pain
Inflammatory pain is the most common type of pain in clinical practice (Tomic et al., Citation2018). Inflammatory pain is usually caused by the release of inflammatory factors (bradykinin, histamine, interleukin 1, interleukin 6, tumor necrosis factor α, 5-hydroxytryptamine, etc.) that stimulate nociceptive receptors (Nazemian et al., Citation2016). The common clinical conditions of frozen shoulder, appendicitis, synovitis, tenosynovitis, and compulsory spondylitis are all inflammatory pain.
Rodrigues da Silva et al. (Citation2021) prepared an inflammatory pain model by injecting carrageenan into the soles of the rat feet. They prepared local anesthetic sustained-release agents by wrapping butamben (BTB) in a NLC. Mechanical pain threshold assay results showed that, compared with BTB suspension, NLC coated BTB pain sensitivity was reduced by another 7%, while the duration of local anesthetics was extended and the cytotoxicity of anesthetics was reduced. This study demonstrated that local anesthetics with sustained release also showed a more effective anesthetic effect in the inflammatory hyperalgesia test.
6.4. Cancer pain
Pain is a common symptom in cancer patients, and studies have shown that patients with cancer experience pain throughout the disease, with 30–50% of cancer patients having moderate to severe pain (Simone et al., Citation2012; Neufeld et al., Citation2017; Wiffen et al., Citation2017). Pain management has traditionally followed the World Health Organization’s (WHO) ‘three-step’ approach to analgesia (Scarborough & Smith, Citation2018). However, according to studies, in 10–30% of cancer patients, traditional analgesic treatment cannot effectively relieve pain or the patients cannot tolerate systemic treatment due to the side effects of drugs (Fumic Dunkic et al., Citation2022). As a result, interventional techniques are becoming an important adjunctive treatment for pain, including autonomic nervous system blockade, peripheral nerve blockade, and intralesional analgesia, among others (Lema, Citation2001). Local anesthetics play an important role in interventional techniques.
N. D. Lafont et al. used liposome-associated bupivacaine administered via a T6-T7 epidural catheter to treat pain in lung cancer patients with T4-T10 segments. Pain was completely relieved after 5 min and lasted up to 11 h, which was much longer than the 4-h analgesia of 0.25% bupivacaine, while liposome-associated bupivacaine did not produce motor blockade (Lafont et al., Citation1996). This effect is well suited to meet the clinical analgesic needs.
7. Summary and outlook
This article briefly outlines the characteristics and shortcomings of various local anesthetic delivery systems in the context of relevant recent studies and describes their clinical applications. The various local anesthetic delivery systems and their applications are summarized in . In recent years, although domestic and foreign research in drug sustained-release formulations has made certain advancements, especially in the fields of antitumor drugs and antibiotics, and the FDA has also approved four types of local anesthetic-related retardant dosage forms, Exparel™, Xaracoll™, Posimir™, and Zynrelef™, for clinical applications, but their clinical translation still faces difficulties (Cusack et al., Citation2012; Hadj et al., Citation2012; Alijanipour et al., Citation2017; Amundson et al., Citation2017; Coppens et al., Citation2019; Velanovich et al., Citation2019; Nair et al., Citation2020; Blair, Citation2021; Chaurasiya et al., Citation2021; Leiman et al., Citation2021; Pedoto et al., Citation2021; Ekelund et al., Citation2022; Otremba et al., Citation2022; Gailey & Ostrum, Citation2023; Yu et al., Citation2023) (). In recent years, local anesthetic control agents have also proliferated, such as mesoporous silica-coated gold nanorods controlled by infrared light and thermosensitive graphene oxide/chitosan-based nanocomposite hydrogels in local anesthetics; however, no FDA-approved local anesthetic control agents are available for clinical applications (Wang H et al., Citation2021; Al Homsi et al., Citation2022). To better meet clinical needs, stimulus-responsive drug delivery systems may be the future direction, which can achieve controllability of drug release and thus achieve ‘on-demand’ release to meet patients’ requirements.
Table 1. Summary of local anesthetic drug delivery systems.
Table 2. Local anesthetic extended-release agents that have been approved for marketing by the FDA.
Authors contributions
Yulu Chen: Writing – Original Draft and drawing the Figures and Tables; Jingmei Xu: Reviewing and Editing; Ping Li, Liyang Shi, Sha Zhang and Qulian Guo: Conceptualization and Editing; Yong Yang: Reviewing and Editing, Supervision. All authors have read and agreed to the published version of the manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
Additional information
Funding
References
- Abd-Elsayed A, Deer TR. (2019). Different types of pain. In: Abd-Elsayed A, ed. Pain. Cham: Springer, 1–18.
- Abu-Dief AM, Salaheldeen M, El-Dabea T. (2021). Recent advances in development of gold nanoparticles for drug delivery systems. J Modern Nanotechnol 1:1. doi: 10.53964/jmn.2021001.
- Al Homsi R, Eltahir S, Jagal J, et al. (2022). Thermosensitive injectable graphene oxide/chitosan-based nanocomposite hydrogels for controlling the in vivo release of bupivacaine hydrochloride. Int J Pharm 621:121786. doi: 10.1016/j.ijpharm.2022.121786.
- Alejo T, Andreu V, Mendoza G, et al. (2018). Controlled release of bupivacaine using hybrid thermoresponsive nanoparticles activated via photothermal heating. J Colloid Interface Sci 523:234–44. doi: 10.1016/j.jcis.2018.03.107.
- Ali A, Ahmed S. (2018). A review on chitosan and its nanocomposites in drug delivery. Int J Biol Macromol 109:273–86. doi: 10.1016/j.ijbiomac.2017.12.078.
- Alijanipour P, Tan TL, Matthews CN, et al. (2017). Periarticular injection of liposomal bupivacaine offers no benefit over standard bupivacaine in total knee arthroplasty: a prospective, randomized, controlled trial. J Arthroplasty 32:628–34. doi: 10.1016/j.arth.2016.07.023.
- Allen TM, Cullis PR. (2013). Liposomal drug delivery systems: from concept to clinical applications. Adv Drug Deliv Rev 65:36–48. doi: 10.1016/j.addr.2012.09.037.
- Amundson AW, Johnson RL, Abdel MP, et al. (2017). A three-arm randomized clinical trial comparing continuous femoral plus single-injection sciatic peripheral nerve blocks versus periarticular injection with ropivacaine or liposomal bupivacaine for patients undergoing total knee arthroplasty. Anesthesiology 126:1139–50. doi: 10.1097/ALN.0000000000001586.
- Andreu V, Arruebo M. (2018). Current progress and challenges of nanoparticle-based therapeutics in pain management. J Control Release 269:189–213. doi: 10.1016/j.jconrel.2017.11.018.
- Baliki MN, Apkarian AV. (2015). Nociception, pain, negative moods, and behavior selection. Neuron 87:474–91. doi: 10.1016/j.neuron.2015.06.005.
- Barletta M, Reed R. (2019). Local anesthetics: pharmacology and special preparations. Vet Clin North Am Small Anim Pract 49:1109–25. doi: 10.1016/j.cvsm.2019.07.004.
- Beiranvand S, Eatemadi A, Karimi A. (2016). New updates pertaining to drug delivery of local anesthetics in particular bupivacaine using lipid nanoparticles. Nanoscale Res Lett 11:307. doi: 10.1186/s11671-016-1520-8.
- Blair HA. (2021). Bupivacaine/meloxicam prolonged release: a review in postoperative pain. Drugs 81:1203–11. doi: 10.1007/s40265-021-01551-9.
- Bragagni M, Gil-Alegre ME, Mura P, et al. (2018). Improving the therapeutic efficacy of prilocaine by PLGA microparticles: preparation, characterization and in vivo evaluation. Int J Pharm 547:24–30. doi: 10.1016/j.ijpharm.2018.05.054.
- Callender SP, Mathews JA, Kobernyk K, et al. (2017). Microemulsion utility in pharmaceuticals: Implications for multi-drug delivery. Int J Pharm 526:425–42. doi: 10.1016/j.ijpharm.2017.05.005.
- Chang HI, Yeh MK. (2012). Clinical development of liposome-based drugs: formulation, characterization, and therapeutic efficacy. Int J Nanomedicine 7:49–60. doi: 10.2147/IJN.S26766.
- Chaurasiya A, Gorajiya A, Panchal K, et al. (2021). A review on multivesicular liposomes for pharmaceutical applications: preparation, characterization, and translational challenges. Drug Deliv Transl Res 12:1569–87. doi: 10.1007/s13346-021-01060-y.
- Chiu C, Aleshi P, Esserman LJ, et al. (2018). Improved analgesia and reduced post-operative nausea and vomiting after implementation of an enhanced recovery after surgery (ERAS) pathway for total mastectomy. BMC Anesthesiol 18:41. doi: 10.1186/s12871-018-0505-9.
- Cloyd C, Moffett BS, Bernhardt MB, et al. (2018). Efficacy of liposomal bupivacaine in pediatric patients undergoing spine surgery. Paediatr Anaesth 28:982–6. doi: 10.1111/pan.13482.
- Colloca L, Ludman T, Bouhassira D, et al. (2017). Neuropathic pain. Nat Rev Dis Primers 3:17002. doi: 10.1038/nrdp.2017.2.
- Coppens SJR, Zawodny Z, Dewinter G, et al. (2019). In search of the Holy Grail: poisons and extended release local anesthetics. Best Pract Res Clin Anaesthesiol 33:3–21. doi: 10.1016/j.bpa.2019.03.002.
- Cordeiro Lima Fernandes P, David de Moura L, Freitas de Lima F, et al. (2021). Lipid nanocapsules loaded with prilocaine and lidocaine and incorporated in gel for topical application. Int J Pharm 602:120675. doi: 10.1016/j.ijpharm.2021.120675.
- Cusack SL, Jaros M, Kuss M, et al. (2012). Clinical evaluation of XaraColl((R)), a bupivacaine-collagen implant, for postoperative analgesia in two multicenter, randomized, double-blind, placebo-controlled pilot studies. J Pain Res 5:217–25. doi: 10.2147/JPR.S33453.
- Daryab M, Faizi M, Mahboubi A, et al. (2022). Preparation and characterization of lidocaine-loaded, microemulsion-based topical gels. Iran J Pharm Res 21:e123787. doi: 10.5812/ijpr.123787.
- de Araújo DR, Ribeiro L. N d M, de Paula E. (2019). Lipid-based carriers for the delivery of local anesthetics. Expert Opin Drug Deliv 16:701–14. doi: 10.1080/17425247.2019.1629415.
- De Melo NF, et al. (2012). Benzocaine-loaded polymeric nanocapsules: study of the anesthetic activities. J Pharm Sci 101:1157–65.
- de Souza Guedes L, Martinez RM, Bou-Chacra NA, et al. (2021). An overview on topical administration of carotenoids and coenzyme Q10 loaded in lipid nanoparticles. Antioxidants 10:1034. doi: 10.3390/antiox10071034.
- Deng W, Yan Y, Zhuang P, et al. (2022). Synthesis of nanocapsules blended polymeric hydrogel loaded with bupivacaine drug delivery system for local anesthetics and pain management. Drug Deliv 29:399–412., doi: 10.1080/10717544.2021.2023702.
- Diez-Pascual AM. (2021). Carbon-based nanomaterials. Int J Mol Sci 22:7726.
- Dowell D, Haegerich TM, Chou R. (2016). CDC guideline for prescribing opioids for chronic pain–United States, 2016. JAMA 315:1624–45. doi: 10.1001/jama.2016.1464.
- Ekelund A, Peredistijs A, Grohs J, et al. (2022). SABER-bupivacaine reduces postoperative pain and opioid consumption after arthroscopic subacromial decompression: a randomized, placebo-controlled trial. J Am Acad Orthop Surg Glob Res Rev 6:e21.00287.
- Eleftheriadis GK, Monou PK, Bouropoulos N, et al. (2020). Fabrication of mucoadhesive buccal films for local administration of ketoprofen and lidocaine hydrochloride by combining fused deposition modeling and inkjet printing. J Pharm Sci 109:2757–66. doi: 10.1016/j.xphs.2020.05.022.
- Estebe JP, Ecoffey C, Dollo G, et al. (2002). Bupivacaine pharmacokinetics and motor blockade following epidural administration of the bupivacaine-sulphobutylether 7-beta-cyclodextrin complex in sheep. EJA 19:308–10. doi: 10.1097/00003643-200204000-00015.
- Euliss LE, DuPont JA, Gratton S, et al. (2006). Imparting size, shape, and composition control of materials for nanomedicine. Chem Soc Rev 35:1095–104. doi: 10.1039/b600913c.
- Fréville JC, Dollo G, Le Corre P, et al. (1996). Controlled systemic absorption and increased anesthetic effect of bupivacaine following epidural administration of bupivacaine-hydroxypropyl-beta-cyclodextrin complex. Pharm Res 13:1576–80. doi: 10.1023/A:1016000217550.
- Fumic Dunkic L, Hostic V, Kustura A. (2022). Palliative treatment of intractable cancer pain. Acta Clin Croat 61:109–14.
- Gailey AD, Ostrum RF. (2023). The use of liposomal bupivacaine in fracture surgery: a review. J Orthop Surg Res 18:267. doi: 10.1186/s13018-023-03583-1.
- Gilron I, Watson CPN, Cahill CM, et al. (2006). Neuropathic pain: a practical guide for the clinician. CMAJ 175:265–75. doi: 10.1503/cmaj.060146.
- Gradzielski M, Duvail M, de Molina PM, et al. (2021). Using microemulsions: formulation based on knowledge of their mesostructure. Chem Rev 121:5671–740. doi: 10.1021/acs.chemrev.0c00812.
- Hadinoto K, Sundaresan A, Cheow WS. (2013). Lipid–polymer hybrid nanoparticles as a new generation therapeutic delivery platform: a review. Eur J Pharm Biopharm 85:427–43. doi: 10.1016/j.ejpb.2013.07.002.
- Hadj A, Hadj A, Hadj A, et al. (2012). Safety and efficacy of extended-release bupivacaine local anaesthetic in open hernia repair: a randomized controlled trial. ANZ J Surg 82:251–7. doi: 10.1111/j.1445-2197.2011.05754.x.
- Hammoud Z, Khreich N, Auezova L, et al. (2019). Cyclodextrin-membrane interaction in drug delivery and membrane structure maintenance. Int J Pharm 564:59–76. doi: 10.1016/j.ijpharm.2019.03.063.
- Hanif S, Sarfraz RM, Syed MA, et al. (2021). Development and optimization of tibezonium iodide and lignocaine hydrochloride containing novel mucoadhesive buccal tablets: a pharmacokinetic investigation among healthy humans. Drug Dev Ind Pharm 47:1209–22. doi: 10.1080/03639045.2021.1988095.
- Harada A, Tsutsuki H, Zhang T, et al. (2020). Preparation of biodegradable PLGA-nanoparticles used for pH-sensitive intracellular delivery of an anti-inflammatory bacterial toxin to macrophages. Chem Pharm Bull 68:363–8. doi: 10.1248/cpb.c19-00917.
- Harrison ZL, Bumgardner JD, Fujiwara T, et al. (2021). In vitro evaluation of loaded chitosan membranes for pain relief and infection prevention. J Biomed Mater Res B Appl Biomater 109:1735–43. doi: 10.1002/jbm.b.34831.
- Hoare T, Bellas E, Zurakowski D, et al. (2010). Rheological blends for drug delivery. II. Prolongation of nerve blockade, biocompatibility, and in vitro-in vivo correlations. J Biomed Mater Res A 92:586–95. doi: 10.1002/jbm.a.32420.
- Hou Y, Meng X, Zhang S, et al. (2022). Near-infrared triggered ropivacaine liposomal gel for adjustable and prolonged local anaesthesia. Int J Pharm 611:121315. doi: 10.1016/j.ijpharm.2021.121315.
- Hutchins J, Delaney D, Vogel RI, et al. (2015). Ultrasound guided subcostal transversus abdominis plane (TAP) infiltration with liposomal bupivacaine for patients undergoing robotic assisted hysterectomy: a prospective randomized controlled study. Gynecol Oncol 138:609–13. doi: 10.1016/j.ygyno.2015.06.008.
- Huynh NT, Passirani C, Saulnier P, et al. (2009). Lipid nanocapsules: a new platform for nanomedicine. Int J Pharm 379:201–9. doi: 10.1016/j.ijpharm.2009.04.026.
- Ivanova N, Gugleva V, Dobreva M, et al. (2019). Silver nanoparticles as multi-functional drug delivery systems. In: Farrukh MA, Koprowski R, eds. Nanomedicines. London: Intech.
- Jacob BC, Peasah SK, Shogbon AO, et al. (2017). Postoperative pain management with liposomal bupivacaine in patients undergoing orthopedic knee and hip arthroplasty at a community hospital. Hosp Pharm 52:367–73. doi: 10.1177/0018578717715382.
- Jeng CL, Torrillo TM, Rosenblatt MA. (2010). Complications of peripheral nerve blocks. Br J Anaesth 105:i97–107. doi: 10.1093/bja/aeq273.
- Jiang Q, Yu S, Li X, et al. (2018). Evaluation of local anesthetic effects of lidocaine-ibuprofen ionic liquid stabilized silver nanoparticles in Male Swiss mice. J Photochem Photobiol B 178:367–70. doi: 10.1016/j.jphotobiol.2017.11.028.
- Joudeh N, Linke D. (2022). Nanoparticle classification, physicochemical properties, characterization, and applications: a comprehensive review for biologists. J Nanobiotechnology 20:262. doi: 10.1186/s12951-022-01477-8.
- Kakran M, Li L. (2012). Carbon nanomaterials for drug delivery. KEM 508:76–80. doi: 10.4028/www.scientific.net/KEM.508.76.
- Karashima K, Taniguchi M, Nakamura T, et al. (2007). Prolongation of intrathecal and sciatic nerve blocks using a complex of levobupivacaine with maltosyl-beta-cyclodextrin in rats. Anesth Analg 104:1121–8, tables of contents. doi: 10.1213/01.ane.0000260309.15034.52.
- Karasulu HY. (2008). Microemulsions as novel drug carriers: the formation, stability, applications and toxicity. Expert Opin Drug Deliv 5:119–35. doi: 10.1517/17425247.5.1.119.
- Karimian A, Parsian H, Majidinia M, et al. (2019). Nanocrystalline cellulose: preparation, physicochemical properties, and applications in drug delivery systems. Int J Biol Macromol 133:850–9. doi: 10.1016/j.ijbiomac.2019.04.117.
- Kaye AD, Armstead-Williams C, Hyatali F, et al. (2020). Exparel for postoperative pain management: a comprehensive review. Curr Pain Headache Rep 24:73. doi: 10.1007/s11916-020-00905-4.
- Kaye AD, Novitch MB, Carlson SF, et al. (2020). The role of exparel plus meloxicam for postoperative pain management. Curr Pain Headache Rep 24:6. doi: 10.1007/s11916-020-0837-2.
- Kp K, R B. (2021). Evaluation and comparison of anti-inflammatory properties of ibuprofen using two drug delivery systems after third molar surgery: using chitosan microspheres as a carrier for local drug delivery in to the third molar socket and through the oral route. Br J Oral Maxillofac Surg 59:191–6. doi: 10.1016/j.bjoms.2020.08.025.
- Lafont ND, Legros FJ, Boogaerts JG. (1996). Use of liposome-associated bupivacaine in a cancer pain syndrome. Anaesthesia 51:578–9. doi: 10.1111/j.1365-2044.1996.tb12569.x.
- Lee B-M, Lee C, Lahiji SF, et al. (2020). Dissolving microneedles for rapid and painless local anesthesia. Pharmaceutics 12:366. doi: 10.3390/pharmaceutics12040366.
- Lee CY, Robinson DA, Johnson CA, et al. (2019). A randomized controlled trial of liposomal bupivacaine parasternal intercostal block for sternotomy. Ann Thorac Surg 107:128–34. doi: 10.1016/j.athoracsur.2018.06.081.
- Leiman D, Niebler G, Minkowitz HS. (2021). Pharmacokinetics and safety of INL-001 (bupivacaine HCl) implants compared with bupivacaine HCl infiltration after open unilateral inguinal hernioplasty. Adv Ther 38:691–706. doi: 10.1007/s12325-020-01565-x.
- Lema MJ. (2001). Invasive analgesia techniques for advanced cancer pain. Surg Oncol Clin North Am 10:127–36. doi: 10.1016/S1055-3207(18)30089-9.
- Leng F, Wan J, Liu W, et al. (2012). Prolongation of epidural analgesia using solid lipid nanoparticles as drug carrier for lidocaine. Reg Anesth Pain Med 37:159–65. doi: 10.1097/AAP.0b013e31823fc058.
- Li A, Yang F, Xin J, et al. (2019). An efficient and long-acting local anesthetic: ropivacaine-loaded lipid-polymer hybrid nanoparticles for the control of pain. Int J Nanomedicine 14:913–20. doi: 10.2147/IJN.S190164.
- Li M, Feng S, Xing H, et al. (2020). Dexmedetomidine and levobupivacaine co-loaded, transcriptional transactivator peptide modified nanostructured lipid carriers or lipid-polymer hybrid nanoparticles, which performed better for local anesthetic therapy? Drug Deliv 27:1452–60. doi: 10.1080/10717544.2020.1831105.
- Li Y, Liao X, Zheng B. (2022). Studies on local anesthetic lidocaine hydrochloride delivery via photo-triggered implantable polymeric microneedles as a patient-controlled transdermal analgesia system. J Biomater Sci Polym Ed 33:155–73. doi: 10.1080/09205063.2021.1981535.
- Lieblich SE, Danesi H. (2017). Liposomal bupivacaine use in third molar impaction surgery: INNOVATE study. Anesth Prog 64:127–35. doi: 10.2344/anpr-64-02-03.
- Liu X, Zhao Q. (2019). Long-term anesthetic analgesic effects: Comparison of tetracaine loaded polymeric nanoparticles, solid lipid nanoparticles, and nanostructured lipid carriers in vitro and in vivo. Biomed Pharmacother 117:109057. doi: 10.1016/j.biopha.2019.109057.
- Lu B, Ma Q, Zhang J, et al. (2021). Preparation and characterization of bupivacaine multivesicular liposome: a QbD study about the effects of formulation and process on critical quality attributes. Int J Pharm 598:120335., doi: 10.1016/j.ijpharm.2021.120335.
- Lu I-J, Fu Y-S, Chang W-Y, et al. (2019). Using microemulsion as carrier for drug transdermal delivery: the effect of surfactants and cosurfactants. Curr Pharm Des 25:1052–8. doi: 10.2174/1381612825666190527091528.
- Ma P, Li T, Xing H, et al. (2017). Local anesthetic effects of bupivacaine loaded lipid-polymer hybrid nanoparticles: in vitro and in vivo evaluation. Biomed Pharmacother 89:689–95. doi: 10.1016/j.biopha.2017.01.175.
- Ma R-R, Xu H-X, Ni L, et al. (2022). Swelling of multilayered calcium alginate microspheres for drug-loaded dressing induced rapid lidocaine release for better pain control. Am J Chin Med 50:2085–102. doi: 10.1142/S0192415X22500896.
- Mantha VRR, Nair HK, Venkataramanan R, et al. (2014). Nanoanesthesia: a novel, intravenous approach to ankle block in the rat by magnet-directed concentration of ropivacaine-associated nanoparticles. Anesth Analg 118:1355–62. doi: 10.1213/ANE.0000000000000175.
- Medhi P, Olatunji O, Nayak A, et al. (2017). Lidocaine-loaded fish scale-nanocellulose biopolymer composite microneedles. AAPS PharmSciTech 18:1488–94. doi: 10.1208/s12249-017-0758-5.
- Mills SEE, Nicolson KP, Smith BH. (2019). Chronic pain: a review of its epidemiology and associated factors in population-based studies. Br J Anaesth 123:e273–e283. doi: 10.1016/j.bja.2019.03.023.
- Mocanu A, Pasca R-D, Tomoaia G, et al. (2012). Selective effect of procaine, tetracaine and dibucaine on gold nanoparticles. J Nanosci Nanotechnol 12:8935–9. doi: 10.1166/jnn.2012.6707.
- Mocanu A, Pasca RD, Tomoaia G, et al. (2013). New procedure to synthesize silver nanoparticles and their interaction with local anesthetics. Int J Nanomedicine 8:3867–74. doi: 10.2147/IJN.S51063.
- Moon RJ, Martini A, Nairn J, et al. (2011). Cellulose nanomaterials review: structure, properties and nanocomposites. Chem Soc Rev 40:3941–94. doi: 10.1039/c0cs00108b.
- Murnion BP. (2018). Neuropathic pain: current definition and review of drug treatment. Aust Prescr 41:60–3. doi: 10.18773/austprescr.2018.022.
- Nafisi S, Samadi N, Houshiar M, et al. (2018). Mesoporous silica nanoparticles for enhanced lidocaine skin delivery. Int J Pharm 550:325–32. doi: 10.1016/j.ijpharm.2018.08.004.
- Nair A, Mantha SSP, Suvvari P, et al. (2020). HTX-011: another game changer multimodal analgesic or an ephemeral, experimental drug! Saudi J Anaesth 14:419–20. doi: 10.4103/sja.SJA_227_20.
- Nazemian V, Shadnoush M, Manaheji H, et al. (2016). Probiotics and inflammatory pain: a literature review study. Middle East J Rehabil Health 3:e36087. doi: 10.17795/mejrh-36087.
- Neufeld NJ, Elnahal SM, Alvarez RH. (2017). Cancer pain: a review of epidemiology, clinical quality and value impact. Future Oncol 13:833–41. doi: 10.2217/fon-2016-0423.
- Noronha VT, Paula AJ, Durán G, et al. (2017). Silver nanoparticles in dentistry. Dent Mater 33:1110–26. doi: 10.1016/j.dental.2017.07.002.
- Otremba B, Dinges H-C, Schubert A-K, et al. (2022). Liposomal bupivacaine-no breakthrough in postoperative pain management. Anaesthesiologie 71:556–64. doi: 10.1007/s00101-022-01118-7.
- Ottoboni T, Quart B, Pawasauskas J, et al. (2020). Mechanism of action of HTX-011: a novel, extended-release, dual-acting local anesthetic formulation for postoperative pain. Reg Anesth Pain Med 45(2):117–123. doi: 10.1136/rapm-2019-100714.
- Pang Q, Zhao J, Zhang S, et al. (2020). Near-infrared triggered on-demand local anesthesia using a jammed microgels system. J Biomater Sci Polym Ed 31:2252–67. doi: 10.1080/09205063.2020.1800904.
- Pathak P, Nagarsenker M. (2009). Formulation and evaluation of lidocaine lipid nanosystems for dermal delivery. AAPS PharmSciTech 10:985–92. doi: 10.1208/s12249-009-9287-1.
- Pedoto A, Noel J, Park BJ, et al. (2021). Liposomal bupivacaine versus bupivacaine hydrochloride for intercostal nerve blockade in minimally invasive thoracic surgery. J Cardiothorac Vasc Anesth 35:1393–8. doi: 10.1053/j.jvca.2020.11.067.
- Prabhu M, Clapp MA, McQuaid-Hanson E, et al. (2018). Liposomal bupivacaine block at the time of cesarean delivery to decrease postoperative pain: a randomized controlled trial. Obstet Gynecol 132:70–8. doi: 10.1097/AOG.0000000000002649.
- Prescott SA, Ratté S. (2017). Somatosensation and pain. In: Michael Conn P, ed. Conn’s translational neuroscience. New York: Academic Press, 517–39.
- Prieto M, Usón L, Garcia-Salinas S, et al. (2022). Light activated pulsatile drug delivery for prolonged peripheral nerve block. Biomaterials 283:121453. doi: 10.1016/j.biomaterials.2022.121453.
- Qi R-Q, Liu W, Wang D-Y, et al. (2021). Development of local anesthetic drug delivery system by administration of organo-silica nanoformulations under ultrasound stimuli: in vitro and in vivo investigations. Drug Deliv 28:54–62. doi: 10.1080/10717544.2020.1856220.
- Qiao Q, Fu X, Huang R, et al. (2022). Ropivacaine-loaded, hydroxypropyl chitin thermo-sensitive hydrogel combined with hyaluronan: an injectable, sustained-release system for providing long-lasting local anesthesia in rats. Reg Anesth Pain Med 47:234–41. doi: 10.1136/rapm-2021-102726.
- Qureshi AT. (2013). Silver nanoparticles as drug delivery systems [doctoral dissertations]. LSU.
- Rajpoot K. (2019). Solid lipid nanoparticles: a promising nanomaterial in drug delivery. Curr Pharm Des 25:3943–59. doi: 10.2174/1381612825666190903155321.
- Ramos Campos EV, Silva de Melo NF, Guilherme VA, et al. (2013). Preparation and characterization of poly(epsilon-caprolactone) nanospheres containing the local anesthetic lidocaine. J Pharm Sci 102:215–26. doi: 10.1002/jps.23350.
- Rideau E, Dimova R, Schwille P, et al. (2018). Liposomes and polymersomes: a comparative review towards cell mimicking. Chem Soc Rev 47:8572–610. doi: 10.1039/c8cs00162f.
- Rodrigues da Silva GH, Lemes JBP, Geronimo G, et al. (2021). Lipid nanoparticles loaded with butamben and designed to improve anesthesia at inflamed tissues. Biomater Sci 9:3378–89. doi: 10.1039/d1bm00077b.
- Sapra B, Thatai P, Bhandari S, et al. (2014). A critical appraisal of microemulsions for drug delivery: part II. Ther Deliv 5:83–94. doi: 10.4155/tde.13.125.
- Scarborough BM, Smith CB. (2018). Optimal pain management for patients with cancer in the modern era. CA Cancer J Clin 68:182–96. doi: 10.3322/caac.21453.
- Schanté CE, Zuber G, Herlin C, et al. (2011). Chemical modifications of hyaluronic acid for the synthesis of derivatives for a broad range of biomedical applications. Carbohydr Polym 85:469–89. doi: 10.1016/j.carbpol.2011.03.019.
- Shankarappa SA, Tsui JH, Kim KN, et al. (2012). Prolonged nerve blockade delays the onset of neuropathic pain. Proc Natl Acad Sci U S A 109:17555–60. doi: 10.1073/pnas.1214634109.
- Simone CB, Vapiwala N, Hampshire MK, 2nd, et al. (2012). Cancer patient attitudes toward analgesic usage and pain intervention. Clin J Pain 28:157–62. doi: 10.1097/AJP.0b013e318223be30.
- Simpson JC, Bao X, Agarwala A. (2019). Pain management in enhanced recovery after surgery (ERAS) protocols. Clin Colon Rectal Surg 32:121–8. doi: 10.1055/s-0038-1676477.
- Singhvi MS, Zinjarde SS, Gokhale DV. (2019). Polylactic acid: synthesis and biomedical applications. J Appl Microbiol 127:1612–26. doi: 10.1111/jam.14290.
- Stayner RS, Copenhaver DJ. (2012). Opioids, pain management and the law. Curr Opin Anaesthesiol 25:566–71. doi: 10.1097/ACO.0b013e328357a24a.
- Su J, Chen X, Liu H, et al. (2021). Ropivacaine magnetic nanoparticles: an efficient local anesthetic nerve conduction blocker. Mat Express 11:1819–25. doi: 10.1166/mex.2021.2098.
- Sun FR, et al. (2022). Application of extended-release local anesthetics based on composite polymer materials in an animal model of chronic pain. Med J Peking Union Med Coll Hosp 13:433–9.
- Suo M, Zhao X, Yu G, et al. (2020). Lidocaine loaded nanostructured lipid carriers for prolonged local anesthesia: in vitro and in vivo studies. J Dispersion Sci Technol 43:682–9. doi: 10.1080/01932691.2020.1844739.
- Tomic M, et al. (2018). Antiepileptic drugs as analgesics/adjuvants in inflammatory pain: current preclinical evidence. Pharmacol Ther 192:42–64.
- Turrina C, Berensmeier S, Schwaminger SP. (2021). Bare iron oxide nanoparticles as drug delivery carrier for the short cationic peptide lasioglossin. Pharmaceuticals 14:405. doi: 10.3390/ph14050405.
- Vadlamudi HC, Narendran H, Nagaswaram T, et al. (2014). Microemulsions based transdermal drug delivery systems. Curr Drug Discov Technol 11:169–80. doi: 10.2174/157016381103141128113034.
- Vangijzegem T, Stanicki D, Laurent S. (2019). Magnetic iron oxide nanoparticles for drug delivery: applications and characteristics. Expert Opin Drug Deliv 16:69–78. doi: 10.1080/17425247.2019.1554647.
- Velanovich V, Rider P, Deck K, et al. (2019). Safety and efficacy of bupivacaine HCl collagen-matrix implant (INL-001) in open inguinal hernia repair: results from two randomized controlled trials. Adv Ther 36:200–16. doi: 10.1007/s12325-018-0836-4.
- Wakaskar RR. (2017). General overview of lipid–polymer hybrid nanoparticles, dendrimers, micelles, liposomes, spongosomes and cubosomes. J Drug Target 26:311–8. doi: 10.1080/1061186X.2017.1367006.
- Wang B, Wang S, Zhang Q, et al. (2019). Recent advances in polymer-based drug delivery systems for local anesthetics. Acta Biomater 96:55–67. doi: 10.1016/j.actbio.2019.05.044.
- Wang C, Yang J, Chang W. (2022). PLGA-based microspheres containing ropivacaine and betamethasone for sciatic nerve block in mice. Pharm Dev Technol 27:503–10. doi: 10.1080/10837450.2020.1871011.
- Wang H, Zhang Y, Xu X, et al. (2021). An injectable mesoporous silica-based analgesic delivery system prolongs the duration of sciatic nerve block in mice with minimal toxicity. Acta Biomater 135:638–49. doi: 10.1016/j.actbio.2021.09.008.
- Wang J, Chen J, Ye N, et al. (2012). Absorption, pharmacokinetics and disposition properties of solid lipid nanoparticles (SLNs). Curr Drug Metab 13:447–56. doi: 10.2174/138920012800166553.
- Wang Y, Zhao Q, Han N, et al. (2015). Mesoporous silica nanoparticles in drug delivery and biomedical applications. Nanomedicine 11:313–27. doi: 10.1016/j.nano.2014.09.014.
- Wang Z, Huang H, Yang S, et al. (2016). Long-term effect of ropivacaine nanoparticles for sciatic nerve block on postoperative pain in rats. Int J Nanomedicine 11:2081–90. doi: 10.2147/IJN.S101563.
- Wiffen PJ, Wee B, Derry S, et al. (2017). Opioids for cancer pain - an overview of Cochrane reviews. Cochrane Database Syst Rev 7:CD012592.
- Woessner JF. (2005). Overview of pain: classification and concepts. In: Boswell MV, Eliot Cole B, eds. Weiner's pain management. Boca Raton, FL: CRC Press.
- Yafout M, Ousaid A, Khayati Y, et al. (2021). Gold nanoparticles as a drug delivery system for standard chemotherapeutics: a new lead for targeted pharmacological cancer treatments. Sci Afr 11:e00685. doi: 10.1016/j.sciaf.2020.e00685.
- Yang H, Kang G, Jang M, et al. (2020). Development of lidocaine-loaded dissolving microneedle for rapid and efficient local anesthesia. Pharmaceutics 12:1067. doi: 10.3390/pharmaceutics12111067.
- Ye Q, Asherman J, Stevenson M, et al. (2000). DepoFoam technology: a vehicle for controlled delivery of protein and peptide drugs. J Control Release 64:155–66. doi: 10.1016/s0168-3659(99)00146-7.
- You P, Yuan R, Chen C. (2017). Design and evaluation of lidocaine- and prilocaine-coloaded nanoparticulate drug delivery systems for topical anesthetic analgesic therapy: a comparison between solid lipid nanoparticles and nanostructured lipid carriers. Drug Des Devel Ther 11:2743–52. doi: 10.2147/DDDT.S141031.
- Yu M, Yuan W, Xia Z, et al. (2023). Characterization of exparel bupivacaine multivesicular liposomes. Int J Pharm 639:122952. doi: 10.1016/j.ijpharm.2023.122952.
- Zhai Y, Yang X, Zhao L, et al. (2014). Lipid nanocapsules for transdermal delivery of ropivacaine: in vitro and in vivo evaluation. Int J Pharm 471:103–11. doi: 10.1016/j.ijpharm.2014.05.035.
- Zhan C, Wang W, McAlvin JB, et al. (2016). Phototriggered local anesthesia. Nano Lett 16:177–81. doi: 10.1021/acs.nanolett.5b03440.
- Zhang J, Ma PX. (2013). Cyclodextrin-based supramolecular systems for drug delivery: recent progress and future perspective. Adv Drug Deliv Rev 65:1215–33. doi: 10.1016/j.addr.2013.05.001.
- Zhang Y, Yue Y, Chang M. (2017). Local anaesthetic pain relief therapy: in vitro and in vivo evaluation of a nanotechnological formulation co-loaded with ropivacaine and dexamethasone. Biomed Pharmacother 96:443–9. doi: 10.1016/j.biopha.2017.09.124.
- Zhao L, Wang Y, Zhai Y, et al. (2014). Ropivacaine loaded microemulsion and microemulsion-based gel for transdermal delivery: preparation, optimization, and evaluation. Int J Pharm 477:47–56. doi: 10.1016/j.ijpharm.2014.10.005.
- Zhao W, Du Y, Ashfaq S, et al. (2022). Evaluation of the efficacy, biocompatibility, and permeation of bupivacaine-loaded poly(epsilon-caprolactone) nano-capsules as an anesthetic. J Biomed Nanotechnol 18:268–76. doi: 10.1166/jbn.2022.3223.
- Zhou L-Y, Wang Y-H, Pan R-R, et al. (2022). Optimized-dose lidocaine-loaded sulfobutyl ether β-cyclodextrin/hyaluronic acid hydrogels to improve physical, chemical, and pharmacological evaluation for local anesthetics and drug delivery systems. J Mater Sci 57:7068–84. doi: 10.1007/s10853-022-07072-4.