Abstract
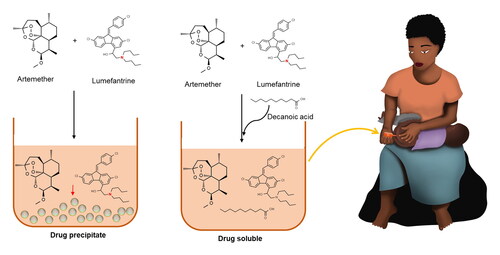
Lipophilic drugs require more advance formulation, especially if the intention is to make solutions or semisolid formulations. This also accounts for most antimalarial drugs. Although some of these antimalarial drugs are soluble in lipid vehicles, few of them, such as lumefantrine (LF), are also poorly soluble in oily vehicles. Trying to dissolve and formulate LF as a liquid formulation together with other antimalarial drugs is, therefore, a major task. When mixed in solution together with artemether (AR), precipitation occurs, sometimes with LF precipitating out on its own, and sometimes with AR precipitating out alongside LF. In this study, it was hypothesized that the use of fatty acids could lead to enhanced solubility in lipid formulation. Addition of the fatty acid solved the dissolution challenges, making LF soluble for over a year at room temperature (21–23 °C); but further research is needed to test the mechanism of action of the fatty acid. In addition, design of experiments (MODDE® 13) revealed that the amount of fatty acid in the formulation was the only significant factor for LF precipitation.
Introduction
Immediate therapy with effective anti-malarial medicines is the major life-saving intervention in the treatment of malaria. However, this treatment is threatened by development of resistance of the malaria parasite to antimalarial drugs (Murambiwa et al., Citation2011). Therefore, there is a need to prevent or postpone that from happening. To treat malaria efficiently, the World Health Organization (WHO) recommends use of a combination therapy, more specifically artemisinin-based combination therapy (ACT) (Bassat et al., Citation2015). When malaria becomes severe or develops into cerebral malaria, the disease becomes life-threatening and every minute counts. There is a need for a simple delivery system, that can be stored in rural areas in hot tropical climate, without refrigeration, and can deliver the ACT effectively into the systemic circulation.
Artemether (AR)–lumefantrine (LF) is one of the ACTs that has been recommended by WHO to treat uncomplicated Plasmodium falciparum malaria (Bassat et al., Citation2015) on the basis that AR has fast onset of action but short half-life (2–3 hours) while LF acts slowly and has a long elimination half-life (3–6 days). Therefore, AR decreases the parasite load quite quickly and LF clears the remaining parasites. Besides this, it is not possible for the parasites to develop resistance simultaneously to both drugs as their modes of action are distinctive (Prabhu et al., Citation2016).
Preformulation and liquid dosage forms are mainly based on solubilizing the drug(s) in aqueous media, and most methods used to estimate solubilization are based on numerous factors connected to aqueous solubility, such as pH, crystal structure, salt formation, etc. However, if the solubility studies are carried out in lipophilic media a different approach is required, focusing on the physical organic chemical properties of the molecule, with respect to different lipophilic solubilizers. Many antimalarial drugs are lipophilic (Aditya et al., Citation2010; Ma et al., Citation2014; Nnamani et al., Citation2014; Omwoyo et al., Citation2016). Some of these antimalarial drugs are soluble in lipid vehicles, while a few of them, such as LF are also poorly soluble in oily vehicles. Trying to dissolve LF together with other antimalarial drugs can, therefore, be a major task. When LF is mixed in a solution together with AR, precipitation occurs. This is probably one of the major reasons, why combination products in solution are not currently available (Patel et al., Citation2013).
Both AR and LF belong to class II drugs based on the biopharmaceutical classification system (BCS) (Patel et al., Citation2013; Lin et al., Citation2016) demonstrating their very low aqueous solubility. Generally, drugs need to be dissolved to cross mucosal barriers and be absorbed. Lipophilic vehicles may open opportunities to deliver the drugs more rapidly and extensively. Lipid based formulations have recently been classified into different classes depending on what is required from the formulation, such as type I, that only contain pure oils; type II that are classified as self-emulsifying drug delivery system (SEDDS); type III that are classified as self-microemulsifying drug delivery system, etc. (Savla et al., Citation2017).
The use of lipids as vehicles for the delivery of drugs with poor aqueous solubility has received more attention. Lipids have been reported to be more cost effective than polymers (Shete & Patravale, Citation2013; Le Bars et al., Citation2015). Examples of such lipids are triglycerides, that are categorized as long chain triglycerides (LCTs), medium chain triglycerides (MCTs), and short chain triglycerides (SCTs); and are used in many lipid based preparations (Le Bars et al., Citation2015).
MCTs consist of saturated fatty acids that have been esterified and have 6–10 carbon atoms. Hydrolysis, filtration, and re-esterification of coconut oil and palm oil produce pure MCTs (Kinsella et al., Citation2017; Rial et al., Citation2020). MCTs show higher solvent capacity than LCTs and SCTs and are less prone to oxidation (Le Bars et al., Citation2015; Salawi, Citation2022). One such lipid, called Miglyol® 812 N is a mixture of MCTs (mostly caprylic and capric acids) and has been regarded as a possible vehicle that could be used to increase the solubility of drugs (Le Bars et al., Citation2015).
In this work, the focus was on AR and LF. Administering AR–LF to infants and children who cannot tolerate oral medications due to severe or cerebral malaria is a challenge. In such situations, rectal drug delivery can be an option. It is important to get rapid and extensive absorption, which require that the drugs should be in a solubilized form.
Therefore, the aim of the study was to screen and identify an excipient or excipients that can solubilize and keep both AR and LF in dissolved stage.
Screening of excipients and optimization of manufacturing conditions to attain reproducible outcomes requires numerous stages and variables. The traditional approach of studying a single factor at a time while holding all others constant can be time-consuming, expensive, unpredictable, and laborious. Screening is used to detect significant factors that may influence the outcome, and to identify the limits in which they ought to be investigated. After screening, a blend of factors that can yield optimal working conditions in a process called optimization have to be identified (Benredouane et al., Citation2016; Mhango et al., Citation2017; Thorsteinsdóttir & Thorsteinsdóttir, Citation2021). MODDE® 13 (Sartorius, Umeå, Sweden) was used to design the experiment and perform statistical analysis.
Materials and methods
Materials
Trifluoroacetic acid, soybean oil, polyethylene glycol 400, and tetraglycol (Glycofurol) were bought from Sigma Aldrich (Gillingham, UK). Medium chain triglyceride (Labrafac Lipophile WL 1349) was kindly provided from Gattefossé SAS (Saint-Priest Cedex, France). Decanoic acid (DCA) and monounsaturated omega-9 fatty acid (oleic acid) were bought from Tokyo Chemical Industry (Zwijndrecht, Belgium). Miglyol® 812 N was kindly provided from Oleochemical (IOI) Pharma GmbH (Hamburg, Germany). Methoxy polyethylene glycol 350 (Carbowax™ Sentry™) was kindly provided from The Dow Chemical Company (Midland, UK). Extra virgin olive oil (100%) was bought from GEA (Ljutomer, Slovenia). Artemether and LF were kindly provided from Mangalam Drugs and Organics LTD (Mumbai, India). Methanol and acetonitrile were purchased from Honeywell (Seelze, Germany). The water was purified using a Milli-Q water purification system.
Methods
Preformulation studies
Initially, a pilot solubility study was conducted by mixing AR with 1 mL of the oily vehicle in a 2 mL microtube. The sample was sonicated for 2 min at 50% amplitude and stored at room temperature. Varying amounts of LF were also weighed and dissolved in 1 mL of the vehicle in a 2 mL microtube by sonicating for 2 min at 50% amplitude using Qsonica sonicators, model CL-18, serial no. 2013061001 (Cole-Parmer, Eaton Socon, UK). Lumefantrine was added in small increments followed by sonication until undissolved particles were seen. Known quantities of both AR and LF were also mixed with different vehicles and sonicated for 2 min at 50% amplitude in a 2 mL microtube.
Saturation solubility study of artemether and lumefantrine in selected lipids
The liquid lipids (1 mL) were put in a 2 mL Eppendorf tube and excess amount of drugs were added in respective tubes. Samples were mixed by vortexing on IKA® VORTEX 4 digital, model: PA1024-3I (Staufen, Germany) and thereafter allowed to shake at a speed of 250 min−1 for 24 hours on an Edmund Bühler GmbH (Bodelshausen, Germany) shaker at room temperature 21–23 °C. Samples were then centrifuged at 5000 rpm for 10 min in a high-speed microcentrifuge (Corning LSE™, Vordingborg, Denmark). The supernatant was diluted appropriately with either acetonitrile or a mixture of methanol and acetonitrile. Genesys 150 UV VIS spectrophotometer (Thermo Fisher Scientific, Lillerød, Denmark) was used. High-performance liquid chromatography (HPLC) system analyses were done using a Dionex Ultimate 3000 HPLC, HPG-3400 pump with degasser, WPS 3000 TSL autosampler, TCC 3100 thermostated column compartment, photodiode array (PDA) 3000 detector (Thermo Fisher Scientific, Lillerød, Denmark) and Chromeleon chromatography workstation. Quantifications were done for LF at 336 nm (Sharma et al., Citation2016) and AR at 218 nm, respectively. A Phenomenex column Luna® 5 µm C18(2) 100 Å, 250 × 4.6 mm was used.
Lumefantrine solubility in solid lipids was done by melting lipids at a temperature slightly higher than the melting point of the lipid and then adding the drug in small increments. The solubility of LF in the molten lipid was determined by observing visually the presence or absence of undissolved drug (Mendes et al., Citation2013).
Interaction between artemether and lumefantrine
Lumefantrine was mixed with 1 mL Miglyol® 812 N and sonicated for 2 min at 50% amplitude. In a different Eppendorf tube, LF and Miglyol® 812 N were mixed first followed by sonication at 50% amplitude for 2 min. After LF was dissolved, AR was added to the solution and the sample was sonicated again for 2 min. Nuclear magnetic resonance (NMR) samples of the pure compounds (LF, AR, Miglyol® 812 N) as well as of the precipitates, both from LF alone in Miglyol® 812 N, and from a mixture of LF and AR in Miglyol® 812 N, were prepared in deuterated chloroform (CDCl3) and their 1H spectra were recorded on a Bruker Avance 400 MHz spectrometer and analyzed using MestReNova software.
Statistically based design of experiments (DoE)
A series of experiments were conducted to screen significant factors that might have influenced the main desired results. A two-level full factorial design with three factors was used, and they were put at two fixed points, i.e. at minimal or a high level. It was an interaction model, that consisted of a total of 13 runs including five center points to assess the reproducibility of the method. The experimental factors and levels used are shown in and the outcome and their goals are used in full factorial design. Design of experiments software, MODDE® 13 from Sartorius (Umeå, Sweden), was used to design the experiments and perform the statistical analysis. Analysis of variance (ANOVA) was used to calculate the significance of the effect. The regression relationship between factors and responses was done using the F-test. Results were considered significant at 95% (p value <.05) significance level.
Table 1. Factors and settings (quantitative factors) used for the two-level full factorial design, where the effects of decanoic acid, Miglyol® 812 N, and temperature were studied.
Results
Preliminary screening of lipid excipients
Artemether dissolved in all the vehicles, producing a clear solution as shown in . Lumefantrine, however, was more difficult to solubilize although it has Log P around 9, but it seemed to be more soluble in Miglyol® 812 N among the tested vehicles. However, precipitation occurred in all the tested vehicles. In samples containing both drugs, precipitation of LF started shortly after sonication.
Table 2. Preliminary solubility of artemether and lumefantrine in different vehicles at 21–23 °C.
Saturated solubility in selected lipids
Selected lipophilic vehicles, including Miglyol® 812 N, monounsaturated omega-9 fatty acid, DCA, Labrafac, and mPEG, were screened for further studies to identify potential lipids that could solubilize both drugs and keep them in solution. shows that the solubility of AR was good in all tested vehicles. LF, however, showed highest solubility in monounsaturated omega-9 fatty acid and lowest in mPEG 350. Generally, the solubility of AR in all the tested lipids was higher than that of LF. In solid lipids, LF was found to be dissolved in 30 mg/g in DCA at 60 °C.
Figure 1. Solubility of artemether and lumefantrine in pure vehicles, compared to the concentrations required to achieve clinically relevant amount per mL. Two brands of medium chain triglycerides (MCT) were compared, Miglyol® 812 N and Labrafac Lipophile WL 1349. mPEG is methoxy-polyethylene glycol. For comparison, clinically relevant concentrations were achieved by using fatty acids (FA) as cosolvents, such as oleic acid or decanoic acid mixed with MCT. Note that clinically relevant amount of artemether is far from its maximum solubility in this graph, or only 20 mg/mL, where lumefantrine was soluble in a concentration of 120 mg/mL, which is significantly higher than in MCT without FA.

Incompatibility between artemether and lumefantrine
When LF and AR were mixed in the same formulation, i.e. Miglyol® 812 N in the absence of DCA, precipitation occurred. NMR analysis was performed on LF by itself (), the precipitate of an AR/LF mixture in Miglyol (), AR by itself (), as well as Miglyol by itself (). When the NMR spectrum of LF by itself was compared with the precipitate from the AR/LF mixture, it could be seen that most of the precipitate was LF, but there was a small peak at 3.43 ppm, indicating the presence of some AR. Other AR peaks were either too small to be observed in the NMR or hidden behind other peaks from LF. Additionally, there was some Miglyol present in that sample as the Miglyol was not completely removed from the precipitate before analyzing it.
Figure 2. (a) NMR spectrum of lumefantrine on its own, (b) NMR spectrum of the precipitate that was formed from a mixture of lumefantrine and artemether, (c) NMR spectrum of artemether on its own, and (d) NMR spectrum of Miglyol® 812 N on its own.

Another NMR comparison is shown in , where the spectra of LF by itself (red), the precipitate of LF by itself in Miglyol (green), and the precipitate from the AR/LF mixture (blue) are overlayed. All spectra are calibrated to the CDCl3 solvent peak at 7.26. When the spectra are compared, a small peak shift can be observed, especially in the aromatic region, for the LF peaks. This shift is small for the precipitate of LF by itself but increases when a slight amount of AR is present as well. shows an overlayed NMR spectrum of LF on its own (blue) and a mixture of LF and AR together in the absence of Miglyol (orange). The top shows a zoomed in version of the aromatic region. Both spectra were calibrated to the CDCl3 solvent peak at 7.26 ppm. An NMR of an AR/LF mixture that had never been dissolved in Miglyol showed significantly less peak shift compared to LF by itself.
Figure 3. Overlayed NMR spectra of lumefantrine on its own (red), the precipitate formed when LF was dissolved in Miglyol® 812 N by itself (green), and the precipitate that formed from a mixture of lumefantrine and artemether in Miglyol® 812 N together (blue). The top shows a zoomed in version of the aromatic region, showing clearer peak shifts that were observed. All spectra were calibrated to the CDCl3 solvent peak at 7.26 ppm.

Figure 4. Overlayed NMR spectra of lumefantrine on its own (blue) and a mixture of LF and AR together in the absence of Miglyol® 812 N (orange). The top shows a zoomed in version of the aromatic region. Both spectra were calibrated to the CDCl3 solvent peak at 7.26 ppm.

Adding an organic acid, such as DCA, to the formulations previously prepared as shown in , resulted in all samples dissolving.
Statistically based design of experiments
The experimental runs designed by MODDE®13 software and relative results are shown in . The amount of DCA and temperature were found to be significant factors for dissolution of LF. A decrease in DCA and/or temperature increased the dissolution time. Decanoic acid amount was found to be the only significant factor for LF precipitation as shown in . Although, lack of significant interactions was observed, the square term of DCA was significant and it was observed that a decrease in DCA amount shortened the precipitation time. The data in show that the shortest dissolution time can be attained by use of high DCA amount and high temperature, where the high stability before precipitation was obtained with high DCA amount, regardless of the temperature used within the range from 35 to 60 °C. The model was valid for both LF precipitation and dissolution times as demonstrated by a Q2 of 0.99 and 0.93, respectively. This also shows the model was very good and therefore can make good future precision predictions. The regression relationship between factors and responses was significant at a p value <.05.
Figure 5. Coefficient plots scaled and centered for lumefantrine precipitation time (a) in months and lumefantrine dissolution time (b) in minutes.

Figure 6. Response contour plots for lumefantrine (LF) precipitation time (a) and lumefantrine dissolution time (b) in a formulation containing Miglyol® 812 N. The scale shows (a) stability (in months) for lumefantrine before precipitation; and (b) time (in minutes) for solubilization of lumefantrine.

Table 3. Proposed factor combination by MODDE and relative responses obtained (n = 3).
Discussion
Artemether is a methyl ether artemisinin derivative and has sesquiterpene lactone rings with an endoperoxide bridge as shown in and LF, formerly known as benflumetol, is a racemic fluorine derivative possessing great activity against blood schizonticides (Deen et al., Citation2008; Murambiwa et al., Citation2011). No precipitation problems were observed with AR on its own. LF, however, exhibited poor solubility and precipitation in the lipids tested.
Recent structural analysis of LF may give an insight into what the major intermolecular forces are at play for LF. Single crystal X-ray studies showed two C-Cl···π interactions and a number of π···π interactions in the crystal structure (Pansuriya et al., Citation2019). Hirshfeld surface analysis also showed that there was a significant contribution of C–H···Cl interactions to the intermolecular forces of LF (Pansuriya et al., Citation2019). These intermolecular forces, may contribute significantly toward the precipitation of LF on its own, but it is unclear, which intermolecular forces are the main ones at play in the instances where AR precipitates out alongside LF.
The NMR data showed evidence of potential interactions between LF and Miglyol or different interactions between LF when in the presence of Miglyol. Even greater interactions were observed when a small amount of AR precipitate was also in the sample. In the NMR spectrum of the precipitate of LF alone in Miglyol, a small peak shift was observed, most notably in the aromatic region where all the peaks of LF being slightly shielded compared to the spectrum with pure LF (, red and green traces). In the NMR spectrum of the AR/LF precipitate in Miglyol, a greater shielding effect was observed for all the aromatic peaks (blue trace). These types of NMR peak shifts have previously been observed and considered evidence of interactions between different molecules, e.g. between DNA and drug molecules that could act as intercalators or groove binders (Feigon et al., Citation1984). Interestingly, this peak shift was not observed when AR and LF were mixed by themselves without any Miglyol present (see Supplementary Information), at least not to the same extent. This raises a question about Miglyol’s role in the precipitation and on the intermolecular forces at play. If the presence of Miglyol results in stronger intermolecular forces between the different LF molecules in solution, and/or between AR and LF, especially compared to the intermolecular forces between the drug molecules and the Miglyol solvent, that could cause these drug molecules to precipitate out together to some extent. Dissolution in the NMR solvent may break some of these interactions, but the presence of the Miglyol solvent seems to be important for these peaks shifts to be observable in the NMR spectra.
Further research is needed to verify exactly what type of intermolecular forces are at play here leading to especially the joint precipitation, but this may help explain why LF is found in solid formulations, such as tablets, capsules, and powder for oral suspension, and thus not available in solubilized liquid form, such as injections.
It was hypothesized that the use of a fatty acid might help to solubilize LF. This hypothesis was tested, initially, using a small amount of DCA as an additive to the solution mixture. When the fatty acid was added, the solubility of LF was successfully enhanced. The inclusion of a fatty acid in the formulation was found to be capable of keeping both drugs in solution, showing that this hypothesis was correct.
The mechanism of solvation is not clear at this point, but LF has shown to be able to form salts when mixed with carboxylic acids in acetone, as the acid can protonate the tertiary amine, leading to ionic forces between the ammonium and the carboxylate ions (Tomar et al., Citation2022). The main question remaining is how this might lead to enhanced solubility for LF in lipid formulations. One possible hypothesis includes that the strong ionic interactions may result in the aliphatic part of the carboxylate blocking other parts of LF from forming as many π···π and/or Cl···π interactions. Another potential hypothesis is that DCA could lead to formation of an inverse micelle structure that would reduce the probability of aggregation of LF by itself. More detailed physical organic chemical evidence is still needed however to verify or disproof whether these are probable mechanisms of action.
The high solubility of AR that was observed in all the tested lipids is expected as it has been reported that less lipophilic drugs can dissolve easily in MCTs (Savla et al., Citation2017). In addition, MCTs show higher solvent capacity than LCTs and SCTs (Le Bars et al., Citation2015). The moderate solubility of LF in triglycerides but its high solubility in fatty acids such as monounsaturated omega-9 fatty acids need further research to understand the mechanism that makes LF to be highly soluble in fatty acids The length of the fatty acid chain is also vital in influencing the dissolution of the drug (Savla et al., Citation2017).
Comparing the solubilities of the two brands of MCTs, i.e. Miglyol® 812 N and Labrafac Lipophile WL 1349, there was no difference, but Miglyol® 812 N was preferred to Labrafac on the basis that it is already being used in a commercial antimalarial product, AR intramuscular injection. Miglyol® 812 N was chosen as the main vehicle but since it could not solubilize the clinical dose of LF, which is 120 mg, a monounsaturated omega-9 fatty acid or/and DCA were chosen as additional excipients. More than 24% w/v DCA is needed to keep both AR and LF in solution for a period of more than a year. A temperature of up to 60 °C is needed to dissolve LF in a minimum amount of DCA (8% w/v) while increasing the amount of DCA will shorten the dissolution time and allow the solubilization process to happen at lower temperature. Medium chain triglyceride was found to be a nonsignificant factor for the precipitation and dissolution time of LF. Medium chain triglycerides are known to be safe and nontoxic, when given orally (Le Bars et al., Citation2015; Murack & Messier, Citation2019).
Mucosal surfaces contain humidity and water, even the rectal mucosa. This humidity is capable of precipitating many drugs, unless there are excipients that can bridge the gap between the lipid formulation and the humid mucosal surface. In this case, both AR and LF were dissolved in clinically relevant concentrations, using Miglyol® 812 N, a monounsaturated omega-9 fatty acid, and/or DCA. In addition, low molecular weight PEG or mPEG (methoxy polyethylene glycol) was added to the mixture for that purpose. Since this formulation could successfully solubilize both drugs, in clinically relevant doses, it will be studied further in preclinical and clinical studies.
Conclusions
The poor solubility of AR and LF makes them difficult to formulate, especially if the intention is to make a solution or a semisolid dosage form, where both drugs need to be in a dissolved form. Screening of vehicles helped identifying potential vehicles; but LF precipitated in all formulation, even in oily solution, both on its own and in the presence of AR. However, when fatty acids were mixed into the lipophilic formulation, everything became soluble. Design of experiment helped in identifying factors that were significant for the dissolution and precipitation time of LF. It also aided in identifying the range or limits in which factors could be investigated to achieve optimal and reproducible working conditions. The inclusion of a fatty acid was necessary to keep LF in solution, but further research is needed to verify the mechanism of action.
Author contributions
Ellen K. G. Mhango: design of experiment, research, analysis, and writing the original draft. Benjamin R. Sveinbjornsson: NMR work and writing of NMR results. Bergthora S. Snorradottir: supervision on design of experiment. Sveinbjorn Gizurarson: main supervisor of the whole project.
Supplemental Material
Download MS Word (618.8 KB)Disclosure statement
Authors declare no conflict of interest with this manuscript.
Data availability statement
Upon reasonable request, data can be obtained from the corresponding author.
Additional information
Funding
References
- Aditya NP, Patankar S, Madhusudhan B, et al. (2010). Artemether-loaded lipid nanoparticles produced by modified thin-film hydration: pharmacokinetics, toxicological and in vivo anti-malarial activity. Eur J Pharm Sci 40:1–10. doi: 10.1016/j.ejps.2010.05.007.
- Bassat Q, Ogutu B, Djimde A, et al. (2015). Tailoring a pediatric formulation of artemether–lumefantrine for treatment of Plasmodium falciparum malaria. Antimicrob Agents Chemother 59:4366–74. doi: 10.1128/AAC.00014-15.
- Benredouane S, Berrama T, Doufene N. (2016). Strategy of screening and optimization of process parameters using experimental design: application to amoxicillin elimination by adsorption on activated carbon. Chemometr Intell Lab Syst 155:128–37. doi: 10.1016/j.chemolab.2016.04.010.
- Deen JL, von Seidlein L, Dondorp A. (2008). Therapy of uncomplicated malaria in children: a review of treatment principles, essential drugs and current recommendations. Trop Med Int Health 13:1111–30. doi: 10.1111/j.1365-3156.2008.02117.x.
- Feigon J, Denny WA, Leupin W, Kearns DR. (1984). Interactions of antitumor drugs with natural DNA: proton NMR study of binding mode and kinetics. J Med Chem 27:450–65. doi: 10.1021/jm00370a007.
- Kinsella R, Maher T, Clegg ME. (2017). Coconut oil has less satiating properties than medium chain triglyceride oil. Physiol Behav 179:422–6. doi: 10.1016/j.physbeh.2017.07.007.
- Le Bars G, Dion S, Gauthier B, et al. (2015). Oral toxicity of Miglyol 812® in the Göttingen® minipig. Regul Toxicol Pharmacol 73:930–7. doi: 10.1016/j.yrtph.2015.09.022.
- Lin W, Heimbach T, Jain JP, et al. (2016). A physiologically based pharmacokinetic model to describe artemether pharmacokinetics in adult and pediatric patients. J Pharm Sci 105:3205–13. doi: 10.1016/j.xphs.2016.06.026.
- Ma Y, Lu T, Zhao W, et al. (2014). Enhanced antimalarial activity by a novel artemether–lumefantrine lipid emulsion for parenteral administration. Antimicrob Agents Chemother 58:5658–65. doi: 10.1128/AAC.01428-13.
- Mendes AI, Silva AC, Catita JA, et al. (2013). Miconazole-loaded nanostructured lipid carriers (NLC) for local delivery to the oral mucosa: improving antifungal activity. Colloids Surf B Biointerfaces 111:755–63. doi: 10.1016/j.colsurfb.2013.05.041.
- Mhango EKG, Kalhapure RS, Jadhav M, et al. (2017). Preparation and optimization of meropenem-loaded solid lipid nanoparticles: in vitro evaluation and molecular modeling. AAPS PharmSciTech 18:2011–25. doi: 10.1208/s12249-016-0675-z.
- Murack M, Messier C. (2019). The impact of lactic acid and medium chain triglyceride on blood glucose, lactate and diurnal motor activity: a re-examination of a treatment of major depression using lactic acid. Physiol Behav 208:112569. doi: 10.1016/j.physbeh.2019.112569.
- Murambiwa P, Masola B, Govender T, et al. (2011). Anti-malarial drug formulations and novel delivery systems: a review. Acta Trop 118:71–9. doi: 10.1016/j.actatropica.2011.03.005.
- Nnamani PO, Hansen S, Windbergs M, Lehr CM. (2014). Development of artemether-loaded nanostructured lipid carrier (NLC) formulation for topical application. Int J Pharm 477:208–17. doi: 10.1016/j.ijpharm.2014.10.004.
- Omwoyo WN, Melariri P, Gathirwa JW, et al. (2016). Development, characterization and antimalarial efficacy of dihydroartemisinin loaded solid lipid nanoparticles. Nanomedicine 12:801–9. doi: 10.1016/j.nano.2015.11.017.
- Pansuriya PB, Maguire GEM, Friedrich HB. (2019). Structural characterization and thermal properties of the anti-malarial drug: lumefantrine. S Afr J Chem 72:253–62. doi: 10.17159/0379-4350/2019/v72a33.
- Patel K, Sarma V, Vavia P. (2013). Design and evaluation of lumefantrine – oleic acid self nanoemulsifying ionic complex for enhanced dissolution. Daru 21:27. doi: 10.1186/2008-2231-21-27.
- Prabhu P, Suryavanshi S, Pathak S, et al. (2016). Artemether–lumefantrine nanostructured lipid carriers for oral malaria therapy: enhanced efficacy at reduced dose and dosing frequency. Int J Pharm 511:473–87. doi: 10.1016/j.ijpharm.2016.07.021.
- Rial SA, Jutras-Carignan A, Bergeron KF, Mounier C. (2020). A high-fat diet enriched in medium chain triglycerides triggers hepatic thermogenesis and improves metabolic health in lean and obese mice. Biochim Biophys Acta Mol Cell Biol Lipids 1865:158582. doi: 10.1016/j.bbalip.2019.158582.
- Salawi A. (2022). Self-emulsifying drug delivery systems: a novel approach to deliver drugs. Drug Deliv 29:1811–23. doi: 10.1080/10717544.2022.2083724.
- Savla R, Browne J, Plassat V, et al. (2017). Review and analysis of FDA approved drugs using lipid-based formulations. Drug Dev Ind Pharm 43:1743–58. doi: 10.1080/03639045.2017.1342654.
- Sharma NPV, Mann S, Khar RK. (2016). Evaluation of combination drugs before the development of self-emulsifying drug delivery system. SPER J Pharm Res 1:18–23.
- Shete H, Patravale V. (2013). Long chain lipid based tamoxifen NLC. Part I: preformulation studies, formulation development and physicochemical characterization. Int J Pharm 454:573–83. doi: 10.1016/j.ijpharm.2013.03.034.
- Thorsteinsdóttir UA, Thorsteinsdóttir M. (2021). Design of experiments for development and optimization of a liquid chromatography coupled to tandem mass spectrometry bioanalytical assay. J Mass Spectrom 56:e4727. doi: 10.1002/jms.4727.
- Tomar D, Lodagekar A, Gunnam A, et al. (2022). The effects of cis and trans butenedioic acid on the physicochemical behavior of lumefantrine. CrystEngComm 24:156–68. doi: 10.1039/D0CE01709D.