Abstract
Transdermal drug delivery systems (TDDS) for antibiotics have seen significant advances in recent years that aimed to improve the efficacy and safety of these drugs. TDDS offer many advantages over other conventional delivery systems such as non-invasiveness, controlled-release pattern, avoidance of first-pass metabolism. The objective of this review is to provide an overview on the recent advances in the TDDS of different groups of antibiotics including β-lactams, tetracyclines, macrolides, and lincosamides, utilized for their effective delivery through the skin and to explore the challenges associated with this field. The majority of antibiotics do not have favorable properties for passive transdermal delivery. Thus, novel strategies have been employed to improve the delivery of antibiotics through the skin, such as the use of nanotechnology (nanoparticles, solid-lipid nanoparticles, nanoemulsions, vesicular carriers, and liposomes) or the physical enhancement techniques like microneedles and ultrasound. In conclusion, the transdermal delivery systems could be a promising method for delivering antibiotics that have the potential to improve patient outcomes and enhance the efficacy of drugs. Further research and development are still needed to explore the potential of delivering more antibiotic drugs by using various transdermal drug delivery approaches.
1. Introduction
Transdermal delivery is a method of administering drugs to pass through the skin and then into the bloodstream. This approach provides an alternative to conventional routes of delivery like oral, intravenous, and intramuscular (Alkilani & Nasereddin, Citation2022). Transdermal drug delivery has gained popularity over other delivery methods such as oral drug delivery. This is primarily attributed to its capacity to ensure a steady and controlled drug delivery, avoid first pass effect, and reduce the need for frequent dosing, resulting in a significant reduction in the probability of undesirable side effects (Prausnitz & Langer, Citation2008).
These advancements improved drug permeation through the skin, allowed for the delivery of a broader range of drugs, enhanced controlled release, and targeted delivery…
The transdermal delivery of the antimicrobial drugs has become increasingly popular as a way to administer antibacterial (Maji et al., Citation2021), antifungal (Gusliakova et al., Citation2021), and antiviral drugs (Puri et al., Citation2019). This method has grown in popularity in recent years due to its ability to provide a controlled and sustained drug release, avoiding problems that can occur with oral administration such as degradation of the drug in the gut and liver and the risk of drug-drug interactions (Rahimpour et al., Citation2016; González-Vázquez et al., Citation2017). TDDS can come in different forms like patches, gels, and creams, and have proven to be effective in delivering various antimicrobial drugs (Liu et al., Citation2023). This approach to drug delivery also showed the potential to increase patient compliance and enhance therapeutic outcomes through the reduction of dosing frequencies (Wang et al., Citation2021). Furthermore, it is particularly valuable for patients who are unconscious, vomiting, or dependent on self-administration. Antibiotics are categorized based on their chemical structures into the following classes: β-lactams, tetracyclines, macrolides, lincosamides, aminoglycosides, sulfonamides, and quinolones (Pancu et al., Citation2021). illustrates the commonly used antibiotic groups and their routes of administration.
Figure 1. Schematic representation illustrating the commonly used antibiotic groups and their available dosage forms.

The field of the transdermal delivery of antimicrobial drugs is expanding, with many promising advances and opportunities for future growth. Therefore, the aim of this review is to focus on the recent advances in TDDS for antibiotics, particularly β-lactams, tetracyclines, macrolides, and lincosamides. While other categories of antibiotics will be explored in subsequent studies. This review provides a comprehensive overview of the recent breakthrough in antibiotics transdermal delivery. In addition, the review aims to investigate the advancements and challenges in this field, with a particular focus on novel technologies, formulations, and approaches utilized for the effective transdermal delivery of antibiotics. Moreover, this review attempts to highlight the potential of the transdermal delivery methods in enhancing antibiotic therapy, resolving issues with traditional administration routes, and encouraging patient adherence through reviewing the latest studies made since 2000. Moreover, the review aims to discuss the key considerations related to antibiotic transdermal delivery systems, such as skin permeation enhancement techniques, formulation optimization, and safety issues. Generally, this review article provide valuable insights into the current state of transdermal drug delivery of antibiotics, paving the way for future research and innovations in this area.
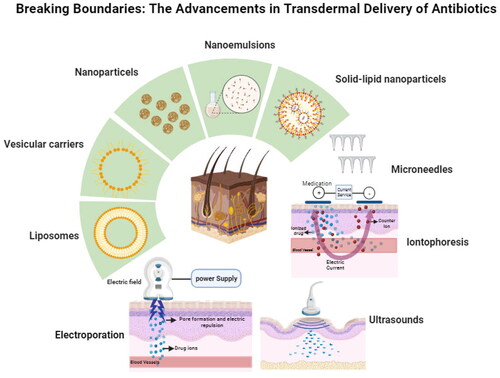
2. Transdermal enhancement technologies
The skin is the largest organ in the human body that provides several important functions. Structurally, the skin has three main layers: epidermis, dermis, and hypodermis. The epidermis is the outermost layer that acts as a barrier to the environment, preventing harmful substances from entering the body (Prausnitz and Langer, Citation2008; Alkilani et al., Citation2015; Alkilani & Nasereddin, Citation2022). The hydration of the skin and the overall condition of the stratum corneum are important factors that can influence the penetration of drugs. It is well known that skin hydration affects its barrier function which consequently has a significant impact on the penetration of both lipophilic and hydrophilic drugs (Batt and Fairhurst, Citation1986). When evaluating the transdermal delivery of drugs, it is essential to consider the dynamic nature of the skin (Batt & Fairhurst, Citation1986). Additionally, hydrophilic drugs frequently experience difficulties in transdermal administration due to the lipophilic characteristic of the stratum corneum, which can hinder their penetration. Amphiphilic molecules with both hydrophilic and hydrophobic properties may have certain advantages in terms of skin permeability, but their success is dependent on their particular properties and formulation (Fanani et al., Citation2022). Amphiphilic drugs can naturally interact with the hydrophobic surface provided by cellular membranes (Fanani et al., Citation2022). Hydrophobic drugs with low molecular weights (less than 500 Da) and low daily doses (less than 100 mg/day) can penetrate the depth of the skin due to the hydrophobic characteristics of the skin (Rahimpour et al., Citation2016). Therefore, these technologies are essential approaches used to broaden the number of drugs administered effectively across the skin (Benson, Citation2005).
Transdermal delivery methods are classified into two types: active and passive. Active methods disrupt the stratum corneum, whereas passive methods rely on diffusion through the skin by modulating drug and vehicle interactions. Physical methods of transdermal drug delivery, such as electroporation, phonophoresis, and laser-assisted procedures, are novel approaches to overcoming the skin’s natural barrier, thereby improving drug absorption and bioavailability (Alkilani et al., Citation2015). Electroporation uses electrical pulses to produce temporary pores in the skin (Ita, Citation2016). Ultrasound, or phonophoresis involves the transport of drugs across the skin using ultrasound waves at frequencies ranging from 20 kHz to 16 MHz that is intense enough to reduce skin resistance (Polat et al., Citation2011). In addition, the laser-assisted technologies disrupt the stratum corneum without injuring deeper tissues. Hence these methods improve drug transport into skin layers. One of the recent advances in TDDS for antibiotics is the development of microneedle patches (Jamaledin et al., Citation2020; Yu et al., Citation2022). These patches consist of tiny needles that pierce the skin and deliver the drug directly into the bloodstream, allowing a controlled release of the drug and reducing the risk of systemic side effects (Zaid Alkilani et al., Citation2022;).
Chemical enhancers are example on passive methods that are used to modify the skin’s lipid structure, increase the fluidity of the stratum corneum, and enhance drug permeation. Nanotechnology has revolutionized transdermal drug administration by providing novel strategies to overcome the skin’s natural barrier characteristics (Rabiei et al., Citation2020). Nano-sized drug carriers, such as liposomes, niosomes, and micelles, are designed to carry drugs through the skin’s outermost layer, the stratum corneum, which is a key challenge in transdermal delivery (Alkilani et al., Citation2015; Leong et al., Citation2023). For example, niosomes are self-assembled vesicles made of nonionic surfactants and cholesterol that have the potential to be used as drug delivery carriers. These nanovesicles mimic liposomes but are much more stable due to their nonionic nature, making them an effective technique of encapsulating and delivering a variety of hydrophilic and hydrophobic drugs (Ge et al., Citation2019). Niosomes have the potential to increase drug solubility, bioavailability, and govern release, making them useful in pharmaceutical applications (Kazi et al., Citation2010; Zaid Alkilani et al., Citation2023). All systems have the ability to administer a wide range of drugs, such as hormones, analgesics, antibiotics, anti-inflammatory and anticancer drugs (Prausnitz and Langer, Citation2008; Alkilani et al., Citation2015; Jeong et al., Citation2021; Basheer et al., Citation2023). However, one of the major challenges in the transdermal delivery of therapeutic agents is the unpredictable absorption rate due to inter-individual variability (Kumar et al., Citation2006; Malinovskaja-Gomez et al., Citation2016; Alkilani and Nasereddin, Citation2022). The technology is continually improving, and new systems are being developed to deliver more complex drugs, including large molecules and biologics (Alkilani and Nasereddin, Citation2022).
summarizes the recent approaches for enhancing transdermal delivery of antibiotics.
3. Applications of TDDS for antibiotics
The majority of antibiotics do not have the favorable properties for passive transdermal delivery, where drug permeation through skin layers is influenced by its physicochemical properties such as pKa which should be near to the skin’s pH (about 4.7–5.7), log P (Prausnitz and Langer, Citation2008; Maji et al., Citation2021 Alkilani and Nasereddin, Citation2022), molecular weight (less than 500 Da), ionization percent, diffusion coefficient, and drug solubility in delivery formulations (Hamed et al., Citation2020; Hamed et al., Citation2023). However, novel strategies such as the use of nanotechnology or physical enhancement techniques like microneedles may improve the delivery of these drugs through the skin (Yu et al., Citation2021). Further research are still needed to explore the potential of these approaches for the transdermal delivery of antibiotics. The following sections describe the different antibiotic classes (β-lactams, tetracyclines, macrolides, and lincosamides) and their transdermal delivery.
4. Transdermal delivery of β-lactam antibiotic
4.1. β-lactam antibiotics
β-lactam are a widely prescribed antibiotic that has several clinical applications. They are estimated to cost around $15 billion yearly, and account for approximately 65% of the total antibiotic market (Thakuria and Lahon, Citation2013). This class of antibiotics have β-lactam ring in their molecular structure (Mehta and Sharma, Citation2016). β-lactams are classified into five classes, penicillins, cephalosporins, cephamycins, monobactams, and carbapenems (Bush and Bradford, Citation2016). Due to their extensive utilization among patients, their use coincidences with the alarming occurrence of β-lactam resistance issue (Eiamphungporn et al., Citation2018; Abu-Sini et al., Citation2023). To address the issue of resistance, the use of β-lactamase inhibitors with broad-spectrum antibiotics has proven to be effective (Thakuria and Lahon, Citation2013). β-lactamases are enzymes secreted by a lot of Gram-positive and Gram-negative bacteria that inactivate β-lactam antibiotics (Mehta and Sharma, Citation2016).
The transdermal delivery may be feasible for some of β-lactam antibiotics with Log P values of 1–3. Some of the β-lactams have negative Log P values, which indicate that they are hydrophilic and less likely to be delivered transdermally. These include amoxicillin, ampicillin, cefadroxil, cefaclor, cefixime, cefepime, cefotaxime, imipenem, meropenem, ertapenem, and doripenem (Lima et al., Citation2020; Bagyalakshmi et al., Citation2007; Shaaban et al., Citation2017). Furthermore, the molecular weight of antibiotics is an important factor in determining their ability to penetrate the skin and be delivered transdermally. Antibiotics with lower molecular weights, typically below 500 Da, have a higher likelihood of passive diffusion through the skin layers (Alkilani et al., Citation2015). Additionally, most of β-lactam antibiotics have a high daily dose, thus depending exclusively on the passive skin diffusion for transdermal delivery may not be adequate to produce the required therapeutic dose (Safdari et al., Citation2014; Bush & Bradford, Citation2016). Consequently, the transdermal delivery of β-lactam antibiotics is challenging. This highlights the need for additional strategies to enhance drug permeation and ensure effective drug delivery through the skin.
β-lactam antibiotics act on enzymes known as penicillin-binding proteins (PBPs), which are responsible for the formation of the bacterial cell wall (Hubschwerlen, Citation2007). As a result, they are only active against rapidly multiplying organisms in which PBPs within the cell wall interferes with the production of cell wall peptidoglycans, resulting in cell lysis (Hubschwerlen, Citation2007). The spectrum of β-lactam antibiotics is impacted by the physicochemical elements related to their structural characteristics. Generally, antibiotics that are more lipophilic tend to have greater activity against Gram-positive bacteria, while those that are more hydrophilic are more effective against Gram-negative bacteria (Lima et al., Citation2020a, Lima et al., Citation2020b).
There are several clinical indications for penicillins, including treatment of syphilis, meningitis, urinary tract infections, respiratory tract infections, bacillary dysentery, typhoid, bones and joints infections, bronchitis and pneumonia, and skin and soft tissue infections (Demain & Elander, Citation1999; A.M., 2022).
4.1.1. Penicillins
β-lactam antibiotics can be delivered trasndermally using a variety of dosage forms, including patches, gels, and creams (Altun et al., Citation2021 Bandyopadhyay, Citation2021; Nasrollahzadeh et al., Citation2022). These systems typically contain the antibiotic in a reservoir or a matrix, which is applied to the skin and releases the drug over time (Altun et al., Citation2021). The rate of drug release can be controlled through the use of different formulations and delivery systems (Kumar et al., Citation2006). One prospective advantage of the transdermal delivery of β-lactam antibiotics is the ability to target localized infections, such as those of the skin and soft tissues (Gao et al., Citation2018).
Pérez-Martínez et al., (Citation2016) has fabricated a polyacrylamide hydrogel with polyaniline nanofibers in order to test its suitability as a material that responds to electrical stimulation. Nanofibers, with a high aspect ratio, were chemically synthesized and loaded with amoxicillin. By incorporating amoxicillin-loaded nanofibers in situ during the polymerization and reticulation of acrylamide, the composite hydrogel system was fabricated. A continuous 3D nanofiber network was visible in transmission electron microscope (TEM) images of cross sections of the composite. When the cathodic electrical stimulation was applied (or removed), the antibiotic molecules were precisely released (or sustained) from the composite hydrogel. A cell viability greater than 80% was observed in an in vitro cytotoxicity evaluation of the composite hydrogel extract on mouse subcutaneous connective tissues. In conclusion, the fabricated material’s potential for electrically controlled drug delivery applications was demonstrated by its minimal toxicity and tuning release profile.
For transdermal drug delivery, a novel drug reservoir as compressed tablets and hydrogel-forming MNs were used and reported by (McAlister et al., Citation2021). MNs patches, made of poly(vinyl alcohol) (PVA), were used when amoxicillin and primaquine precipitate in the hydrogel-forming MNs matrix. After one hour of in vivo studies, amoxicillin concentration that is therapeutically appropriate (2 µg/ml) was successfully achieved. Therefore, the usage of directly compressed tablets raises the possibility of increasing the number of drug molecules that may be applied transdermally via MNs patches.
Altun et al., Citation2021 developed bacterial cellulose polycaprolactone patches loaded with antibiotics such as amoxicillin, ampicillin, and kanamycin for transdermal delivery. The encapsulation efficiency of amoxicillin, ampicillin, and kanamycin was 97.4 ± 0.9, 96.8 ± 1.6, and 98.6 ± 1.4%, respectively. The release curves were modeled using the Higuchi square root model, which showed a diffusion-controlled and time-dependent release from the patches. The patches also displayed potent antibacterial action against both Gram-positive Staphylococcus aureus, (S. aureus) and Gram-negative Escherichia coli (E. coli) pathogens, indicating their potential application in the management of chronic infected wounds after closure.
4.1.2. Amoxicillin
A novel transdermal delivery system utilizing gelatin methacryloyl MNs containing amoxicillin was developed by Erkus et al., Citation2023 using 3D printing technology based on the digital light processing. The MNs with a regular structure and pointed tips were successfully fabricated. The designed MNs showed excellent mechanical characteristics in line with 0.1 N/needle without breaking. Additionally, the results of the in vitro antibacterial activity assays showed that the growth of S. aureus and E. coli was significantly inhibited by amoxicillin-loaded MNs, indicating that 3D-printing MNs are a potential method for the transdermal delivery of amoxicillin.
4.1.3. Ampicillin
(Bagyalakshmi et al., Citation2007) investigated the effectiveness of the transdermal delivery of ampicillin sodium as a transdermal patch, against E. coli. In this study, an in vitro infection model was used to simulate human pharmacokinetics and assess the drug’s ability to combat E. coli. The researchers examined the drug release capacity by exposing E. coli strains to transdermal patches containing various polymers, including hydroxypropyl methylcellulose (HPMC), methylcellulose (CMC), cellulose acetate phthalate, chitosan, sodium alginate, and sodium carboxymethyl cellulose (Na CMC). The results indicated that HPMC was the most effective polymer for reducing colony-forming units. The in vivo results of the sodium alginate patch showed that it reached a maximum plasma concentration (Cmax) of 126 μg/mL after 4 h (Tmax). Based on these findings, the transdermal delivery of ampicillin can be a substitute for the intravenous (IV) administration that is associated with minimal negative impacts.
The process of electrospinning and crosslinking was used by Cui et al., Citation2018 to develop ampicillin-loaded PVA/chitosan (PVA/CS) composite nanofibers that served as a matrix for transdermal drug delivery. The Young’s modulus and tensile strength of the crosslinked PVA/CS composite nanofibers were found to increase with increasing the concentration of the crosslinker (glulataraldehyde), reaching a maximum Young’s modulus of 198.1 MPa and the tensile strength of 4.6 MPa for the composite nanofibers. According to the drug release studies, Korsmeyer-Peppas model best fits the results of the kinetic analysis of the drug delivery system from the loaded drug. These findings showed how the crosslinked PVA/CS composite nanofibers could be used as a transdermal delivery device.
4.1.4. Cephalosporins
Cephalosporins belong to the β-lactam antibiotics. They are naturally derived from fungi, Acremonium. Cephalosporins are classified into five generations, as described in . The first generation of cephalosporins is active against Gram-positive bacteria, whereas other generations have higher activity against Gram-negative bacteria (Mehta & Sharma, Citation2016).
4.1.5. Cephalexin
Hatanaka et al., Citation2000 investigated how cephalexin diffuses through the skin when paired with different ions and solvents. The findings showed that adding certain ions can help the transport of cephalexin more effectively by creating an ion pair. To optimize the transport of drugs through skin with both positive and negative charges, it is better to pick an ion pair of a small size and high lipophilicity, and use a solvent of a pH that works well and of low electrical conductivity (Hatanaka et al., Citation2000).
Salatin and Jelvehgari, Citation2020 developed an in situ hydrogel forming system of cephalexin transfersomes. A thin-film hydration technique has been employed to formulate cephalexin transfersomes, which were then embedded in a 3D hydrogel network. The resulting cephalexin transfersomes showed a vesicle size of 192 nm and encapsulation efficiency of 66.23%. The antibacterial effectiveness of cephalexin was improved when it was loaded into transfersomes and tested against S. aureus. When the cephalexin transfersomes-loaded hydrogel was applied to rat skin for 10 days, the antibacterial activity was improved without negatively affecting hair growth or skin appearance. These results indicated that the cephalexin transfersomes-loaded hydrogel could be a valuable platform for delivering drugs to combat bacterial infections (Salatin and Jelvehgari, Citation2020).
A transdermal patch was constructed by Nasrollahzadeh et al., Citation2022 using cephalexin as a model antibiotic drug. Solid-lipid nanoparticles (SLNs) prepared with α-tocopherol succinate were loaded with cephalexin. The optimal formulation of SLNs loaded with cephalexin, with a drug/lipid ratio of 20%, showed the largest zone of inhibition of S. aureus and highest skin permeation abilities. The best developed transdermal patch, which contained 90% adhesive solution of polyisobutylene, 7% cephalexin, and 3% cephalexin SLNs, showed better inhibition against S. aureus than the formulation containing 10% cephalexin.
4.1.6. Cefadroxil
Electrospun nanofibers made of chitosan and PVA and loaded with cefadroxil monohydrate were evaluated for their antibacterial activity (Iqbal et al., Citation2020). The electrospun nanofibers demonstrated a sustained release of cefadroxil, with a more potent antibacterial effect than free cefadroxil. These findings suggested that the electrospun cefadroxil-loaded nanofibers could be a promising drug delivery system for transdermal use, promoting wound healing, and providing a cost-effective treatment option for S. aureus-induced skin infections. Zakrewsky et al., Citation2014 used ionic liquids for pathogen neutralization and transdermal delivery of cefadroxil. The ability of ionic liquids in improving the delivery of cefadroxil, treating biofilms in a wound model, cytotoxicity, and skin irritability were examined. Choline-geranate stood out among the tested ionic liquids for its excellent antimicrobial activity, low toxicity to skin and cells, and efficient permeation enhancement for drug delivery. Cefadroxil was delivered more effectively by choline-geranate, penetrating the skin deeply without irritating it. Using a biofilm-infected wound model, the researchers confirmed the effectiveness of choline-geranate in vivo by showing that after a 2-hour treatment, over 95% of the bacteria had died.
4.1.7. Cefuroxime
A study by Mannem et al., Citation2014 investigated the use of iontophoresis to deliver amoxicillin and cefuroxime antibiotics for the treatment of bacterial skin infections. The study aimed to reduce the side effects and increase the therapeutic effectiveness. The concentration of antibiotics in skin dermis of a rabbit model was determined via microdialysis. The results showed that iontophoresis is a promising method to achieve fast and sustained concentrations of the antibiotics in the skin. Therapeutically effective skin concentrations were immediately detected for amoxicillin. Whereas, the concentrations of cefuroxime only rose above the minimum inhibitory concentration (MIC) at higher current densities which was then decreased at the end of the experiment.
4.1.7. Cefixime
Alotaibi et al., (Citation2022), tested the antibacterial activity of cefixime films against three different types of bacteria (E. coli, Klebsiella pneumonia (K. pneumonia), and Acetobacter aceti) and conducted the in vitro and ex vivo permeation studies using rat skin. Films were prepared with chitosan and starch using the solvent casting technique. The optimized films (which had not been irradiated) showed the highest antibacterial effect against two of the tested bacteria (E. coli and K. pneumonia), whereas the antibacterial activity decreased significantly after the films were irradiated. The optimized film showed the highest amount of drug permeation after 24 h. The mechanism of drug release from all films was governed by non-Fickian diffusion. It was found that the mechanical properties of the films improved after cross-linking by radiation. The non-irradiated films with a 50:50 polymeric blend of chitosan and starch had the highest percentage swelling ratio, while the water uptake of irradiated films was lower than the non-irradiated ones. In conclusion, the bionanocomposite film is a potential delivery system for cefixime by improving its permeability and antibacterial activity.
In summary, several β-lactam antibiotics have negative Log P values and are hence less appropriate for passive transdermal administration. Furthermore, the high daily doses required for many β-lactam antibiotics make transdermal delivery challenging. Consequently, more innovative strategies are essential to improve drug penetration for successful transdermal delivery. In various research studies, transdermal delivery systems for β-lactam antibiotics have relied mostly on chemical approaches. Despite the promise of these technologies, there is growing interest in the potential benefits of using physical techniques for transdermal delivery. To significantly enhance the efficiency of β-lactam transdermal delivery, forthcoming research will have to explore physical techniques.
5. Transdermal delivery of tetracyclines antibiotics
5.1. Tetracyclines antibiotics
Tetracyclines are antibiotics with a broad-spectrum of action used to treat a wide range of bacterial infections (Kostrzębska et al., Citation2023). Natural antibiotics of this class include tetracycline, chlortetracycline, oxytetracycline, and demeclocycline, and semi-synthetic tetracyclines like lymecycline, methacycline, minocycline, rolitetracycline, and doxycycline. Newer drugs in the tetracycline antibiotics family includes ervacycline, sarecycline, and omadacycline. Tetracyclines act by inhibiting the connection between aminoacyl-tRNA and bacterial ribosomes, ultimately preventing the synthesis of bacterial proteins (Chopra & Roberts, Citation2001).
Despite its effectiveness, tetracyclines antibiotics can cause gastrointestinal disturbances (nausea, vomiting, and diarrhea), modifications to gut ecology, vaginal candidiasis, and permanent changes in tooth structure in fetuses (Chopra and Roberts, Citation2001). Tetracyclines can also cause liver and renal damage, as well as local tissue toxicity and photosensitivity (Odorici et al., Citation2021; Antagonists, Citation2012).
Although tetracyclines are available in a variety dosage forms, including tablets and oral suspensions, there have been attempts to deliver them transdermally (Zhao et al., Citation2023). However, the transdermal delivery of tetracycline is limited by their hydrophilicity with log P ranges between negative values and nearly zero. Hence, the passive delivery of tetracycline through skin still remains a major challenge. To overcome this issue, several approaches have been developed to enhance the transdermal delivery of tetracyclines, including the use of vesicular carriers (Honary & Zahir, Citation2012), liposomes (Hasanpouri et al., Citation2018), and solid-lipid microparticles (Rahimpour et al., Citation2016).
5.1.1. Doxycycline
Fan et al., Citation2009 developed a transdermal delivery system for doxycycline HCl using a hydrophilized polyvinylidene fluoride (PVDF) membrane. They immobilized a thermo-sensitive polymeric gel of poly(N-isopropylacrylamide) (PNIPAAM) or PNIPAAM-co-2 mol% acrylic acid (AA) on the surface and into the pores of the membrane to control the release of doxycycline HCl. The results of the permeation experiments, using mouse skin, showed that the release of doxycycline could be turned on and off based on the lower critical solution temperature (LCST) of the gel of 33 °C. When the temperature was 33 °C, which represents the ailing state, 30 μg/cm2 of doxycycline HCl was accumulated in the receptor phase after 24 h, with permeability and flux similar to those obtained from a standard PVDF/mouse skin composite. It should be noted that assumptions were made regarding the normal and diseased skin temperatures, which may vary based on various factors, such as body parts, age, and health conditions.
Nagy et al., 2022 fabricated a hybrid-bilayer patch for delivering doxycycline. The patch was comprised of electrospun nanofibers made of polyamide 6/tallow-modified clay and hydrogels composed of either sodium alginate, PVA, or a combination of sodium alginate and PVA. The hydrogels were physically crosslinked through repeated freezing-thawing cycles or ionically using CaCl2 solution. Doxycycline HCl was loaded into the patch by either active loading or adsorption techniques. Hydrogels showed porous structures. The in vitro drug release tests were performed in phosphate-buffered saline (PBS, pH 7.4) and acetate buffer (pH 5.5). The results demonstrated that the drug release rate was influenced by the hydrogel structure and crosslinking method. The physically crosslinked hydrogels of PVA or sodium alginate and PVA displayed a slow release rate. In addition, doxycycline loaded hydrogels retained its antimicrobial activity. Therefore, the fabricated patches exhibited a promising potential for the transdermal delivery of doxycycline (Shehab-ElDin et al., Citation2023).
Honary and Zahir, Citation2012 investigated the potential of using ascorbyl palmitate niosomes as a nanocarrier for doxycycline, either for transdermal or systemic administration. The niosomes were prepared by incorporating ascorbyl palmitate, cholesterol, and dicetyl phosphate (a negatively charged lipid) and then encapsulating the aqueous doxycycline solution within the vesicles before subjecting them to sonication. The vesicles were analyzed using UV-vis spectroscopy, differential scanning calorimetry (DSC), and scanning electron microscopy to evaluate their entrapment efficiency, in vitro release, thermal properties, and morphology. The study also examined the effect of sonication time, pH, hydration temperature, and centrifugation speed on the characteristics of niosomes. The results of the study revealed that niosomes were spherical in shape, retained more than 90% of the drug that released it slowly, with 60% being released after 8 h. The size of the vesicles was influenced by the sonication time and hydration temperature, with shorter sonication time and lower pH resulting in smaller vesicles. However, high hydration temperatures could cause drug degradation. Overall, ascorbyl palmitate, cholesterol, and dicetyl phosphate were found to be effective in forming niosomes suitable for doxycycline delivery.
Kashani-Asadi-Jafari and Hadjizadeh, Citation2022 fabricated niosome-encapsulated doxycycline hyclate for enhanced acne therapy. Different doxycycline-loaded niosomal formulations were developed and assessed for several parameters, including particle size, drug entrapment efficiency, zeta potential, drug penetration, skin deposition, cell viability, and antibacterial activity. The optimal formulation had a particle size of 362.88 ± 13.05 nm, an entrapment efficiency of 56.3 ± 2.1%, a zeta potential of −24.46 ± 1.39 mV, and a drug release of 54.93 ± 1.99% after 32 h. Compared to free drugs, the niosomal formulation exhibited improved cell viability, higher antibacterial activity, and approximately three-fold increased drug deposition. These findings showed that the doxycycline- loaded niosomes were more effective in treating acne than the free drug.
5.1.2. Tetracycline
A study conducted by Hamishehkar et al., 2018 investigated the potential of nanoliposomes and nanotransfersomes as means of delivering tetracycline hydrochloride through the skin to treat acne. The study utilized thin film hydration to create vesicular nanostructures, which were then evaluated for their physical properties such as size, zeta potential, and entrapment efficiency. The MIC values for tetracycline-loaded vesicles were compared to tetracycline in aqueous solution that were tested against skin infection. The effectiveness of drug delivery was evaluated by testing the in vitro drug release and ex vivo drug permeation through rat skin. The results showed that the particle size, zeta potential, and entrapment efficiency of liposomes were 75 nm, +17 mV, and 45%, respectively, and those of transfersomes were 78 nm, +7 mV, and 55%, respectively. The antimicrobial testing revealed no difference between the vesicular formulations and tetracycline in aqueous solution, which was used as a control. The skin permeation study demonstrated that tetracycline-loaded transfersomes had 1.6 times higher permeability than tetracycline-loaded liposomes, as confirmed by fluorescence microscope imaging.
In another study, Rahimpour et al., Citation2016 utilized solid-lipid microparticles (SLMs) to load tetracycline. SLMs were prepared using the spray drying method. The in vitro and ex vivo release behavior of tetracycline through SLMs and control formulations (aqueous Carbopol gels) was assessed over a period of 24 h using vertical Franz diffusion cells through cellulose acetate membranes and excised rat skin, respectively. SLMs exhibited high encapsulation efficiency of > 97% with no significant difference among formulations (p < .05). The in vitro release study demonstrated the sustained release pattern of tetracycline through SLMs. Additionally, the ex vivo skin permeation analysis revealed that the dermal deposition of tetracycline from the optimal SLMs formulation was nearly seven times that of the control formulations. The increased efficiency of acne therapy and reduced side effects associated with tetracycline were attributed to the enhanced skin penetration observed for tetracycline-loaded SLMs.
To tackle the antibiotic resistance problem, hydrogel-forming microarray patch (HF-MAP) was fabricated by Zhao et al., Citation2023 for the transdermal delivery system of tetracycline hydrochloride. The microarray was fabricated using PVP/poly(vinylpyrrolidone) (PVA/PVP) which showed excellent swelling properties of more than 600% swelling in PBS (pH 7.4) over 24 h. In addition, HF-MAP showed acceptable mechanically properties, where the tips of the microarray were able to penetrate the neonatal porcine skin. The results of the in vivo studies showed that the administration of tetracycline using microarrays achieved a sustained release profile compared to tetracycline administered by oral and IV administration. At 24 h, the maximum drug plasma concentration for microarray group was 7.40 ± 4.74 µg/mL, whereas the plasma concentrations for oral and IV groups were 5.86 ± 1.48 and 8.86 ± 4.19 µg/mL, respectively. The tetracycline plasma concentration in the oral and IV groups peaked immediately after drug administration and then dropped below the limit of detection after 24 h, suggesting that HF-MAP is a potential platform for delivering tetracycline in a sustained manner.
5.1.3. Minocycline
Guo et al., Citation2021 evaluated the effect of minocycline hydrochloride combined with photodynamic therapy on skin barrier function to treat acne. This evaluation was done by taking eighty-eight acne patients admitted to the hospital that were randomized into two groups, a research group (n = 44) that was photodynamically treated on the basis of minocycline hydrochloride and a control group (n = 44) that was orally treated with minocycline hydrochloride (50 mg/twice a day for 4 weeks). The transdermal water loss (TEWL), stratum corneum water content, and pH value were evaluated as skin barrier function indexes. TEWL was measured by TM300 skin moisture loss tester and the stratum corneum water content was measured by CM825 skin moisture tester, and the improvement of acne was evaluated by the Global Acne Grading System (GAGS). The results showed an improvement in skin barrier functions after treatment compared with those before treatment. In addition, the GAGS scores were lower than those before treatment. The study concluded that minocycline hydrochloride and photodynamic therapy significantly increased patients’ barrier function, reduced clinical symptoms, and enhanced overall efficacy and quality of life.
Kircik et al., Citation2020 assessed the topical foam formulation of minocycline (FMX101 4%) for the treatment of lesions in acne vulgaris. FMX101 4% was approved by the United States Food and Drug Administration (FDA) which was developed to reduce the systemic adverse effects associated with oral administration of minocycline. FMX101 4% is a hydrophobic formulation composed of soybean oil, coconut oil, light mineral oil, and cyclomethicone as suitable carriers for the formulation. The components of the foam-based products and the rationale for their selection, were explored as well as microbiologic data for optimal formulation relating sebum permeation, and disposition into skin layers. The effects of FMX101 4% formulation and an oil-in-water emulsion (OIWE) on the physical properties of the model human sebum were compared with those of several commercially available acne preparations. The artificial human sebum was developed to simulate the content and properties of human sebum. Three techniques were used to test the two formulations including DSC, rheometer, and light microscopy and assess the miscibility of test formulations with a model human sebum. The results showed that FMX101 4% achieved high amount of minocycline permeated into the receptor solution after 12 h of skin application (ranged between 24 and 417 ng/cm2) and the amount in dermis layer was 1680–2407 ng. According to the antimicrobial resistance study, the delivery of minocycline to the epidermis and sebaceous appendage was above the minimum inhibitory concentration for 90% of organisms (MIC90) of 0.25 µg/mL for minocycline, suggesting the low potential of developing antimicrobial resistance. Therefore, FMX101 4% provided high concentrations of the active ingredient in the epidermis and sebaceous appendage, where Cutibacterium acnes (C. acnes) is found. In addition, the topical treatment appeared to be well-tolerated while giving high skin concentrations of minocycline and minimizing systemic exposure to the drug.
Siddiqui et al., 2022 formulated nanoemulgel of minocycline for an improved drug delivery and longer retention time in the targeted area. Different oil-in-water (O/W) nanoemulsions were prepared by the oil phase titration method using eucalyptus oil, Tween 20, and Transcutol HP. Nanoemulsions were optimized using pseudo-ternary phase diagrams. The droplet size, viscosity, and refractive index were determined for the thermodynamically stable nanoemulsion. To formulate the nanoemulgel, the optimal nanoemulsion was suspended in 1.0% w/v of Carbopol 940 gel and the pH, viscosity, and spreadability of the nanoemulgel were measured. In addition, the texture analysis and ex vivo permeation for nanoemulgel and nanoemulsion were investigated. The results showed that the cumulative amounts of the drug permeated after 6 h from the minocycline nanoemulsion and nanoemulgel were 75% and 58%, indicating that the nanoemulgel showed a sustained release behavior. Therefore, the minocycline nanoemulgel is expected to treat acne rosacea with superior therapeutic results.
Finally, tetracycline antibiotics are known to be highly effective when used orally, yet side effects include skin irritation and gastrointestinal distress. Despite being available in a variety of oral formulations, their hydrophilic nature makes transdermal delivery challenging and presents a big obstacle. To address this, novel techniques such as vesicular carriers, liposomes, and solid-lipid microparticles have been developed to improve tetracycline transdermal delivery and before considering the potential advantages and safety issues of these techniques, preclinical studies should be conducted. In addition, physical approaches should be also explored for tetracycline antibiotics.
6. Transdermal delivery of macrolides antibiotics
6.1. Macrolides antibiotics
Macrolides are a group of natural compounds that consist of lactone ring and deoxy-sugars. Some macrolides have antimicrobial properties and are used in pharmaceutical antimicrobial therapy. Erythromycin (ERT), the first macrolide was discovered in 1952, which was isolated from the soil actinomycete Saccharopolyspora erythraea (Patel & Hashmi, Citation2022). ERT was frequently prescribed for infections in patients who were allergic to penicillin or whose infections were resistant to penicillin (Roberts, Citation2014). Other antibiotics in this class include, clarithromycin (CLR), roxithromycin (ROX), dirithromycin, azithromycin (AZT), josamycin, and spiramycin. Because of their superior pharmacokinetic properties, and lower gastrointestinal side effects, CLR and AZT are widely prescribed in respiratory tract infection (Whitman & Tunkel, Citation1992).
Macrolides have some advantages over other classes of antibiotics in the treatment of bacterial infections since they are effective against both obligatory and facultative intracellular infections. Furthermore, they have the ability to penetrate the acidic environment of macrophages and polymorph nuclear leukocytes. AZT is the most effective macrolide for the treatment of Helicobacter pylori (H. pylori) and Chlamydia trachomatis infections (Charles & Segreti, Citation1997).
All macrolides are poorly water soluble and have a weak base properties (Grübel and Cave, Citation1998; Fassbender et al., Citation1996; Periti et al., Citation1989). Based on the physicochemical properties, it appears that the transdermal delivery of macrolides may be challenging due to their relatively high molecular weight. ERT and CLR have a relatively high molecular weight of 733.9 and 748.0 Da, respectively (Hutton et al., Citation2020). In addition, ROX has a low water solubility and a relatively high molecular weight of 837 Da (Koopaei et al., Citation2012). However, the use of various transdermal delivery strategies such as the use of chemical enhancers (Yang et al., Citation2013; Główka et al., Citation2014) and ethosomes (Godin and Touitou, Citation2005) may help in enhancing the transdermal delivery of these drugs (Hutton et al., Citation2020). These strategies can help in increasing the solubility and permeability of macrolides across the skin barrier, leading to improved therapeutic outcomes.
The FDA has approved the use of ERT, CLR, and AZT in pediatrics with minor skin infections and otitis media (Parsad et al., Citation2003). Furthermore, CLR is utilized to treat H. pylori infections in the course of normal triple therapy (Matsumoto et al., Citation2019). Additionally, a recent study by Vitiello et al. (Vitiello & Ferrara, Citation2022) revealed that AZT had in vitro activity against a wide range of respiratory tract viruses, including SARS-CoV-2, which is responsible for the current global pandemic COVID-19 (Vitiello & Ferrara, Citation2022). Moreover, there have been several hypothesized antiviral modes of action for AZT against SARS-CoV-2 (Khoshnood et al., Citation2022; Yang and Hughes, Citation2020).
Macrolides exert their bacteriostatic or bactericidal effect by impeding bacterial protein synthesis (Vázquez-Laslop & Mankin, Citation2018). Specifically, macrolides bind to the 50S subunit of the bacterial ribosome, preventing the elongation of the peptide chain during protein synthesis (Vázquez-Laslop & Mankin, Citation2018). This ultimately cause an inhibition in bacterial growth. The most prevalent side effects of macrolides include gastrointestinal effects and hepatotoxicity. Recent studies on macrolides have also indicated a link between their use is and ototoxicity (Vanoverschelde et al., Citation2021). Macrolides can be delivered in a variety of forms depending on the intended drug and the purpose of its use. Oral formulations are the most common form, and they are also available as topical creams, IV formulations, and ocular preparations.
6.1.1. Erythromycin (ERT)
Godin & Touitou, Citation2005 were able to formulate erythromycin (ERT) ethosomes. The ERT ethosomes were stable for at least a year at room temperature without showing cytotoxicity against cultured fibroblasts. When tested on three bacterial strains, ERT ethosomes in hydroethanolic solutions were significantly more effective than ERT alone, resulting in greater inhibitory zones. In addition, ERT ethosomes reduced the MIC of ERT against S. aureus ATCC 29213 and the clinically isolated resistant S. aureus strain. These findings indicated that ethosomes can successfully deliver ERT to deep skin layers to eradicate staphylococcal infections.
In an attempt to treat acne, Kwon et al. Citation2014 investigated the use of PVA film as a transdermal drug carrier for ERT. The films were prepared with varying concentrations of ERT and different additives such as glycerin, polyethylene glycol (PEG), Tween 80, caprylic/capric triglyceride oil, and CMC. Both glycerin and PEG were suitable for fabricating films with a consistent thickness of 46 ± 5.4 μm. The results showed that ERT released from the PVA films exhibited a sustained release behavior, where adjusting the amount of ERT and additives allowed for controlling the amount of ERT released from films. It was found that ERT films continuously delivered ERT to the acne region and sustained its effect for 15 h.
6.1.2. Azithromycin (AZT)
Yang et al., Citation2013 conducted an investigation to assess how effective the transdermal administration of AZT could be in preventing skin flap infection in rats. In this study, S. aureus and E. coli were injected beneath the ischemic rat flaps to cause bacterial infection. The researchers administered AZT transdermal gels daily for seven days by combining the appropriate amount of AZT with ethanol, azone, and propylene glycol (PG) to produce 1, 2, and 3% AZT gels. Over time, the levels of AZT increased in flap tissues and the accumulative penetration quantities in a time-dependent manner. The AZT gels reduced the inflammation and increased the survival area of the flaps.
Qin et al., Citation2015 developed and evaluated AZT gels for transdermal application to enhance skin flap survival and decrease the potential risks associated with systemic drug delivery. The researchers dissolved AZT (1%), amlodipine besylate (0.5%), and low molecular weight heparin (300 IU/g) in 25 mL of 95% ethanol, 3% azone, and 10% PG in compound gels. In vitro, all three drugs penetrated the skin efficiently and in a time-dependent manner. Whereas in vivo, amlodipine or low molecular weight heparin enhanced blood supply in ischemic flaps. Additionally, gels containing AZT showed less flap inflammation. Moreover, the surviving areas after treatment with AZT/low molecular weight heparin or AZT/amlodipine were significantly larger than those treated with AZT only gel. Furthermore, treatment with all three drugs produced the largest surviving area. Gels that lacked AZT were ineffective in reducing flap inflammation or improving flap survival.
Zaid Alkilani et al., Citation2022 conducted a study with the objective of developing, characterizing, and optimizing niosomal gel of AZT. Niosomes were prepared using Span 60 and cholesterol at varying concentrations using the ether injection method. The particle size, PDI, and zeta potential of niosomes were assessed. The niosomes were found to have a size range of 288 to 394 nm with narrow size distributions (PDI < .95). The niosomal gel showed lower viscosity than its conventional counterpart. In the ex vivo skin permeation studies, the niosomal gel demonstrated significantly higher (p < .001) drug release (90.83 ± 3.19%) compared to the conventional gel (1.25 ± 0.12%) over 24 h. Overall, these findings suggested that the niosomal gel could be an effective nanocarrier for AZT transdermal delivery, offering an alternative route of administration for this BCS class III drug.
6.1.3. Clarithromycin (CLR)
A study was conducted by Zaid Alkilani and colleagues (Zaid Alkilani et al., Citation2023) to develop transdermal patches loaded with CLR niosomes, using the solvent casting technique. The niosomes were spherical in shape with a size ranged between 109.0 and 552.1 nm, based on the ratio of cholesterol:surfactant (Span 60). The niosomal size was uniform with PDI < 0.36 and the zeta potential of the niosomes ranged between −21.59 and −62.87 mV. The CLR encapsulation efficiency of niosomes was high of 86%. The optimal ratio of cholesterol:Span 60 for drug release was determined to be 0.5:1. The antimicrobial activity of CLR was not affected by the encapsulation into the niosomes. The niosomal patch showed significantly higher permeability coefficient of 102.1 ± 0.6 × 1 0 −3 cm/h and a flux of 380.2 ± 2.3 µg/cm2/h than those of the conventional patch. Thus, the niosomal-loaded transdermal patches could be a promising method for delivering class II drugs and drugs with gastrointestinal side effects through the skin.
6.1.4. Roxithromycin (ROX)
Glowka et al., 2014 (88) conducted a study to design and evaluate an organogel loaded with roxithromycin nanoparticles (ROX NPs) for follicular targeting. ROX was effectively loaded into polymeric nanoparticles, prepared with poly(epsilon caprolactone), with a particle size range of 277 ± 6 to 338 ± 5 nm, facilitating the delivery of ROX to hair follicles. The encapsulation efficiency of ROX was 61.7 ± 10.9%. Lyophilized ROX NPs were loaded into organogel, prepared using pluronic-lecithin at 1:5 (v/v) ratio. ROX NPs loaded organogels showed non-Newtonian, shear-thinning (pseudoplastic), and thixotropic behavior. The findings of the ex vivo human skin penetration studies showed that targeting the pilosebaceous unit can be achieved through ROX NPs (∼300 nm) formulated in either an aqueous suspension or a semi-solid topical organogel formulation. Several studies have shown that drug carriers, such as nanoparticles, can selectively penetrate and accumulate in hair follicles within the skin, leading to high concentrations of drugs in that area (Gu et al., Citation2022; Fang et al., Citation2014).
Generally, all macrolides are poorly soluble in water and have relatively high molecular weight and weak basic characteristics. Because of their physicochemical properties, delivering macrolides transdermally is difficult. However, using techniques such as chemical enhancers and solid lipid nanoparticles may help in improving transdermal drugs delivery. Since these methods have the potential to improve macrolide solubility and permeability across skin barrier. But preclinical studies should be conducted to evaluate the safety and effectiveness of these techniques.
7. Transdermal delivery of lincosamide antibiotics
7.1. Lincosamides
Lincosamides are a small group of antibiotics that include two antibiotics, lincomycin and clindamycin (Spížek & Řezanka, Citation2017). These antibiotics are active against Gram-positive cocci as well as the majority of an anaerobes. Lincosamides can be given orally, intramuscularly, or intravenously (Spížek & Řezanka, Citation2017). This type of antibiotics acts by inhibiting the synthesis of proteins (Spížek and Řezanka, Citation2017). Lincosamides can cause a range of adverse effects including; diarrhea, nausea, vomiting, and abdominal pain, that may vary from mild to severe side effects (Zaidi & Weier, Citation2019). Lincosamides may cause redness, itching, and contact dermatitis when applied topically (Kreft & Wohlrab, Citation2022). Patients who receive prolonged treatment with lincosamides should do complete blood count, liver and renal function monitored (Zaidi & Weier, Citation2019).
In terms of their physicochemical properties, both lincomycin and clindamycin exhibit physicochemical characteristics that could make them suitable for transdermal delivery. For instance, lincomycin, with a moderate log P value of .56 and a low molecular weight of 406.5 Da, may have good skin permeability (Arai and Niikura, Citation2021). Whereas, clindamycin has a higher log P value of 2.16 and a slightly higher molecular weight of 425.0 Da. The high water solubility of clindamycin of 30.61 mg/L suggested that it may be a candidate for the transdermal delivery (Khodaeiani et al., Citation2013). In general, the physicochemical properties of both drugs indicate that they have a great potential for transdermal delivery.
7.1.1. Clindamycin (CLI)
A study conducted by Abdellatif and Tawfeek, Citation2016 investigated the potential of delivering clindamycin (CLI) phosphate through transfersomes for transdermal delivery. The CLI transfersomes were prepared by the thin-film hydration technique. Then they were incorporated into a Carbopol 934 gel. Additionally, the properties of gels like pH, spreadability, viscosity, homogeneity, skin irritation, in vitro and ex vivo release and permeation studies, and stability were assessed. CLI transfersomes showed a uniform size of 351 ± 0.4 nm and a high entrapment efficiency of 93.3 ± 0.8%. Moreover, the surface charge of CLI transfersomes exhibited a high negative charge of −40 ± 0.5 mV, indicating the stability of CLI transfersomes by preventing their aggregation. The CLI transfersomal gel has a pH of 6.9 ± 0.2 and higher spreadability compared to the control gel formulation (6.5 ± 0.8 vs. 5.5 ± 0.7 g.cm/s). In addition, the CLI transfersomal gel exhibited a higher viscosity than the control gel formulation, attributed to the incorporation of CLI transfersomes, which are formulated with Span 80. Moreover, CLI transfersomal gel showed higher cumulative drug penetration and flux than CLI transfersomal suspension and the control CLI Carbopol gel. The findings of this study indicated that CLI transfersomes are promising carriers for improving the dermal delivery of CLI.
Tiraton et al., Citation2022 developed CLI transdermal microneedle patch for the treatment of acne vulgaris. The microneedle patch was fabricated from sodium alginate-gelatin containing different concentrations of CLI hydrochloride (6, 8, and 10 μmol/L). CLI microneedles showed acceptable mechanical properties, where the addition of gelatin increased the mechanical properties of microneedles. Whereas the addition of CLI did not affect the mechanical properties of microneedles. It has been shown that as the loading amount of CLI increased, the released amount of CLI was increased. The microneedles successfully penetrated the piglet skin and inhibited the growth of C. acnes. Furthermore, CLI microneedles were safe to normal human skin fibroblasts. Therefore, sodium alginate-gelatin transdermal microneedle patch could be a potential platform for delivering CLI for the treatment of acne vulgaris.
It is worth-noting that there have been limited studies on the transdermal delivery of lincosamides, exploring the use of microneedles and transfersomes for this purpose. For instance, the development of micron-sized needles loaded with CLI for the treatment of acne is an innovative approach, as it allows for the targeted delivery of the medication directly to the affected area (Tiraton et al., Citation2022). Transfersomes, on the other hand, are lipid-based vesicles that can encapsulate drugs and enhance their transdermal delivery. The incorporation of transfersomes into a gel formulation further enhanced their suitability for the transdermal delivery, as gels can provide a sustained release of drug over a longer period of time (Abdellatif and Tawfeek, Citation2016).
Further research in this area may help to identify new approaches for the transdermal delivery of lincosamide antibiotics, which could potentially improve the treatment outcomes and patient adherence. Upon conducting literature survey, it has been shown that the transdermal delivery of lincomycin has not been explored, necessitating more research studies for delivering it transdermally.
8. Challenges and strategies
Several obstacles stand out in the area of transdermal delivery of antibiotics. Novel approaches have been used to overcome these obstacles. These include the use of nanotechnology, chemical enhancers, iontophoresis and electroporation, and microneedles. However, the stratum corneum barrier, dynamic nature of the skin, skin penetration inconsistencies, safety concerns, user-friendly delivery systems and regulatory issues are all challenges. Preclinical and clinical research are still essential to evaluate the efficacy and safety of various delivery strategies while considering various patient profiles and needs. In addition, the transdermal delivery of antibiotics is limited by their physicochemical properties such as low log P that ranges between negative values and nearly zero and hydrophilicity. Hence, the passive delivery of most of them through skin still remains a major challenge. To overcome this issue, further research in this area is still needed to identify new approaches for the transdermal delivery of antibiotics, which could potentially improve the treatment outcomes and patient adherence.
9. Conclusion and future perspectives
The issue of effectively delivering antibiotics through the skin in large daily doses remains a problem that has yet to be fully addressed. In addition, the serious adverse-effects and the increased incidence of bacterial resistance, associated with the long-term usage of antibiotics, warrant stringent action. This suggests new advancement in the field of transdermal delivery strategies for antibiotics. Although several strategies have been developed to deliver many antibiotic drugs transdermally, further research is still needed to transdermally deliver more antibiotic drugs such as lincomycin, cefuroxime and monobactams. Many chemical (such as nano-systems) and physical (like micronnedles and iontophoresis) strategies have been separately used to deliver antibiotics into the skin, yet using a combination of these two strategies, owing to their synergistic delivery effects, is still limited and need further elucidation.
Owing to the complex structure of the skin and its barrier function which hindered the delivery of the antibiotics-loaded nanosystems into deep skin layers, novel nanosystems are currently needed. This opens the doors for researchers to develop novel nanosystem formulations of ideal size (∼ 300 nm) and shape (spherical, triangular, or rod NPs). In addition, the use of biocompatible and nontoxic nanocarriers expanded the scope of developing novel nanosystems, where additives can either enhance the antibacterial activity or may cause skin irritation and dermatitis. This may ultimately achieve different antibiotics-loaded nanosystems with specific skin penetration mechanism and highest drug efficacy, where the physicochemical properties and composition of nanosystems impact their skin penetration depth based and consequently their specific therapeutic action ().
Table 1. The structures and mechanisms of action for the reported antibiotics.
Ethical approval
NA.
Authors’ contribution
Ahlam Zaid Alkilani, Rania Hamed, Batool Musleh and Zaina Sharaire.; writing-original draft preparation; Batool Musleh and Zaina Sharaire; drawing the Figures; Ahlam Zaid Alkilani and Rania Hamed; writing-review and editing. All authors have read and agreed to the published version of the manuscript.
Acknowledgements
The authors thank the Deanship of Scientific Research at Zarqa University.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Data sharing not applicable.
Additional information
Funding
References
- Abdellatif AA, Tawfeek HM. (2016). Transfersomal nanoparticles for enhanced transdermal delivery of clindamycin. AAPS PharmSciTech 17:1–15. doi: 10.1208/s12249-015-0441-7.
- Abu-Sini MK, Maharmah RA, Abulebdah DH, et al. (2023). Isolation and identification of coliform bacteria and multidrug-resistant Escherichia coli from water intended for drug compounding in community pharmacies in Jordan. Healthcare 11:299. doi: 10.3390/healthcare11030299.
- Alfei S, Schito AM. and A.M. (2022). Schito, β-Lactam Antibiotics and β-Lactamase enzymes inhibitors, Part 2: our limited resources. Pharmaceuticals 15:476. doi: 10.3390/ph15040476.
- Alkilani AZ, McCrudden MT, Donnelly RF. (2015). Transdermal drug delivery: innovative pharmaceutical developments based on disruption of the Barrier Properties of the stratum corneum. Pharmaceutics 7:438–70. doi: 10.3390/pharmaceutics7040438.
- Alkilani AZ, Nasereddin J. (2022). Beneath the Skin: A Review of Current Trends and Future Prospects of Transdermal Drug Delivery Systems 14:1152.
- Alotaibi BS, Ashames A, Buabeid M, et al. (2022). A new approach for the management of Escherichia coli and Klebsiella pneumonia by using cefixime-based bionanocomposite films. J Exp Nanosci 17:389–419. doi: 10.1080/17458080.2022.2080197.
- Altun E, Yuca E, Ekren N, et al. (2021). Kinetic release studies of antibiotic patches for local transdermal delivery. Pharmaceutics 13:613., doi: 10.3390/pharmaceutics13050613.
- Antagonists TNF. (2012). LiverTox: Clinical and research information on drug-Induced liver injury. Bethesda, MD, USA: National Institute of Diabetes and Digestive and Kidney Diseases,
- Arai J, Niikura R. (2021). Use of antibiotics and probiotics reduces the risk of metachronous gastric cancer after endoscopic resection. Biology 10:455.
- Bagyalakshmi J, Vamsikrishna RP, Manavalan R, et al. (2007). Formulation development and in vitro and in vivo evaluation of membrane-moderated transdermal systems of ampicillin sodium in ethanol: pH 4.7 buffer solvent system. AAPS PharmSciTech 8:7–E55. doi: 10.1208/pt0801007.
- Bandyopadhyay D. (2021). Topical antibacterials in dermatology. Indian J Dermatol 66:117–25. doi: 10.4103/ijd.IJD_99_18.
- Basheer HA, Alhusban MA, Zaid Alkilani A, et al. (2023). Niosomal delivery of Celecoxib and Metformin for targeted breast cancer treatment. Cancers 15:5004. doi: 10.3390/cancers15205004.
- Batt MD, Fairhurst E. (1986). Hydration of the stratum corneum. Int J Cosmet Sci 8:253–64. doi: 10.1111/j.1467-2494.1986.tb00583.x.
- Benson HA. (2005). Transdermal drug delivery: penetration enhancement techniques. Curr Drug Deliv 2:23–33. doi: 10.2174/1567201052772915.
- Bush K, Bradford PA. (2016). β-Lactams and β-lactamase inhibitors: an overview. Cold Spring Harb Perspect Med 6:a025247. doi: 10.1101/cshperspect.a025247.
- Charles L, Segreti J. (1997). Choosing the right macrolide antibiotic. A guide to selection. Drugs 53:349–57. doi: 10.2165/00003495-199753030-00002.
- Chopra I, Roberts M. (2001). Tetracycline antibiotics: mode of action, applications, molecular biology, and epidemiology of bacterial resistance. Microbiol Mol Biol Rev 65:232–60 ; second page, table of contents. doi: 10.1128/MMBR.65.2.232-260.2001.
- Craig WA, Andes DR. (2015). Chapter 1. 21 - Cephalosporins, in Mandell, Douglas, and Bennett’s Principles and practice of infectious diseases (eighth edition), J.E. Bennett, R. Dolin, and M.J. Blaser, Editors. W.B. Saunders: Philadelphia, 278–92.e4.
- Cui Z, Zheng Z, Lin L, et al. (2018). Electrospinning and crosslinking of polyvinyl alcohol/chitosan composite nanofiber for transdermal drug delivery. Adv Polym Technol 37:1917–28. doi: 10.1002/adv.21850.
- Demain AL, Elander R. P. (1999). The beta-lactam antibiotics: past, present, and future. Antonie Van Leeuwenhoek 75:5–19. doi: 10.1023/a:1001738823146.
- Eiamphungporn W, Schaduangrat N, Malik AA, et al. (2018). Tackling the antibiotic resistance caused by class A β-lactamases through the use of β-lactamase inhibitory protein. Int J Mol Sci 19:2222. doi: 10.3390/ijms19082222.
- Erkus H, Bedir T, Kaya E, et al. (2023). Innovative transdermal drug delivery system based on amoxicillin-loaded gelatin methacryloyl microneedles obtained by 3D printing. Materialia 27:101700. doi: 10.1016/j.mtla.2023.101700.
- Fan Q, Sirkar KK, Wu J. (2009). A thermo-sensitive release system based on polymeric membrane for transdermal delivery of doxycycline HCl. J Membr Sci 337:175–81. doi: 10.1016/j.memsci.2009.03.032.
- Fanani ML, Nocelli NE, Zulueta Díaz YdlM. (2022). What can we learn about amphiphile-membrane interaction from model lipid membranes? Biochim Biophys Acta Biomembr 1864:183781. doi: 10.1016/j.bbamem.2021.183781.
- Fang C-L, Aljuffali IA, Li Y-C, et al. (2014). Delivery and targeting of nanoparticles into hair follicles. Ther Deliv 5:991–1006. doi: 10.4155/tde.14.61.
- Fassbender M, Lode H, Schiller C, et al. (1996). Comparative pharmacokinetics of macrolide antibiotics and concentrations achieved in polymorphonuclear leukocytes and saliva. Clin Microbiol Infect 1:235–43., doi: 10.1016/S1198-743X(15)60281-6.
- Gao W, Chen Y, Zhang Y, et al. (2018). Nanoparticle-based local antimicrobial drug delivery. Adv Drug Deliv Rev 127:46–57. doi: 10.1016/j.addr.2017.09.015.
- Ge X, Wei M, He S, et al. (2019). Advances of non-ionic surfactant vesicles (Niosomes) and their application in drug delivery. Pharmaceutics 11:55. doi: 10.3390/pharmaceutics11020055.
- Główka E, Wosicka-Frąckowiak H, Hyla K, et al. (2014). Polymeric nanoparticles-embedded organogel for roxithromycin delivery to hair follicles. Eur J Pharm Biopharm 88:75–84.
- Godin B, Touitou E. (2005). Erythromycin ethosomal systems: physicochemical characterization and enhanced antibacterial activity. CDD 2:269–75. doi: 10.2174/1567201054367931.
- González-Vázquez P, Larrañeta E, McCrudden MTC, et al. (2017). Transdermal delivery of gentamicin using dissolving microneedle arrays for potential treatment of neonatal sepsis. J Control Release 265:30–40. doi: 10.1016/j.jconrel.2017.07.032.
- Grübel P & Cave D R (1998). Factors affecting solubility and penetration of clarithromycin through gastric mucus. Aliment Pharmacol Ther 12:569–76. doi: 10.1046/j.1365-2036.1998.00329.x.
- Gu Y, Bian Q, Zhou Y, et al. (2022). Hair follicle-targeting drug delivery strategies for the management of hair follicle-associated disorders. Asian J Pharm Sci 17:333–52.
- Guo K, et al. (2021). Effect of minocycline hydrochloride combined with photodynamic therapy on skin barrier function of patients with acne. Am J Transl Res 13:8427–32.
- Gusliakova O, Verkhovskii R, Abalymov A, et al. (2021). Transdermal platform for the delivery of the antifungal drug naftifine hydrochloride based on porous vaterite particles. Mater Sci Eng C Mater Biol Appl 119:111428. doi: 10.1016/j.msec.2020.111428.
- Hamed R, Abu Alata W, Abu-Sini M, et al. (2023). Development and comparative evaluation of ciprofloxacin nanoemulsion-loaded bigels prepared using different ratios of oleogel to hydrogels. Gels 9:592. doi: 10.3390/gels9070592.
- Hamed R, Mahmoud NN, Alnadi SH, et al. (2020). Diclofenac diethylamine nanosystems-loaded bigels for topical delivery: development, rheological characterization, and release studies. Drug Dev Ind Pharm 46:1705–15. doi: 10.1080/03639045.2020.1820038.
- Hasanpouri A, et al. (2018). Improvement of dermal delivery of tetracycline using vesicular nanostructures. Res Pharm Sci 13:385–93.
- Hatanaka T, Kamon T, Morigaki S, et al. (2000). Ion pair skin transport of a zwitterionic drug, cephalexin. J Control Release 66:63–71. doi: 10.1016/S0168-3659(99)00259-X.
- Honary S, Zahir F. (2012). Effect of process factors on the properties of doxycycline nanovesicles. Trop J Pharm Res 11:169–75. doi: 10.4314/tjpr.v11i2.1.
- Hubschwerlen C. (2007). 7.17 - β-Lactam Antibiotics. In: Comprehensive medicinal chemistry II, J.B. Taylor and D.J. Triggle, editors. Elsevier: Oxford. 479–518.
- Hutton ARJ, McCrudden MTC, Larrañeta E, et al. (2020). Influence of molecular weight on transdermal delivery of model macromolecules using hydrogel-forming microneedles: potential to enhance the administration of novel low molecular weight biotherapeutics. J Mater Chem B 8:4202–9. doi: 10.1039/D0TB00021C.
- Iqbal H, Khan BA, Khan ZU, et al. (2020). Fabrication, physical characterizations and in vitro antibacterial activity of cefadroxil-loaded chitosan/poly(vinyl alcohol) nanofibers against Staphylococcus aureus clinical isolates. Int J Biol Macromol 144:921–31. doi: 10.1016/j.ijbiomac.2019.09.169.
- Ita K. (2016). Perspectives on Transdermal Electroporation. Pharmaceutics 8:9. doi: 10.3390/pharmaceutics8010009.
- Jamaledin R, et al. (2020). Advances in antimicrobial microneedle patches for combating infections. Adv Mater 32:e2002129.
- Jeong WY, Kwon M, Choi HE, et al. (2021). Recent advances in transdermal drug delivery systems: a review. Biomater Res 25:24. doi: 10.1186/s40824-021-00226-6.
- Kanoh S, Rubin BK. (2010). Mechanisms of action and clinical application of macrolides as immunomodulatory medications. Clin Microbiol Rev 23:590–615. doi: 10.1128/CMR.00078-09.
- Kashani-Asadi-Jafari F, Hadjizadeh A. (2022). Niosome-encapsulated doxycycline hyclate for potentiation of acne therapy: formulation and characterization. PNT 10:56–68. doi: 10.2174/2211738510666220224103406.
- Kazi KM, et al. (2010). Niosome: a future of targeted drug delivery systems. J Adv Pharm Technol Res 1:374–80.
- Khodaeiani E, Fouladi RF, Amirnia M, et al. (2013). Topical 4% nicotinamide vs. 1% clindamycin in moderate inflammatory acne vulgaris. Int J Dermatol 52:999–1004. doi: 10.1111/ijd.12002.
- Khoshnood S, Shirani M, Dalir A, et al. (2022). Antiviral effects of azithromycin: a narrative review. Biomed Pharmacother 147:112682. doi: 10.1016/j.biopha.2022.112682.
- Kircik L, et al. (2020). Formulation and profile of FMX101 4% minocycline topical foam for the treatment of Acne Vulgaris. J Clin Aesthet Dermatol 13:14–21.
- Koopaei MN, et al. (2012). Enhanced antibacterial activity of roxithromycin loaded pegylated poly lactide-co-glycolide nanoparticles. DARU J. Pharm. Sci 20:1–8.
- Kostrzębska A, et al. (2023). Effect of hydrogel substrate components on the stability of tetracycline hydrochloride and swelling activity against model skin sebum. Int J Mol Sci 24:2678.
- Kreft B, Wohlrab J. (2022). Contact allergies to topical antibiotic applications. ALS 6:18–26. doi: 10.5414/ALX02253E.
- Kumar TR, Soppimath K, Nachaegari SK. (2006). Novel delivery technologies for protein and peptide therapeutics. Curr Pharm Biotechnol 7:261–76. doi: 10.2174/138920106777950852.
- Kwon JS, Kim DY, Seo HW, et al. (2014). Preparation of erythromycin-loaded poly(vinylalcohol) film and investigation of its feasibility as a transdermal delivery carrier. Tissue Eng Regen Med 11:211–6. doi: 10.1007/s13770-014-0018-7.
- Leong MY, Kong YL, Burgess K, et al. (2023). Recent development of nanomaterials for transdermal drug delivery. Biomedicines 11:1124. doi: 10.3390/biomedicines11041124.
- Lima LM, Silva B. N M d, Barbosa G, et al. (2020). β-lactam antibiotics: an overview from a medicinal chemistry perspective. Eur J Med Chem 208:112829. doi: 10.1016/j.ejmech.2020.112829.
- Liu L, Zhao W, Ma Q, et al. (2023). Functional nano-systems for transdermal drug delivery and skin therapy. Nanoscale Adv 5:1527–58. doi: 10.1039/d2na00530a.
- Maji R, Omolo CA, Jaglal Y, et al. (2021). A transferosome-loaded bigel for enhanced transdermal delivery and antibacterial activity of vancomycin hydrochloride. Int J Pharm 607:120990. doi: 10.1016/j.ijpharm.2021.120990.
- Malinovskaja-Gomez K, Labouta HI, Schneider M, et al. (2016). Transdermal iontophoresis of flufenamic acid loaded PLGA nanoparticles. Eur J Pharm Sci 89:154–62. doi: 10.1016/j.ejps.2016.04.034.
- Mannem V, Nanjarapalle C, Stagni G. (2014). Iontophoresis of amoxicillin and cefuroxime: rapid therapeutic concentrations in skin. Drug Dev Ind Pharm 40:325–9. doi: 10.3109/03639045.2012.760579.
- Matsumoto H, Shiotani A, Graham DY. (2019). Current and future treatment of helicobacter pylori infections. Adv Exp Med Biol 1149:211–25.
- McAlister E, Dutton B, Vora LK, et al. (2021). Directly compressed tablets: a novel drug-containing reservoir combined with hydrogel-forming microneedle arrays for transdermal drug delivery. Adv Healthcare Materials 10:2001256. doi: 10.1002/adhm.202001256.
- Mehta D, Sharma AK. (2016). Cephalosporins: a review on imperative class of antibiotics. Inventi Rapid: Mol Pharmacol 1:1–6.
- Nasrollahzadeh M, Ganji F, Taghizadeh SM, et al. (2022). Drug in adhesive transdermal patch containing antibiotic-loaded solid lipid nanoparticles. J Biosci Bioeng 134:471–6.
- Odorici G, Monfrecola G, Bettoli V. (2021). Tetracyclines and photosensitive skin reactions: a narrative review. Dermatol. Ther 34:e14978.
- Pancu DF, Scurtu A, Macasoi IG, et al. (2021). Antibiotics: conventional therapy and natural compounds with antibacterial activity—a pharmaco-toxicological screening. Antibiotics 10:401. doi: 10.3390/antibiotics10040401.
- Parsad D, Pandhi R, Dogra S. (2003). A guide to selection and appropriate use of macrolides in skin infections. Am J Clin Dermatol 4:389–97. doi: 10.2165/00128071-200304060-00003.
- Patel PH, Hashmi MF. (2022). Macrolides, in StatPearls. Treasure Island (FL): StatPearls Publishing Copyright © 2022, StatPearls Publishing LLC.:
- Pérez-Martínez CJ, Morales Chávez SD, del Castillo-Castro T, et al. (2016). Electroconductive nanocomposite hydrogel for pulsatile drug release. React Funct Polym 100:12–7. doi: 10.1016/j.reactfunctpolym.2015.12.017.
- Periti P, Mazzei T, Mini E, et al. (1989). Clinical pharmacokinetic properties of the macrolide antibiotics. Clin Pharmacokinet 16:193–214. doi: 10.2165/00003088-198916040-00001.
- Polat BE, Hart D, Langer R, et al. (2011). Ultrasound-mediated transdermal drug delivery: mechanisms, scope, and emerging trends. J Control Release 152:330–48. doi: 10.1016/j.jconrel.2011.01.006.
- Prausnitz MR, Langer R. (2008). Transdermal drug delivery. Nat Biotechnol 26:1261–8. doi: 10.1038/nbt.1504.
- Puri A, et al. (2019). Development of a transdermal delivery system for tenofovir alafenamide, a prodrug of tenofovir with potent antiviral activity against HIV and HBV. Pharmaceutics 11:173.
- Qin Y-H, Jiao H-S, Li A-S, et al. (2015). Transdermal application of azithromycin-amlodipine-heparin gel enhances survival of infected random ischaemic flap. J Plast Surg Hand Surg 49:319–26. doi: 10.3109/2000656X.2015.1042386.
- Rabiei M, Kashanian S, Samavati SS, et al. (2020). Nanomaterial and advanced technologies in transdermal drug delivery. J Drug Target 28:356–67. doi: 10.1080/1061186X.2019.1693579.
- Rahimpour Y, Javadzadeh Y, Hamishehkar H. (2016). Solid lipid microparticles for enhanced dermal delivery of tetracycline HCl. Colloids Surf B Biointerfaces 145:14–20. doi: 10.1016/j.colsurfb.2016.04.034.
- Roberts DJ. (2014). Erythromycin. In: P. Wexler, editor Encyclopedia of toxicology (third edition),. Academic Press: Oxford, 453–8.
- Safdari H, Neshani A, Sadeghian A, et al. (2014). Potent and selective inhibitors of class A β-lactamase: 7-prenyloxy coumarins. J Antibiot 67:373–7. doi: 10.1038/ja.2014.9.
- Salatin S, Jelvehgari M. (2020). Desirability function approach for development of a thermosensitive and bioadhesive nanotransfersome-hydrogel hybrid system for enhanced skin bioavailability and antibacterial activity of cephalexin. Drug Dev Ind Pharm 46:1318–33. doi: 10.1080/03639045.2020.1788068.
- Shaaban MI, Shaker MA, Mady FM. (2017). Imipenem/cilastatin encapsulated polymeric nanoparticles for destroying carbapenem-resistant bacterial isolates. J Nanobiotechnol 15:29. doi: 10.1186/s12951-017-0262-9.
- Shehab-ElDin AN, Sobh RA, Rabie AM, et al. (2023). Polyamide 6/tallow modified clay nanofibrous mat coupled with hydrogels for potential topical/transdermal delivery of doxycycline hydrochloride. J Pharm Investig 53:307–21. doi: 10.1007/s40005-022-00598-4.
- Siddiqui A, Jain P. (2022). Investigation of a minocycline-loaded nanoemulgel for the treatment of Acne Rosacea. Biochem Pharmacol 14:20–8.
- Spížek J, Řezanka T. (2017). Lincosamides: chemical structure, biosynthesis, mechanism of action, resistance, and applications. Biochem. Pharmacol 133:20–8. doi: 10.1016/j.bcp.2016.12.001.
- Tenson T, Lovmar M, Ehrenberg M. (2003). The mechanism of action of macrolides, lincosamides and streptogramin B reveals the nascent peptide exit path in the ribosome. J Mol Biol 330:1005–14. doi: 10.1016/s0022-2836(03)00662-4.
- Thakuria B, Lahon K. (2013). The beta lactam antibiotics as an empirical therapy in a developing country: an update on their current status and recommendations to counter the resistance against them. J Clin Diagn Res 7:1207–14.
- Tiraton T, Suwantong O, Chuysinuan P, et al. (2022). Biodegradable microneedle fabricated from sodium alginate-gelatin for transdermal delivery of clindamycin. Mater Today Commun 32:104158. doi: 10.1016/j.mtcomm.2022.104158.
- Vanoverschelde A, Oosterloo BC, Ly NF, et al. (2021). Macrolide-associated ototoxicity: a cross-sectional and longitudinal study to assess the association of macrolide use with tinnitus and hearing loss. J Antimicrob Chemother 76:2708–16. doi: 10.1093/jac/dkab232.
- Vázquez-Laslop N, Mankin AS. (2018). How Macrolide Antibiotics Work. Trends Biochem Sci 43:668–84. doi: 10.1016/j.tibs.2018.06.011.
- Vitiello A, Ferrara F. (2022). A short focus, azithromycin in the treatment of respiratory viral infection COVID-19: efficacy or inefficacy? Immunol Res 70:129–33. doi: 10.1007/s12026-021-09244-x.
- Wang F-Y, Chen Y, Huang Y-Y, et al. (2021). Transdermal drug delivery systems for fighting common viral infectious diseases. Drug Deliv Transl Res 11:1498–508. doi: 10.1007/s13346-021-01004-6.
- Whitman MS, Tunkel AR. (1992). Azithromycin and clarithromycin: overview and comparison with erythromycin. Infect Control Hosp Epidemiol 13:357–68.
- Yang P, Qin Y-H, Zhong L, et al. (2013). Prevention of skin flap infection by transdermal penetration of azithromycin in rats. Ann Plast Surg 71:214–8. doi: 10.1097/SAP.0b013e31823dce96.
- Yang Q, Hughes TA. (2020). Inhibition of SARS-CoV-2 Viral Entry upon Blocking N- and O-Glycan Elaboration. elife 9:e61552.
- Yu X, Zhao J, Fan D. (2022). A dissolving microneedle patch for antibiotic/Enzymolysis/Photothermal triple therapy against bacteria and their biofilms. J Chem Eng 437:135475. doi: 10.1016/j.cej.2022.135475.
- Yu Y-Q, Yang X, Wu X-F, et al. (2021). Enhancing permeation of drug molecules across the skin via delivery in nanocarriers: novel strategies for effective transdermal applications. Front Bioeng Biotechnol 9:646554. doi: 10.3389/fbioe.2021.646554.
- Zaid Alkilani A, Abo-Zour H, Basheer HA, et al. (2023). Development and evaluation of an innovative approach using niosomes based polymeric microneedles to deliver dual antioxidant drugs. Polymer 15:1962. doi: 10.3390/polym15081962.
- Zaid Alkilani A, Abu-Zour H, Alshishani A, et al. (2022). Formulation and evaluation of niosomal alendronate sodium encapsulated in polymeric microneedles: in vitro studies, stability study and cytotoxicity study. Nanomaterials 12:3570. doi: 10.3390/nano12203570.
- Zaid Alkilani A, Hamed R, Abdo H, et al. (2022). Formulation and evaluation of azithromycin-loaded niosomal gel: optimization, in vitro studies, rheological characterization, and cytotoxicity study. ACS Omega 7:39782–93. doi: 10.1021/acsomega.2c03762.
- Zaid Alkilani A, Musleh B, Hamed R. (2023). Preparation and characterization of patch loaded with clarithromycin nanovesicles for transdermal drug delivery. J Funct Biomater 14:57.
- Zaid Alkilani A, Nimrawi S, Al-Nemrawi NK, et al. (2022). Microneedle-assisted transdermal delivery of amlodipine besylate loaded nanoparticles. Drug Dev Ind Pharm 48:322–32. doi: 10.1080/03639045.2022.2112694.
- Zaidi STR, Weier NE. (2019). bacterial infections and the role of the pharmacist. In: Z.-U.-D. Babar, Editor. Encyclopedia of pharmacy practice and clinical pharmacy. Elsevier: Oxford. 730–41.
- Zakrewsky M, Lovejoy KS, Kern TL, et al. (2014). Ionic liquids as a class of materials for transdermal delivery and pathogen neutralization. Proc Natl Acad Sci USA 111:13313–8. doi: 10.1073/pnas.1403995111.
- Zhao L, Vora LK, Kelly SA, et al. (2023). Hydrogel-forming microarray patch mediated transdermal delivery of tetracycline hydrochloride. J Control Release 356:196–204.