Abstract
High intensity focused ultrasound (HIFU) has demonstrated its safety, efficacy and noninvasiveness in the ablation of solid tumor. However, its further application is limited by its inherent deficiencies, such as postoperative recurrence caused by incomplete ablation and excessive intensity affecting surrounding healthy tissues. Recent research has indicated that the integration of nanomaterials with HIFU exhibits a promising synergistic effect in tumor ablation. The concurrent utilization of nanomaterials with HIFU can help overcome the limitations of HIFU by improving targeting and ablation efficiency, expanding operation area, increasing operation accuracy, enhancing stability and bio-safety during the process. It also provides a platform for multi-therapy and multi-mode imaging guidance. The present review comprehensively expounds upon the synergistic mechanism between nanomaterials and HIFU, summarizes the research progress of nanomaterials as cavitation nuclei and drug carriers in combination with HIFU for tumor ablation. Furthermore, this review highlights the potential for further exploration in the development of novel nanomaterials that enhance the synergistic effect with HIFU on tumor ablation.
1. Introduction
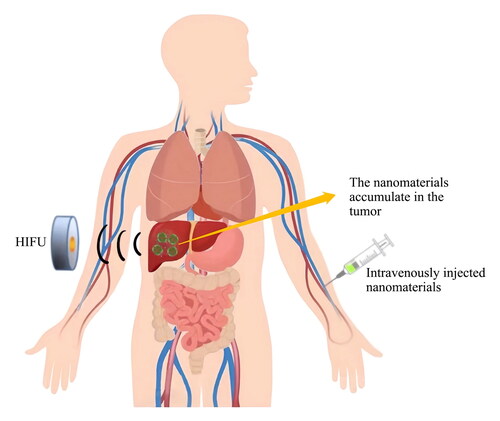
High intensity focused ultrasound (HIFU) presents a viable approach for entirely noninvasive cancer treatment, with real-time monitoring and management possible through diagnostic ultrasound or magnetic resonance imaging (Al-Bataineh et al., Citation2012). In recent years, HIFU ablation technology has developed rapidly. It was initially used mainly for the treatment of benign tumors such as uterine fibroids and uterine adenomyosis, and subsequently expanding to the treatment of liver cancer (Tsang et al., Citation2021), pancreatic cancer (Sofuni et al., Citation2022), breast cancer (Feril et al., Citation2021), kidney cancer (Cranston et al., Citation2021), prostate cancer (Napoli et al., Citation2020) and soft tissue tumors in multiple locations, etc. It has demonstrated its wide application prospects and become an important means of local ablation treatment for solid tumors.
HIFU has great potential due to its noninvasive nature as a treatment method. However, its low efficiency and unsatisfactory security limit its application in clinical settings (Dai et al., Citation2017). Thermal effect, due to acoustic attenuation, is difficult to achieve the desired ablation effect. Increasing the intensity of HIFU can improve the therapeutic effect, but it also raises the risk of damage to the acoustic tract and surrounding normal tissues. Conventional microbubble contrast agents can enhance the ultrasound signal and act as cavitation bubbles to enhance the ultrasound cavitation effect. However, microbubbles in the micron sizes has a poor stability, making it difficult to pass through the endothelium and deposit in tumor tissue. Therefore, there is an urgent need to explore a safer method to improve the efficiency of HIFU treatment.
The concept of combining HIFU with nanomaterials provides a new perspective That could enhance HIFU efficacy. This combination could lead to several benefits, including: (i) improved ultrasound imaging resolution, such as visualization of angiogenic vessels, (ii) attachment or encapsulation of tumor chemotherapeutic agents or gases and a controlled release under ultrasound application, and (iii) gentler heating extent at the tumor site (Alphandéry, Citation2022). Moreover, research indicates that ultrasonic treatment enhances the precision of chemotherapy drug administration, boosts the effectiveness of drug absorption, and escalates the cell-killing impact on designated cancerous cells (Panzone et al., Citation2022).
This article provides a review of research on the combination of HIFU with nanomaterials. This aim is to enhance our understanding of this field and continuously update the current status. The ultimate goal is to promote the application of HIFU in tumor treatment, and achieve better localization accuracy and ablation effect.
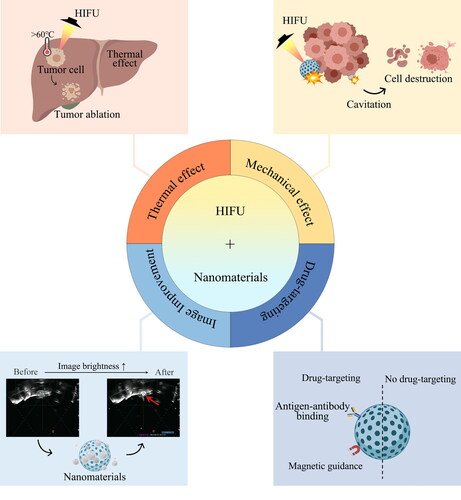
2. Mechanism of synergistic application of HIFU and nanomaterials
The Mechanism of synergistic application of HIFU and nanomaterials includes thermal effects, mechanical effects, image brightness improvement and targeting ().
Thermal effect is the main mechanism of HIFU (Zhou YF, Citation2011; Khokhlova et al., Citation2015). Through the in vivo use of low energy in vitro ultrasonic focus, HIFU can rapidly produce 60 °C or higher temperature at the treatment site (Dubinsky et al., Citation2008), leading to the necrosis and coagulation of diseased tissue within a few seconds. However, the relatively low therapeutic efficacy has emerged as a key limiting factor for the further clinical use of HIFU. The ultrasonic energy decreases exponentially with the increase of the propagation (Kim et al., Citation2014; Cohen et al., Citation2016) distance, which leads to underdose of ultrasound energy in deep tumor tissues (Kim et al., Citation2014; Cohen et al., Citation2016). Furthermore, the presence of blood stream also contributes to the absorption of heat. Increase of HIFU output power merely for accumulating adequate energy in the target region for the treatment of deep tumors or tumors with impediments in the beam route, would raise significant danger to healthy tissues along the beam path. The application of HIFU combined with nanomaterials provides the possibility to solve this problem. Nanomaterials synergize with the thermal effect of HIFU in two ways. Firstly, due to their high thermal conductivities, metallic nanoparticles can raise effective thermal conductivity of the tumor (Beik, Abed, Ghoreishi, et al., Citation2016). Therefore, metal-containing nanoparticles will increase heating rate and regional ablation of the tumor under the action of HIFU. When exposed to ultrasound, tumors with gold nanoparticles (AuNPs), iron oxide nanoparticles (IONPs) are heated faster than those without (Beik, Abed, Ghadimi-Daresajini, et al., Citation2016). Therefore, such nanomaterials can improve heating efficiency and reduce the risk of surrounding tissues damage due to excessive power. Secondly, HIFU can also be used as an external heating device for nanomaterials. Nanomaterials with temperature-sensitive liposomes as shells maintain stable configurations at physiological temperatures and transition to an unstable state at higher temperatures (40–43 °C) (Alphandéry, Citation2022). At physiological temperature, drugs are encapsulated in the liposomes, while at phase transition temperature, the permeability of phospholipid bilayer membrane increases by several orders of magnitude, thereby releasing its contents (Grüll & Langereis, Citation2012).
Another important mechanism of HIFU is its mechanical effect. The mechanical effects of ultrasound originate from various sources, among which, cavitation effect is most mentioned. Cavitation is the process of formation, vibration and collapse of microbubbles in liquid under the action of ultrasound. The presence of cavitation microbubbles in the focused area of HIFU enhances cavitation effect, which as an effective method of energy gathering, would significantly enhance the thermal effect of HIFU. Temperature change will also affect cavitation effect. Encapsulating gas or phase changeable substances in nanomaterials as exogenous cavitation nuclei can increase cavitation effect and improve the efficiency of tumor ablation. Tumor tissue compared with normal tissue, has a distinctively different structure. Extracellular matrix (ECM) is in high density and is typically overexpressed in tumor structures along with abnormal vasculature. (Tharkar et al., Citation2019). The extensive ECM of heterogeneous tumor tissues can restrict drug-loaded nanoparticles (NPs) from penetrating deeply into the tumor, which restricts therapeutic efficacy in cancer treatment (Choi et al., Citation2020). In addition, high interstitial fluid pressure, the high permeability of tumor vasculature, and the absence of the lymphatic drainage system, collectively known as the enhanced permeability and retention (EPR) effect, block the nanoparticles from penetrating into the tumor. Additionally, these circumstances make it difficult for nanoparticles to be evenly distributed across the total tumor volume (Tharkar et al., Citation2019). HIFU can overcome the shortcomings mentioned above by facilitating accurate drug delivery to the tumor through ultrasound and enhancing penetration of nanoparticles into the tumor. Sonoporation can be generated during the cavitation process. Sonoporation is a ultrasound-triggered process of forming transient pore in cell membrane (Przystupski & Ussowicz, Citation2022). The vibration and collapse of the microbubble apply a shear pressure on the cell membrane, enhancing its permeability. Sonoporation is useful for drug delivery as the created pore allows for increased particle uptake in target tissues and increased particle crossing through intercellular and intracellular barriers. (Bachu et al., Citation2021) Therefore, HIFU not only promotes the release of drugs in desired region, but also increased cellular uptake and accumulation of the nanoparticles.
The synergistic effect of nanomaterials and HIFU is also shown in the improvement of image brightness. As a contrast agent, microbubbles provide better contrast due to their low acoustic impedance and higher echo. However, microvesicles cannot be selectively placed in tumor tissues for targeted diagnosis and therapy because their microscopic size is too large to cross vascular endothelium and enter extravascular space (Hernot & Klibanov, Citation2008). The development of nanomaterials with dual size can solve this problem. This duality enhances EPR effect generated by nanomaterials at nanoscale passive diffusion to the tumor, obtaining good echo at micron scale (Rapoport, Citation2012). Phase change substances can be encapsulated in nanomaterials, and HIFU can act as external stimulus to convert it into gaseous states. For example, a perfluorocarbon (PFC) liquid core (Rapoport, Citation2012; Park & Na, Citation2015; Ishijima et al., Citation2016), stabilized by a lipid, polymer or protein shell, can transform from liquid to gaseous state when heated by HIFU. The vaporized nanoparticles can improve the backscatter frequency echo signal, echo enhancement and lesion image clarity (Zeng et al., Citation2022). They can also be effectively used as contrast enhancers (Strohm et al., Citation2011).
Systemic administrated nanoparticles accumulate into solid tumors due to EPR effects (Yu JJ et al., Citation2007; Prabhakar et al., Citation2013). However, studies have shown that on average, less than 1% of the nanoparticle dose reaches malignant tissue. As aforementioned, the combination of nanoparticles with HIFU can improve the situation. Sonoporation enhances the accumulation and deeper penetration of nanoparticles within the diseased tissue. The stimulation produced by ultrasound can promote drugs from endovascular lumen enter intercellular space. The change of tumor microenvironment brought about by HIFU boosts the accumulation of nanodrugs, improving treatment results noticeably (Nicolson et al., Citation2020). Additionally, biological or physiochemical modification of nanomaterials is frequently used to increase the number of nanomaterials deposited in the tumor area, which can improve targeting ability of nanoparticles and enhance HIFU ablation impact. Biological targeting is based on the connected targeting agents on the surface of nanoparticles that combine with the receptors specifically expressed or overexpressed on the surface of tumor cells, where the nanoparticles localize and release. Physical and chemical targeting is based on the environmental-sensitive characteristic of nanomaterials, so as to promote the targeted release of targeted nanodrugs under specific external environment. For example, the nanomaterials that encapsulate magnetic substances that target tumor cells through an external magnetic field, exert curative effect by inducing drug release on the focused lesion site, have the characteristics of high efficiency and low toxicity.
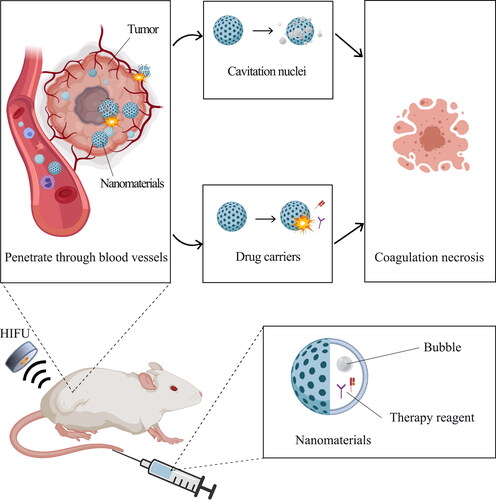
3. Application of nanomaterials as cavitation nuclei in HIFU
HIFU can produce many small bubbles and cause cavitation effect when applied to tissues as its energy reaches a certain threshold, contributing to tumor ablation. Enhancement of ultrasonic intensity could promote cavitation effect; however, excessive ultrasonic intensity would damage the surrounding healthy tissue. Nevertheless, the method of introducing the cavitation nucleus in vitro can be utilized to alleviate lesion with higher efficiency and reduce ultrasonic intensity required for ablation (), showing a promising clinical application prospect (Hill & ter Haar, Citation1995; Roberts et al., Citation2006).
The classical external cavitation nuclei are microbubbles, but their large particle size prevents them from permeating the tumor site and gives them short half-life time. Moreover, microbubbles have uncontrollable cavitation scope, which limits their further clinical application. Therefore, it is urgent to explore a novel material with tiny diameters, accurate localization and controllable gas release strategy. Fortunately, varied nanomaterials above have been elaborately designed in recent years. These nanomaterials have longer circulation time and can accumulate efficiently in tumor sites because of its tiny diameters. In addition, these nanomaterials can achieve controllable nanobubbles or microbubbles formation through accurate localization and controllable gas release strategy, enhancing ultrasound contrast signals and therapeutic efficacy of HIFU more precisely (Exner & Kolios, Citation2021). According to the composition of the gas core, these nanomaterials are classified into four categories as shown in .
Table 1. The utilization of bubble-generating nanoparticles to induce cavitation effect and combine with high-intensity focused ultrasound enhances the effectiveness of the treatment.
3.1. Perfluorocarbon
Perfluorocarbon (PFC) are compounds in which all the hydrogen atoms in a hydrocarbon molecule are replaced by fluorine atoms. PFC are widely used in many aspects (Krafft & Riess, Citation2007). In the field of ultrasound, as shown in , PFC is the one of the most classical sources of cavitation nuclei in nanoparticles for ultrasound contrast enhancers. The common types of perfluorocarbon include perfluoropropane (Abdalkader et al., Citation2017), perfluoropentane (PFP) (Rapoport et al., Citation2007), perfluorohexane(PFH) (Li W et al., Citation2018), perluorooctyl bromide (Bérard et al., Citation2022) etc.
Perfluoropentane (PFP) has the boiling point of 29 °C, which makes PFP vaporize on physiologic temperatures, conversing nanodroplets to nano or/and microbubbles, which gives PFP the ability of real-time imaging and could enhance ultrasound contrast signals (Krafft & Riess, Citation2007; Rapoport et al., Citation2007). For example, the nanoparticles with PFP cores designed by Rapoport et al. could provide a long-lasting, strong and selective ultrasound contrast upon the intravenous injections in tumor sites. In addition, bubbles formed by vaporization of PFP could cavitate and collapse under tumor-directed ultrasound, demonstrating its prospect of utilization on HIFU ablation (Rapoport et al., Citation2007). In 2013, the liquid PFC phase-shifted nanodroplets were proposed by Phillips et al., with cores of highly volatile decafluorobutane (DFB) and less volatile dodecafluoropentane (DDFP) to balance the stability and acoustic sensitivity. These nanodroplets can control the location of enhanced thermal energy deposition by controlled vaporization, resulting in shorter treatment times, decreasing risk to healthy tissue along the ultrasound path, deepening access to tumors and thus improving the ablation effect efficiently. (Phillips et al., Citation2013)
Perflurohexane (PFH) has gained strong attention of researchers for its suitable boiling temperature of 56 °C, which make the emulsions not only stable at room temperature, but also able to trigger vaporization whenever needed. Zhou et al. carefully designed temperature-responsive organic targeted nanoemulsions (TNEs) with PFH cores. TNEs can achieve temperature-responsive phase transitions. The liquid core can be triggered to release sufficient microbubbles via the acoustic droplet vaporization (ADV) under the HIFU stimulation, which is favorable for enhancing the capabilities of ultrasound imaging and therapy. The temperature sensitive ultrasonic behavior of TNEs was demonstrated by electron microscopy, ultrasonography and sound intensity examination. TNEs can remain unchanged at 60 °C , and the nano-emulsion begins to expand and fuze when the temperature rises to 70 °C , indicating the beginning of phase transition and the formation of microbubbles. When it reaches 80 °C , almost all particles become larger and produce a large number of microbubbles, which greatly enhances the ablation ability of HIFU on tumors. This represents the first successful demonstration of nanobiotechnology in stimuli-responsive ultrasound imaging and HIFU therapy, offering an alternative approach to enhance ultrasonography precision and ultrasound therapy efficiency, thereby demonstrating significant potential for clinical translation toward effective ultrasound-based imaging and therapy (Zhou Y et al., Citation2013).
Excellent features give PFC a promising future for clinical application. Apart from being ultrasound enhancers, PFCs are good carriers for many antitumor drugs such as doxorubicin (Rapoport et al., Citation2007; Li W et al., Citation2018) and curcumin (Ji et al., Citation2014), showing us great possibility of the cooperation of HIFU and chemotherapy (Ma M et al., Citation2014). In addition, PFC is a suitable carrier of oxygen (Song et al., Citation2016), a kind of tumor sensitive gas, which not only increases the source of the nuclei to enhance HIFU ablation but also makes a great contribution to alleviating the tumor hypoxia, solving the issue of tumor multidrug resistance (MDR) and tumor metastasis (Ma X et al., Citation2020; Tang R et al., Citation2023).
In general, the application of PFC in nanomaterials to assist HIFU therapy is popular and commonly adapted in the research field. Studies have shown that PFC can be metabolically cleared by our body. For example, PFP released upon microbubble collapse is expected to be eliminated through lungs (Rapoport et al., Citation2007), perfluorooctyl bromide and perfluorodichlorooctane could be eliminated through exhalation from lungs or skin pores, with respective elimination half-lives of three to four days and eight days (Song et al., Citation2016). However, this does not guarantee biosafety of PFCS completely. People still show great concerns about PFC’s biosecurity (Srivastava et al., Citation2022). When used in ophthalmic surgery for the management of vitreoretinal diseases (Yu Q et al., Citation2014; Chan et al., Citation2017), severe episodes of ocular toxicity were reported in Spain throughout Europe since 2013 (Pastor et al., Citation2017; Srivastava et al., Citation2018), which is thought to be due to the presence of reactive and underfluorinated impurities (Waxman, Citation1986; Gatto et al., Citation2023). Additionally, some studies showed that PFC may be developmentally toxic to pancreas (Liu S et al., Citation2020), demonstrating that careful consideration is needed for its application in the future.
3.2. Oxygen
Oxygen is a kind of colorless, odorless and tumor sensitive gas with high biological safety and application value. Recently, it has been adapted to strengthen cavitation effect, enhance ultrasonic pre-imaging and alleviate tumor hypoxia during HIFU therapy (). At present, there are two ways to increase the amount of oxygen in the tumor microenvironment, namely endogenous and exogenous oxygen generation (Liu T et al., Citation2017; Wang C et al., Citation2022; Sun L et al., Citation2023).
Endogenous production of oxygen has been studied first, involving substance such as catalase or metal ions with catalase activity like MnO2 (Hu et al., Citation2020; Murphy et al., Citation2021), Pt (Ren SZ et al., Citation2021), Fe-PDAP (Jiang Q et al., Citation2022) etc., catalyzing decomposition of hydrogen peroxide in situ in tumor microenvironment. A novel nanomaterial has been synthesized by Liu et al. which integrates dendritic mesoporous organic silica nanoparticles and catalase as contrast agent and synergist in ultrasound-guided HIFU cancer surgery. The catalase on the nanoparticle takes advantage of the characteristic of rich H2O2 within the malignant tumor microenvironment and thus generate O2 continuously in a relatively mild way. This realizes the on-demand release of tumor sensitive gas during HIFU surgery and significantly increases the necrotic area of the tumor (Liu T et al., Citation2017).
Nevertheless, due to the limited H2O2 content in tumor area, oxygen from endogenous production such as catalase-induced oxygen mentioned above is still insufficient to completely ablate tumor. Recently, some substances are used to achieve endogenous oxygen production. CaO2@SiO2 NPs was developed by Wang et al., which generates high-potency O2 bubble assisting HIFU surgery for tumor treatment (Wang C et al., Citation2022). The calcium peroxide nanoparticles (CaO2 NPs) are used as an enhanced O2 body and H2O2 resource for HIFU therapy, and the unstable hydrogen peroxide is further converted to O2 after triggering the thermal effect of HIFU. Therefore, the nanoparticles can effectively improve the therapeutic efficacy of HIFU, opening up a way for the application of calcium peroxide in nanomedicine (Wang C et al., Citation2022). There are still many substances or combinations of substances that have not been developed for HIFU therapy but can be used to provide exogenous oxygen, such as CuO (Chen Z et al., Citation2018), a fast-growing cyanobacterium Synechococcus (Sun T et al., Citation2020; Wang B et al., Citation2022), the combination of the CaO2 and (NH4)2CO3 (Yu Q et al., Citation2019), the combination of CaO2 and MnO2 (Hu et al., Citation2020), the combination of the PFC and O2 (Tang R et al., Citation2023) etc., showing us its great potential application of this therapy.
Oxygen, as a tumor sensitive gas, a raw material of reactive oxygen species and a great source of cavitation nuclei, also can be a kind of promising substance to cooperate with the combined therapy based on HIFU. That is, Oxygen can cooperate with the combined therapy of HIFU therapy and chemotherapy to alleviate tumor MDR and prevent tumor metastasis (Sun L et al., Citation2023). Oxygen can also cooperate with the combined therapy of HIFU and immunotherapy to promote transition of the protumor M2-type macrophages into antitumor M1-type and enhance HIFU’s killing of tumor tissue (Tang R et al., Citation2023).
In a word, the versatility of oxygen gives it superior utilization value, which is of great guiding significance for subsequent research about the improvement of nanomaterials. Many oxygen-based nanomaterials for tumor ablation based on HIFU have been developed in recent years (Mai et al., Citation2021). Although the application of oxygen has broad prospects, the biosafety of inorganic nanocases that catalyze oxygen production or oxygen-carrying carriers like PFC has not been fully confirmed, which leads to some problems in clinical conversion (Liu S et al., Citation2020; Wang P et al., Citation2021; Ren X et al., Citation2022). Fortunately, Liu S et al. had proposed a more biosafe oxygen-producing organic nanozyme for the treatment of acute kidney injury (Liu S et al., Citation2020), which has implications for alleviating hypoxic microenvironment of tumors in a more biosafe manner. We believe that more research is needed in the future.
3.3. Carbon dioxide
Carbon dioxide (CO2), as a kind of safe nontoxic fat-soluble gas with relatively low production cost, could be put into practice as composition of cavitation nuclei. Moreover, it also has advantages stabilizing lipid shell, thereby stabilizing vesiculation and enhancing biosecurity (). Nowadays, various methods have been explored to produce CO2 (Wang et al., Citation2016; Feng et al., Citation2017; Wang D et al., Citation2021; Gao et al., Citation2022).
Ammonium bicarbonate solution has been utilized to produce CO2. Feng et al. successfully prepared nanoscale bubble producing liposomes that are temperature sensitive and contains ammonium bicarbonate solution (Lip-ABC). The liposomes are stable at 37 °C while decomposing slowly. However, when the temperature exceeds 40 °C during the HIFU irradiation, the ABC solution in the liposomes would rapidly decompose and release large amounts of carbon dioxide. Since CO2 bubbles are easily diffused from the liposomes, the diameter of the liposomes does not change significantly during decomposition and gas production. This significantly reduces the possibility of gas embolization and ensures the safety of the liposomes during the HIFU synergistic process (Feng et al., Citation2017).
CaCO3 are utilized to produce CO2 as well. Gao et al. constructed Ca@H NPs with cores consisting of CaCO3. CaCO3 is pH-responsive, stable in neutral environment and unstable in a weakly acidic tumor microenvironment where it decomposes to produce CO2 (Gao et al., Citation2022). In addition, other familiar substances such as NaHCO3 (Wang D et al., Citation2021), effervescent disintegrants etc. could also be used to provide CO2 making the production process simpler and the cost lower, demonstrating its great possibilities for clinical application.
Additionally, the production of CO2 can be coupled with the release of antitumor chemicals. In Ca@H NPs, a typical acoustic-sensitive agent, hemaporphyrin-monommethyl ether (HMME) is coupled to CaCO3, in order to guide and promote tumor ablation through a series of combined therapeutic mechanisms and multi-mode effects (Gao et al., Citation2022). This successful combination shows its great application value in multi-therapy combined ablation. Nevertheless, compared to oxygen, the tumor-sensitive gas, CO2’s functional effectiveness and diversity for ablation mechanisms seem to be slightly inferior and it can’t solve the significant problems, MDR and tumor metastasis, caused by tumor hypoxia and thus limiting its further application in clinic.
3.4. L-menthol
L-menthol (LM) has been reported as a replacement for traditional fluorocarbons. It is a highly volatile natural substance which stabilizes bubble production and enhances biosafety. LM has been first used as a gas core in a nano-HIFU enhancer by Zhang et al. in 2014. In addition, they demonstrate that this nano-HIFU enhancer has an advantage to repeatedly enhance HIFU therapy by a single intravenous injection because LM could continuously generate volatile gases in a relatively mild manner (), showing its great potential in enhanced ultrasound imaging (Zhang K et al., Citation2014).
Recently, LM has been put into practice in many fields as bubble source and imaging enhancer to assist tumor ablation, such as a novel theranostic agent based on gold nanoshell cerasome-encapsulated L-menthol (GNC-LM) prepared by Guan et al. (Guan et al., Citation2019) and a photothermal conversion agent based on liposome load LM/IR-780 developed by Zhang et al. (Zhang C et al., Citation2019). Furthermore, although LM was predicted to be nontoxic, Sidhu et al. had demonstrated that LM is cytotoxic toward cervical cancer CaSki cells through their in vitro analysis last year, confirming LM a potential therapeutic drug against cervical cancer, which indicates its potential value in treating other tumors (Sidhu et al., Citation2023).
Additionally, nano-HIFU enhancers with LM cores can load multiple drugs, which would facilitate a series of combination therapies. Unfortunately, there are fewer reports about that, especially in HIFU therapy. But this might be a potential direction for future research based on LM’s capacity of gas generation, multiple drug delivery and wide cytotoxicity toward tumors.
4. Application of nanomaterials as drug carriers in HIFU
At present, it’s difficult to eliminate tumor radically with HIFU therapy due to its internal deficiencies such as the lack of effective targeting methods making nanomaterials as drug delivery system popularly adapted as accessory to HIFU treatment. Treatments of atherosclerosis (Sha et al., Citation2021), dental caries antibacterial (Zhu T et al., Citation2022) and tumor microenvironment regulation (Wu M et al., Citation2022) were coordinated by applications of drug-loaded nanomaterials, such as various drugs for chemotherapy, tumor toxic, and free radical enhancers, etc. (Alphandéry, Citation2022). Various targeting drugs can also be added to nanomaterials to enhance the targeting of HIFU therapy.
The construction of drug carriers can be divided into the following three ideas: equipping nanoparticles with chemotherapy drugs, combining nanoparticles with targeted drugs and making nano-bombs (). To comply with specific requirements, different construction methods of nanomaterials were adapted for their properties and advantages. For the various nanomaterials listed in , we focused on their advantages and inspiring ideas of building platforms and looked into directions in future development.
Table 2. The utilization of drug-loaded nanoparticles for targeted delivery of drugs to tumor sites enhances the effectiveness of high-intensity focused ultrasound treatment.
4.1. Nanomaterials equipped with chemotherapy drugs could combine with HIFU treatment
Nanomaterials can act as drug carriers, which can be triggered by HIFU to release drugs and thus inhibit tumor growth. An earlier study confirmed that HIFU could be used for temperature-responsive drug delivery (Zhang K et al., Citation2014). On this basis, Choi et al. synthesized glycol chitosan nanoparticles (CNPs) loaded with Doxorubicin (DOX) and triggered the release of DOX-CNP by HIFU, effectively inhibiting the lung adenocarcinoma (LUAD) tumor growth of A549 tumor-bearing mice (Choi et al., Citation2020). They successfully used HIFU in vitro to trigger and accelerate drug release from DOX-CNPs. DOX can be effectively accumulated in tumor tissues through deep tumor penetration by using this HIFU-triggered drug carriers, and the ECM with dense tumor can be destroyed by external HIFU, effectively inhibiting tumor growth.
In addition to simple drug delivery, nanomaterials can be used to treat tumors that cannot be reached by normal drug delivery routes. Nanomaterials, being able to cross the blood-brain barrier that prevents many pharmaceuticals from brain damage treatment, have been adapted to carry drugs to the afflicted brain area. Luo et al. designed a HIFU-activated nanoparticle drug delivery system that uses angiopep-2-modified small poly (lactic-co-glycolic acid) (PLGA) as its shell and doxorubicin/perfluorooctyl bromide (ANP-D/P) as its drug to treat tumors of intracranial glioblastoma mice (Luo et al., Citation2017). This drug delivery system enables angiopep-2 modified ANP-D/P to cross the blood-brain barrier and specifically accumulate in glioblastoma tissues 17 times and 13.4 times respectively higher than unmodified nanoparticles, largely solving the problem of inadequate drug delivery to glioblastoma. This nanomaterial enhances the treatment of glioblastoma by using HIFU to activate a drug delivery system, providing a new approach to tackling glioblastoma and treating tumors across barriers in vivo.
Except for barriers, the tumor microenvironment is also one of the factors limiting the effectiveness of HIFU therapy. The clinical efficacy of HIFU in combination with chemotherapy is often affected by MDR induced by the preexisting anoxic tumor microenvironment, which can also be solved by nanomaterials. Li et al. developed a highly stable nanoparticle that was simultaneously loaded with doxorubicin prodrugs (borate-Dox, BDOX) and β-laparone onto active targeted pH (low) insertion peptides (pHLIPs) -polyethylene glycol and nitrogluconic acid copolymers (Li Q et al., Citation2021). In vivo and in vitro experiments have shown that this material can produce large levels of NO and ROS in tumor tissues where GSH is present, improve tumor hypoxic environment, and enhance the therapeutic effect of HIFU and chemotherapy. In particular, HIFU combined with P@BDOX/β-lapachone-NO-NP has been shown to down-regulate p53 gene expression and hypoxia-associated protein (HIF-1α) levels, further inhibiting tumor growth. The therapeutic strategy of using HIFU with nanomaterials to deal with the tumor microenvironment provides new insights to the development of nanomaterials for tumor and specific therapies targeting the characteristics and gene expression levels of the tumor microenvironment.
4.2. Nanomaterials combined with targeted drugs participate in HIFU treatment
Targeted therapy means the delivery of a drug specifically to a target for action. As a main point of nanoparticle, targeting ability including passive targeting strategy, active targeting strategy, and environmental targeting strategy. Passive targeting strategies can be thought of as delivering nanomaterials to the tumor site through EPR effects. Although the EPR effect can theoretically enable particles smaller than 200 nm to enter the tissue space through the vascular wall, it may not be sufficient to guarantee the targeting effect of nanomaterials in practical experimental studies and clinical applications due to heterogeneity (Zi et al., Citation2022). Therefore, the construction of nanomaterials relies more on active targeting and environmental targeting, and the particle size of the nanoparticles in also illustrates this problem from another aspect. Active targeting strategy is the most commonly used strategy for constructing nanomaterials. Common ways to construct targeted effects include magnetic effects (such as Fe3O4), antigen-antibody specific binding, etc. Environmental targeting strategy mainly focuses on tumor microenvironment. The tumor microenvironment contains many unique physiological and physicochemical properties (such as pH, inflammation, etc.), which can be used as the basis for targeted construction.
Magnetic effects were used to transport nanomaterials to the tumor site. A common targeting agent for magnetic effects is Fe3O4. Perfluoropentane (PFP) coated PAA-F127 thermal nanofoam (PFP-PAA-F127) for RG2 glioma tumors in rats is one example of the combined use of HIFU with ultrasonic and magnetic effects. The material features nanoparticles with Fe3O4 (Superparamagnetic iron oxide) on its shell (Huang et al., Citation2013) allowing the nanovesicles to be magnetically guided to the tumor site releasing high concentrations of anticancer drugs from the nanovesicles on HIFU. This construction idea is an active targeting strategy. These drugs can temporarily increase the permeability of tumor cell membranes, facilitating the uptake of drugs across membranes of tumor cells. Such nanomaterials utilizing magnetic effects can be used as potential vectors for image-guided, magnetically targeted and ultrasound-mediated drug delivery, providing a new approach for HIFU targeted cancer therapy.
Fe3O4 not only acts as a drug localization agent, but also participates in drug delivery reaction. Yang et al. reported a polylactide-coethylene glycol (PLGA) nanoparticle (Fe3O4@PLGA/LA NPs) based on superparamagnetic iron oxide (SPIO, Fe3O4NPs) as shell and L-arginine (LA) as core for synergistic HIFU treatment of breast cancer (Yang, Jiang, Zhang, et al., Citation2021). Fe3O4@PLGA/LA NPs are accumulated through magnetic field guidance and retained at the tumor site for a long time. The released LA can spontaneously react with hydrogen peroxide (H2O2) to produce NO in the tumor microenvironment for gas therapy. Compared with previous studies, Fe3O4 NPs can not only promote tumor ablation through magnetic effect guidance and combined with HIFU, but also react with H2O2 to produce highly reactive oxygen species (ROS) to accelerate the oxidation of LA and release of NO, so as to achieve better gas therapeutic effect. In this nanomaterial construction scheme, the targeted agents not only play a role of targeting guidance, but also promote drug release. It realizes the multi-utilization of single drug in nano platform, and has reference significance for building more concise and more powerful nano platform.
Antigen-antibody specific response is also a common way of active targeting guidance. Specific targeting drugs on nanomaterials can also be used to guide the targeting of drugs. A methotrexate (MTX) loaded polylactic acid co-glycolic acid (PLGA) coated nanobubbles (NBs) were designed to carry surfacing coupled active tumor-targeting monoclonal anti-HLA-G antibodies (mAbHLA-G) (Zhang X et al., Citation2014). These mAbHLA-G/MTX/PLGA NBs can specifically target choriocarcinoma (CC) tumor cells in vivo and in vitro, and the blood circulation time in vivo is significantly longer. HIFU stimulates the release of MTX in nanocolles in tumor tissues to promote solid necrosis, specifically to kill the remaining tumor cells after HIFU ablation, to improve the therapeutic effect of HIFU, and to prevent tumor recurrence and metastasis. In this design scheme, monoclonal antibodies are added to the nanomaterial shell for targeted guidance, which combines drug delivery to deal with participating tumor cells, and solves the defect of incomplete HIFU treatment.
In addition to loading targeted drugs, targeted guidance can also be completed through tumor cell membrane. A nanomaterial, M@P-SOP, with PLGA encapsulating SPIO and PFH (P-SP), modified with 4T1 cell membrane (CCM) on P-SP by co-extrusion and thus gaining the ability of homologous tumor targeting capability (Tang R et al., Citation2023). M@P-SOP homologous targeted and enriched in tumor, which could greatly enhance in situ killing of tumor tissue and the total energy required for HIFU. The number of M1-type macrophages and cytotoxic T cells in tumor tissue were significantly increased. The combination of HIFU and M@P-SOP against PD-L1 also demonstrated a powerful synergistic anticancer effect against both primary and remote tumors. Moreover, M@P-SOP enables complementary multi-mode imaging, including PFH ultrasound imaging (USI), T2-weighted MRI, and superparamagnetic iron oxide (SPIO) photoacoustic imaging (PAI). M@P-SOP, based on homologous targeting, and rational combination of nanomaterials, HIFU and anti-PD-L1 blocking, can improve cancer therapy efficiency.
The process of HIFU treatment is accompanied by the generation of inflammatory environment at the tumor site, and inflammation will promote the migration of inflammatory cells. This process can also be used to build environmental targeting. Shen et al. selected neutrophils as the carrier, and pegylated liposome doxorubicin (PLD) as the model chemotherapy nanodrug to form an innovative cell therapy drug (PLD@NEs) (Shen et al., Citation2021). HIFU ablation creates an inflammatory environment at the tumor site, stimulating the migration of neutrophils in order to carry PLD@NEs to the tumor site, penetrate the endodermis of blood vessels and into the tumor and thus overcoming the biological barrier encountered by traditional drug administration. Once PLD@NEs entered the tumor tissue, PLD is released through a reticular structure and is then internalized by the tumor cells. HIFU ablation-induced inflammation is used to achieve active targeting, enabling this drug delivery strategy to provide the clinical drug PLD with good tumor targeting ability and permeability, and the ability to exert anti-tumor effects at doses below recommended. This effective integrated approach leverages the benefits of HIFU, chemotherapy, and neutrophils.
The anaerobic and acidic characteristics of the tumor microenvironment can be colonized by some special bacterial groups, and they can also become targeted supporters. Bifidobacterium bifidum (B. bifidum) selectively colonizes the anoxic region of solid tumors due to its anaerobic growth characteristics. Disen Wang et al. designed Polyethylenimine (PEI) -Modified Poly (lactic-co-glycolic) acid nanoparticles loaded sodium bicarbonate (PEI-PLGA-NaHCO3-NPS), a nanomaterial that carries a positive charge (via PEI) (Wang et al., Citation2021). They injected B. bifidum into the tail vein of 4T1 breast cancer bearing-BALB/c mice in advance, and B. bifidum with negative surface potential can guide nanomaterials to target the tumor microenvironment through Electrostatic adsorption. In vivo and in vitro experiments confirmed that through B. bifidum’s mediations, positively charged NPs entered solid tumors faster, more in quantity and longer in residence. This approach utilizes B. bifidum as a pioneer, amplifying the advantages of the tumor’s special microenvironment. Since B. bifidum is a probiotic, experiments have also confirmed that B. bifidum will not have a significant impact on the body before and after colonization in the tumor microenvironment, so this is a reference method. The inspiration of this method is that researchers can transform inconvenient tumor microenvironmental conditions into usable targeted electrostatic adsorption, which provides a new idea for the construction of targeted nanomaterials.
4.3. Nano bombs combined with targeted drugs participate in HIFU therapy
Nanoparticles can form stable interactions with ligands, for its characteristic of size and shape variability, high carrier capacity, and the ability to easily bind with hydrophilic and hydrophobic substances. These characteristics make it favorable for specific and controlled delivery of micro- and macro- molecular targets in therapy (Yetisgin et al., Citation2020). Nano-bombs can also be combined with targeted drugs to participate in HIFU therapy. It can also trigger phase change response at specific sites and enhance the effect of HIFU therapy. Tang et al. designed a temperature-sensitive treatment nanoplatform (PFH/DOX@PLGA/Fe3O4-FA) with PLGA as shell and PFH and DOX as core, conjugating folic acid on the surface and integrating Fe3O4 NPs for efficient dual channel ultrasound/magnetic resonance imaging (Tang H et al., Citation2018). These nanocomposites can target liver cancer cells and promote the accumulation of nanoplatforms at tumor sites. Simultaneously, superparamagnetic iron oxide (Fe3O4) was used as the contrast agent for magnetic resonance imaging. HIFU induces phase transition and make encapsulated DOX act on specific tumor sites with controlled release, which effectively inhibits tumor growth. This kind of nano-bomb utilizes magnetic effect, targeting drugs for imaging and guidance, directing itself to tumor cells for phase transition reaction and drug release and thus significantly improving the ablation effect of HIFU. It achieves phase transition reaction at controlled sites and provides a feasible multifunctional nano platform for tumor treatment.
Targeting not only enables the targeted release of nanomaterials, but also enables the on-demand release of drugs at the tumor site, minimizing the impact on other parts of the body. Mai et al. coated PLGA nanoparticles with ultra-thin silica shell, loaded with perfluorocarbon (PFOB), hydrophobic anti-tumor compound RuPOP and superparamagnetic Fe3O4, to obtain a multifunctional organic-inorganic hybrid nano-system (PFeRu-PL@SiO2(R) NPs) (Mai et al., Citation2021). This system showed excellent tumor growth inhibition, better biodegradability and biocompatibility in ultrasonic/MR-guided HIFU treatment of HeLa-bearing nude mice. This nanomaterial is characterized by targeted drugs to induce HIFU-stimulated nanomaterials to release RuPOP on demand at tumor sites. This mode of action can inhibit residual tumor to the maximum extent, avoid toxic side effects on other major organs, reduce systemic side effects of HIFU significantly, provide a new scheme for noninvasive cancer treatment and overcome tumor recurrence, which is difficult to avoid with conventional methods.
Cells can also be used as targeting tools. Doxorubicin (DOX) and phase change perfluoropentane (PFP) were embedded in hollow mesoporous organic silica nanoparticles (HMONs) to prepare DOX/PFP loaded HMONs (DPH) before internalizing them into macrophages (RAW 264.7 cells) in order to develop ultrasound inactivated cell bombs (Xu et al. Citation2020). These cell bombs (DPH-RAWs) can remain active and return to the tumor cells spontaneously. During cell incubation at 37 °C, a small fraction of the vaporized PFP can be tracked by real-time ultrasound. After the remaining PFP accumulates at the tumor site, it can be triggered to further vaporize by HIFU, creating several large microvesicles that destroy DPH-RAWs and release the drug. DPH-RAWs combined with HIFU significantly inhibited tumor growth and prolonged the survival of tumor-bearing mice. This study provides a new approach for cell-based drug delivery systems to track tumor migration in real time and enable targeted cancer therapies.
In addition to traditional nanoparticles and nano-bombs, some other forms of nano-targeted drugs also have good efficacy. A perfluorooctyl bromide (PFOB) nano emulsion holding MnO2 nanoparticles (MBP) was developed to coordinate cancer immunotherapy (Shen et al., Citation2021). MBP nano emulsion shows excellent MRI/CT dual-modal imaging capability, which can more accurately monitor tumor targeting ability of nano emulsion. MBP nanomaterials induce ICD effects by participating in HIFU therapy while depleting GSH and elevating intracellular ROS. The tumor immune microenvironment is regulated by inducing dendritic cell (DC) maturation and promoting the activation of CD8 and CD4 T cells. Synergistic GSH depletion and HIFU ablation also amplified inhibition of tumor growth and lung metastasis. This study pioneered a new strategy for tumor targeted immunotherapy and realized a novel therapeutic paradigm of important clinical significance.
Bacteria can also be engineered into nano bombs. Different from Wang et al., who used bacteria to create targeting conditions (Wang et al., Citation2021), Yang et al., made bacteria as materials to construct nano bombs (Yang et al., Citation2021). They used a genetically engineered acoustic reporter gene (ARG) that was successfully expressed in Escherichia coli (E. coli) to produce protein nanoparticle-gas vesicles (GV) in vitro. After intravenous injection, GVs-E. coli could specifically target the tumor site and continuously colonized in the tumor microenvironment, which could significantly inhibit tumor growth. Moreover, the GVs-E. coli protein nanoparticles could act as the cavitation nuclei to synergize HIFU therapy efficiently. Both in vivo and in vitro experiments confirmed that GVs-E. coli could greatly increase the tumor inhibition rate after HIFU treatment. Unlike normal nanomaterials, this nanomaterial is a biological agent produced by E. coli bacteria. The tumor targeting process of the drug is assigned to E. coli, and the cavitating action of the drug turns the E. coli into a nanobomb that enhances the effect of HIFU.
All the nanomaterials mentioned above have been confirmed to have significant therapeutic effects on tumors by in vitro and in vivo experiments, enhancing the effect of HIFU therapy. The combination of nano-drug and macrovesicle complex strategies with HIFU is a promising cancer treatment tool (Han et al., Citation2017). At present, drugs carried by nanomaterials are still classic anti-tumor drugs, and their mechanism is mainly to cooperate with HIFU for tumor therapy through HIFU triggering release, so as to overcome deficiencies such as inadequate tumor treatment and difficulty in preventing recurrence. In terms of the targeting of nanomaterials at the present stage, specific binding of various antigen-antibodies and magnetic targeting guidance are adapted to enhance treatment pertinency and reduce the impact on normal body. There still remained to be much room for the development of drug loading nanomaterials binding HIFU. In the future, there will be more sophisticated and powerful nanomaterial platforms, such as improved drug models for nanomaterials, or more targeted drug delivery systems, and simpler and more efficient ways to complete nanomaterial architectures. We have also noticed that some of the treatments in the laboratory do not match the current clinical direction of cancer treatments. We assume further development of nanomaterials in the future could broaden the application of HIFU to more types of cancer. In conclusion, nanomaterials have a promising development stage that opens up new ideas for tumor therapy.
5. Conclusion and prospect
Causing thermal and non-thermal (cavitation) effects to the focal region (Maloney & Hwang, Citation2015), HIFU has proven to be an efficient approach to noninvasive treatment for solid malignant tumors due to its positive attributes of less pain and quick recovery (Al-Bataineh et al., Citation2012). In previous studies, micrometer-sized micro-bubbles were intervened to enhance cavitation effect. However, its size was incompetent to cross the internals of vascular endothelial cells or penetrate the tumor vessels (Sokka et al., Citation2003). HIFU’s therapeutic effect was also affected by its low aggregation rate and ambiguous ultrasound or MRI image.
In recent studies, nanoparticles that provide cavitation nuclei were introduced to overcome the said deficiencies, such as NPs with micro-bubbles (Blum et al., Citation2017), NPs with phase-changeable perfluorochemical substances (Schutt et al., Citation2003) such as PFH (You et al., Citation2016), PFP (Chen J et al., Citation2021), PFOB (Kuai et al., Citation2022), NPs with peroxide such as CaO2 (Wang C et al., Citation2022), enzymes-synergistic NPs such as catalase (Liu T et al., Citation2017; Zhu J et al., Citation2019), tert-butoxycarbonyl (Li H et al., Citation2020) etc. Among those NPs, various materials, both inorganic and organic, were used as coating. Lipid material such as liposome (Maples et al., Citation2015) and phospholipid used as stabilizer (Yildirim et al., Citation2017) were adapted widely recently due to its stability and bio-compatibility. Heat sensitive materials such as low temperature sensitive liposomes (Maples et al., Citation2015) and mesporous organosilica (Li H et al., Citation2020) were adapted to ease the cavity on HIFU treatment. These NPs with cavitation nuclei effectively enhanced HIFU ablation by improving heating and cavitation effect, permeation and accumulation efficiency and ultrasound imaging, simultaneously alleviating damage to nearby healthy tissues.
HIFU, though advantageous on its noninvasive treatment, has difficulties in eliminating tumor cells in the affected area. In order to extinct residue of tumor cells after HIFU ablation, scientists developed NPs that could active immune system and induce immunogenic cell death. (Kuai et al., Citation2022) Such nanoparticles as ‘nano-bomb’ was also introduced to accomplish the mission of chemo-therapy to eliminate tumor cells yet to be ablated after HIFU treatment, of which distinct pharmaceuticals targeting specific types of tumor were packed as core, such as DOX (Choi et al., Citation2020), paclitaxel and PFH (Du et al., Citation2022), methotrexate MTX (Zhang X et al., Citation2014). Besides chemotherapy, radio-therapy is also widely applied in cancer treatment (Allen et al., Citation2017). Bismuth-based NPs was reported to combine HIFU treatment with radio-therapy (Wen et al., Citation2022). These NPs targeted specific tumor cell, benefit in treatment for kidney, breast, prostate, liver, pancreas, thyroid, parathyroid, connective tissue and brain cancer (Maloney & Hwang, Citation2015).
To accurately target tumor cells, scientists developed peptide-modified (Li Y et al., Citation2019), biotin-avidin-modified (Zhang Y et al., Citation2019), low PH environment sensitive (Li Q et al., Citation2021), Bfidobacteria bounded (Jiang BL et al., Citation2020) and magnetic Fe3O4 loaded (Sadeghi-Goughari et al., Citation2020) NPs, targeting the diseased area chemically, biochemically, physically or any combination to these above (Jiang F et al., Citation2022). Postoperative infection was also a major problem of post-therapy recovery (Fernández-Ugidos et al., Citation2019), which diclofenac was reported capsuled to reverse (Wu H et al., Citation2022).
Although the diagnosis of ultrasound works in almost every hospital/clinic, its use in treatment has long been underestimated. Recently, the US Food and Drug Administration (FDA) approved the use of ultrasound together with microbubbles for diagnostic applications (Mullick Chowdhury et al., Citation2017). Furthermore, there are many ultrasound-responsive microbubbles and nanoparticle preparations that are in clinical trials or have been used clinically as ultrasound contrast agents and to enhance ultrasound-triggered drug release applications. For example, SonoVue, Definity, Optison, and Sonazoid were used as ultrasound contrast agents, and ThermoDox (liposomal doxorubicin) was used to enhance doxorubicin release triggered by temperature induced by high-intensity focus ultrasound (Anselmo & Mitragotri, Citation2019). The potential toxicity of nanomaterials is one of the keys limiting clinical applications, which comes not only from the nanomaterials themselves, but also from the solvents and chemicals involved in the synthesis process. Successfully prepared nanoparticles may exhibit low toxic side effects in a short time, but their long-term cytotoxicity and associated immune response remained to be further studied. Moreover, in basic studies, small animal models cannot fully mimic complex human in vivo microenvironments. Therefore, only if toxicity assessment of nanomaterials on different animal models could provide valuable clues for their potential clinical translation applications should it possibly move toward clinical trials and applications.
In future studies, the direction of research should be focusing on finding more bio-compatible, heat-sensitive and stable material as shell, combining with effective phase-changing substance, peroxide, gas-releasing enzyme and micro-bacteria, coating specific targeting marker and carrying chemo- and radio pharmaceuticals, thus accomplishing HIFU treatment intra-surgeryly and post-surgeryly. With the development of nanotechnology, the application of HIFU in the biomedical field will flourish in the future.
Author contributions
Conceptualization: Shangyong Li and Ning Yu; Investigation and formal analysis: Xuehui Zhang, Ningning He; Writing-original draft preparation: Xuehui Zhang, Ningning He, Liang Zhang, Tong Dai, Zihan Sun, Yuqing Shi; Writing-review and editing: Ningning He, Shangyong Li and Ning Yu; Supervision, project administration, and funding acquisition: Shangyong Li and Ning Yu. All authors have read and agreed to the published version of the manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
Additional information
Funding
References
- Abdalkader R, Kawakami S, Unga J, et al. (2017). The development of mechanically formed stable nanobubbles intended for sonoporation-mediated gene transfection. Drug Deliv 24:1–16. doi:10.1080/10717544.2016.1250139.
- Al-Bataineh O, Jenne J, Huber P. (2012). Clinical and future applications of high intensity focused ultrasound in cancer. Cancer Treat Rev 38:346–53. doi:10.1016/j.ctrv.2011.08.004.
- Allen C, Her S, Jaffray DA. (2017). Radiotherapy for cancer: present and future. Adv Drug Deliv Rev 109:1–2. doi:10.1016/j.addr.2017.01.004.
- Alphandéry E. (2022). Ultrasound and nanomaterial: an efficient pair to fight cancer. J Nanobiotechnology 20:139. doi:10.1186/s12951-022-01243-w.
- Anselmo AC, Mitragotri S. (2019). Nanoparticles in the clinic: an update. Bioeng Transl Med 4:e10143. doi:10.1002/btm2.10143.
- Bachu VS, Kedda J, Suk I, et al. (2021). High-intensity focused ultrasound: a review of mechanisms and clinical applications. Ann Biomed Eng 49:1975–91. doi:10.1007/s10439-021-02833-9.
- Beik J, Abed Z, Ghadimi-Daresajini A, et al. (2016). Measurements of nanoparticle-enhanced heating from 1MHz ultrasound in solution and in mice bearing CT26 colon tumors. J Therm Biol 62:84–9. doi:10.1016/j.jtherbio.2016.10.007.
- Beik J, Abed Z, Ghoreishi FS, et al. (2016). Nanotechnology in hyperthermia cancer therapy: From fundamental principles to advanced applications. J Control Release 235:205–21. doi:10.1016/j.jconrel.2016.05.062.
- Bérard C, Desgranges S, Dumas N, et al. (2022). Perfluorocarbon nanodroplets as potential nanocarriers for brain delivery assisted by focused ultrasound-mediated blood-brain barrier disruption. Pharmaceutics 14:1498. doi:10.3390/pharmaceutics14071498.
- Blum NT, Yildirim A, Chattaraj R, Goodwin AP. (2017). Nanoparticles formed by acoustic destruction of microbubbles and their utilization for imaging and effects on therapy by high intensity focused ultrasound. Theranostics 7:694–702. doi:10.7150/thno.17522.
- Chan YK, Lu Y, Czanner G, et al. (2017). In vitro experiment to elucidate the mechanism of the ‘soft shell technique’ for preventing subretinal migration of perfluoro-octane. Br J Ophthalmol 101:389–94. doi:10.1136/bjophthalmol-2016-309856.
- Chen J, Nan Z, Zhao Y, et al. (2021). Enhanced HIFU theranostics with dual-frequency-ring focused ultrasound and activatable perfluoropentane-loaded polymer nanoparticles. Micromachines 12:1324. doi:10.3390/mi12111324.
- Chen Z, Niu M, Chen G, et al. (2018). Oxygen production of modified core-shell CuO@ZrO(2) nanocomposites by microwave radiation to alleviate cancer hypoxia for enhanced chemo-microwave thermal therapy. ACS Nano 12:12721–32. doi:10.1021/acsnano.8b07749.
- Choi Y, Han H, Jeon S, et al. (2020). Deep tumor penetration of doxorubicin-loaded glycol chitosan nanoparticles using high-intensity focused ultrasound. Pharmaceutics 12:974. doi:10.3390/pharmaceutics12100974.
- Cohen JL, Weiner SF, Pozner JN, et al. (2016). Multi-center pilot study to evaluate the safety profile of high energy fractionated radiofrequency with insulated microneedles to multiple levels of the dermis. J Drugs Dermatol 15:1308–12.
- Cranston D, Leslie T, Ter Haar G. (2021). A review of high-intensity focused ultrasound in urology. Cancers 13:5696. doi:10.3390/cancers13225696.
- Dai H, Chen F, Yan S, et al. (2017). In vitro and in vivo investigation of high-intensity focused ultrasound (HIFU) hat-type ablation mode. Med Sci Monit 23:3373–82. doi:10.12659/msm.902528.
- Du Y, Lin L, Zhang Z, et al. (2022). Drug-loaded nanoparticles conjugated with genetically engineered bacteria for cancer therapy. Biochem Biophys Res Commun 606:29–34. doi:10.1016/j.bbrc.2022.03.049.
- Dubinsky TJ, Cuevas C, Dighe MK, et al. (2008). High-intensity focused ultrasound: current potential and oncologic applications. AJR Am J Roentgenol 190:191–9. doi:10.2214/AJR.07.2671.
- Exner AA, Kolios MC. (2021). Bursting microbubbles: how nanobubble contrast agents can enable the future of medical ultrasound molecular imaging and image-guided therapy. Curr Opin Colloid Interface Sci 54:101463. doi:10.1016/j.cocis.2021.101463.
- Feng G, Hao L, Xu C, et al. (2017). High-intensity focused ultrasound-triggered nanoscale bubble-generating liposomes for efficient and safe tumor ablation under photoacoustic imaging monitoring. Int J Nanomed 12:4647–59. doi:10.2147/IJN.S135391.
- Feril LB, Fernan RL, Tachibana K. (2021). High-intensity focused ultrasound in the treatment of breast cancer. Curr Med Chem 28:5179–88. doi:10.2174/0929867327666201111143206.
- Fernández-Ugidos P, Barge-Caballero E, Gómez-López R, et al. (2019). In-hospital postoperative infection after heart transplantation: risk factors and development of a novel predictive score. Transpl Infect Dis 21:e13104. doi: 10.1111/tid.13104.
- Gao H, Wang Z, Tan M, et al. (2022). pH-responsive nanoparticles for enhanced antitumor activity by high-intensity focused ultrasound therapy combined with sonodynamic therapy. Int J Nanomed 17:333–50. doi:10.2147/IJN.S336632.
- Gatto C, Ruzza P, Giurgola L, et al. (2023). Comparison of perfluorocarbon liquids cytotoxicity tests: direct contact versus the test on liquid extracts. ACS Omega 8:365–72. doi:10.1021/acsomega.2c04697.
- Grüll H, Langereis S. (2012). Hyperthermia-triggered drug delivery from temperature-sensitive liposomes using MRI-guided high intensity focused ultrasound. J Control Release 161:317–27. doi:10.1016/j.jconrel.2012.04.041.
- Guan Q, Wang C, Wu D, et al. (2019). Cerasome-based gold-nanoshell encapsulating L-menthol for ultrasound contrast imaging and photothermal therapy of cancer. Nanotechnology 30:015101. doi:10.1088/1361-6528/aae6aa.
- Han H, Lee H, Kim K, Kim H. (2017). Effect of high intensity focused ultrasound (HIFU) in conjunction with a nanomedicines-microbubble complex for enhanced drug delivery. J Control Release 266:75–86. doi:10.1016/j.jconrel.2017.09.022.
- Hernot S, Klibanov AL. (2008). Microbubbles in ultrasound-triggered drug and gene delivery. Adv Drug Deliv Rev 60:1153–66. doi:10.1016/j.addr.2008.03.005.
- Hill CR, ter Haar GR. (1995). Review article: high intensity focused ultrasound–potential for cancer treatment. Br J Radiol 68:1296–303. doi:10.1259/0007-1285-68-816-1296.
- Hu Y, Wang X, Zhao P, et al. (2020). Nanozyme-catalyzed oxygen release from calcium peroxide nanoparticles for accelerated hypoxia relief and image-guided super-efficient photodynamic therapy. Biomater Sci 8:2931–8. doi:10.1039/d0bm00187b.
- Huang HY, Hu SH, Hung SY, et al. (2013). SPIO nanoparticle-stabilized PAA-F127 thermosensitive nanobubbles with MR/US dual-modality imaging and HIFU-triggered drug release for magnetically guided in vivo tumor therapy. J Control Release 172:118–27. doi:10.1016/j.jconrel.2013.07.029.
- Ishijima A, Tanaka J, Azuma T, et al. (2016). The lifetime evaluation of vapourised phase-change nano-droplets. Ultrasonics 69:97–105. doi:10.1016/j.ultras.2016.04.002.
- Ji G, Yang J, Chen J. (2014). Preparation of novel curcumin-loaded multifunctional nanodroplets for combining ultrasonic development and targeted chemotherapy. Int J Pharm 466:314–20. doi:10.1016/j.ijpharm.2014.03.030.
- Jiang BL, Gao X, Xiong J, et al. (2020). Experimental study on synergistic effect of HIFU treatment of tumors using Bifidobacterium bound with cationic phase-change nanoparticles. Eur Rev Med Pharmacol Sci 24:5714–25. doi: 10.26355/eurrev_202005_21363.
- Jiang F, Wang L, Tang Y, et al. (2022). US/MR bimodal imaging-guided bio-targeting synergistic agent for tumor therapy. Int J Nanomed 17:2943–60. doi:10.2147/IJN.S363645.
- Jiang Q, Qiao B, Lin X, et al. (2022). A hydrogen peroxide economizer for on-demand oxygen production-assisted robust sonodynamic immunotherapy. Theranostics 12:59–75. doi:10.7150/thno.64862.
- Khokhlova VA, Fowlkes JB, Roberts WW, et al. (2015). Histotripsy methods in mechanical disintegration of tissue: towards clinical applications. Int J Hyperthermia 31:145–62. doi:10.3109/02656736.2015.1007538.
- Kim H, Kang J, Chang JH. (2014). Thermal therapeutic method for selective treatment of deep-lying tissue by combining laser and high-intensity focused ultrasound energy. Opt Lett 39:2806–9. doi:10.1364/OL.39.002806.
- Krafft MP, Riess JG. (2007). Perfluorocarbons: life sciences and biomedical uses - dedicated to the memory of Professor Guy Ourisson, a true RENAISSANCE man. J Polym Sci A Polym Chem 45:1185–98. doi:10.1002/pola.21937.
- Kuai X, Zhu Y, Yuan Z, et al. (2022). Perfluorooctyl bromide nanoemulsions holding MnO(2) nanoparticles with dual-modality imaging and glutathione depletion enhanced HIFU-eliciting tumor immunogenic cell death. Acta Pharm Sin B 12:967–81. doi:10.1016/j.apsb.2021.07.025.
- Li H, Gascó C, Delalande A, et al. (2020). Periodic mesoporous organosilica nanoparticles with BOC group, towards HIFU responsive agents. Molecules 25:974. doi:10.3390/molecules25040974.
- Li Q, Zhang J, Li J, et al. (2021). Glutathione-activated NO-/ROS-generation nanoparticles to modulate the tumor hypoxic microenvironment for enhancing the effect of HIFU-combined chemotherapy. ACS Appl Mater Interfaces 13:26808–23. doi:10.1021/acsami.1c07494.
- Li W, Hou W, Guo X, et al. (2018). Temperature-controlled, phase-transition ultrasound imaging-guided photothermal-chemotherapy triggered by NIR light. Theranostics 8:3059–73. doi:10.7150/thno.23885.
- Li Y, Hao L, Liu F, et al. (2019). Cell penetrating peptide-modified nanoparticles for tumor targeted imaging and synergistic effect of sonodynamic/HIFU therapy. Int J Nanomed 14:5875–94. doi:10.2147/IJN.S212184.
- Liu S, Yang R, Yin N, Faiola F. (2020). Effects of per- and poly-fluorinated alkyl substances on pancreatic and endocrine differentiation of human pluripotent stem cells. Chemosphere 254:126709. doi:10.1016/j.chemosphere.2020.126709.
- Liu T, Zhang N, Wang Z, et al. (2017). Endogenous catalytic generation of O(2) bubbles for in situ ultrasound-guided high intensity focused ultrasound ablation. ACS Nano 11:9093–102. doi:10.1021/acsnano.7b03772.
- Luo Z, Jin K, Pang Q, et al. (2017). On-demand drug release from dual-targeting small nanoparticles triggered by high-intensity focused ultrasound enhanced glioblastoma-targeting therapy. ACS Appl Mater Interfaces 9:31612–25. doi:10.1021/acsami.7b10866.
- Ma M, Xu H, Chen H, et al. (2014). A drug-perfluorocarbon nanoemulsion with an ultrathin silica coating for the synergistic effect of chemotherapy and ablation by high-intensity focused ultrasound. Adv Mater 26:7378–85. doi:10.1002/adma.201402969.
- Ma X, Yao M, Shi J, et al. (2020). High intensity focused ultrasound-responsive and ultrastable cerasomal perfluorocarbon nanodroplets for alleviating tumor multidrug resistance and epithelial-mesenchymal transition. ACS Nano 14:15904–18. doi:10.1021/acsnano.0c07287.
- Mai X, Chang Y, You Y, et al. (2021). Designing intelligent nano-bomb with on-demand site-specific drug burst release to synergize with high-intensity focused ultrasound cancer ablation. J Control Release 331:270–81. doi:10.1016/j.jconrel.2020.09.051.
- Maloney E, Hwang JH. (2015). Emerging HIFU applications in cancer therapy. Int J Hyperthermia 31:302–9. doi:10.3109/02656736.2014.969789.
- Maples D, McLean K, Sahoo K, et al. (2015). Synthesis and characterisation of ultrasound imageable heat-sensitive liposomes for HIFU therapy. Int J Hyperthermia 31:674–85. doi: 10.3109/02656736.2015.1057622.
- Mullick Chowdhury S, Lee T, Willmann JK. (2017). Ultrasound-guided drug delivery in cancer. Ultrasonography 36:171–84. doi:10.14366/usg.17021.
- Murphy DA, Cheng H, Yang T, et al. (2021). Reversing hypoxia with PLGA-encapsulated manganese dioxide nanoparticles improves natural killer cell response to tumor spheroids. Mol Pharm 18:2935–46. doi:10.1021/acs.molpharmaceut.1c00085.
- Napoli A, Alfieri G, Scipione R, et al. (2020). High-intensity focused ultrasound for prostate cancer. Expert Rev Med Devices 17:427–33. doi:10.1080/17434440.2020.1755258.
- Nicolson F, Ali A, Kircher MF, Pal S. (2020). DNA nanostructures and DNA-functionalized nanoparticles for cancer theranostics. Adv Sci 7:2001669. doi: 10.1002/advs.202001669.
- Panzone J, Byler T, Bratslavsky G, Goldberg H. (2022). Applications of focused ultrasound in the treatment of genitourinary cancers. Cancers 14:1536. doi:10.3390/cancers14061536.
- Park W, Na K. (2015). Advances in the synthesis and application of nanoparticles for drug delivery. Wiley Interdiscip Rev Nanomed Nanobiotechnol 7:494–508. doi:10.1002/wnan.1325.
- Pastor JC, Coco RM, Fernandez-Bueno I, et al. (2017). Acute retinal damage after using a toxic perfluoro-octane for vitreo-retinal surgery. Retina 37:1140–51. doi:10.1097/IAE.0000000000001680.
- Phillips LC, Puett C, Sheeran PS, et al. (2013). Phase-shift perfluorocarbon agents enhance high intensity focused ultrasound thermal delivery with reduced near-field heating. J Acoust Soc Am 134:1473–82. doi:10.1121/1.4812866.
- Prabhakar U, Maeda H, Jain RK, et al. (2013). Challenges and key considerations of the enhanced permeability and retention effect for nanomedicine drug delivery in oncology. Cancer Res 73:2412–7. doi:10.1158/0008-5472.CAN-12-4561.
- Przystupski D, Ussowicz M. (2022). Landscape of cellular bioeffects triggered by ultrasound-induced sonoporation. Int J Mol Sci 23:11222. doi:10.3390/ijms231911222.
- Rapoport N. (2012). Phase-shift, stimuli-responsive perfluorocarbon nanodroplets for drug delivery to cancer. Wiley Interdiscip Rev Nanomed Nanobiotechnol 4:492–510. doi:10.1002/wnan.1176.
- Rapoport N, Gao Z, Kennedy A. (2007). Multifunctional nanoparticles for combining ultrasonic tumor imaging and targeted chemotherapy. J Natl Cancer Inst 99:1095–106. doi:10.1093/jnci/djm043.
- Ren SZ, Zhu XH, Wang B, et al. (2021). A versatile nanoplatform based on multivariate porphyrinic metal-organic frameworks for catalytic cascade-enhanced photodynamic therapy. J Mater Chem B 9:4678–89. doi:10.1039/d0tb02652b.
- Ren X, Chen D, Wang Y, et al. (2022). Nanozymes-recent development and biomedical applications. J Nanobiotechnol 20:92. doi:10.1186/s12951-022-01295-y.
- Roberts WW, Hall TL, Ives K, et al. (2006). Pulsed cavitational ultrasound: a noninvasive technology for controlled tissue ablation (histotripsy) in the rabbit kidney. J Urol 175:734–8. doi:10.1016/S0022-5347(05)00141-2.
- Sadeghi-Goughari M, Jeon S, Kwon HJ. (2020). Magnetic nanoparticles-enhanced focused ultrasound heating: size effect, mechanism, and performance analysis. Nanotechnology 31:245101. doi:10.1088/1361-6528/ab7cea.
- Schutt EG, Klein DH, Mattrey RM, Riess JG. (2003). Injectable microbubbles as contrast agents for diagnostic ultrasound imaging: the key role of perfluorochemicals. Angew Chem Int Ed Engl 42:3218–35. doi:10.1002/anie.200200550.
- Sha X, Dai Y, Song X, et al. (2021). The opportunities and challenges of silica nanomaterial for atherosclerosis. Int J Nanomed 16:701–14. doi:10.2147/IJN.S290537.
- Shen J, Hao J, Chen Y, et al. (2021). Neutrophil-mediated clinical nanodrug for treatment of residual tumor after focused ultrasound ablation. J Nanobiotechnol. 19:345. doi:10.1186/s12951-021-01087-w.
- Sidhu H, Gautam LK, Capalash N. (2023). Unraveling the molecular mechanism of l-menthol against cervical cancer based on network pharmacology, molecular docking and in vitro analysis. Mol Divers 27:323–40. doi:10.1007/s11030-022-10429-1.
- Sofuni A, Asai Y, Mukai S, et al. (2022). High-intensity focused ultrasound therapy for pancreatic cancer. J Med Ultrason (2001). doi:10.1007/s10396-022-01208-4.
- Sokka SD, King R, Hynynen K. (2003). MRI-guided gas bubble enhanced ultrasound heating in in vivo rabbit thigh. Phys Med Biol 48:223–41. doi:10.1088/0031-9155/48/2/306.
- Song X, Feng L, Liang C, et al. (2016). Ultrasound triggered tumor oxygenation with oxygen-shuttle nanoperfluorocarbon to overcome hypoxia-associated resistance in cancer therapies. Nano Lett 16:6145–53. doi:10.1021/acs.nanolett.6b02365.
- Srivastava GK, Alonso-Alonso ML, Fernandez-Bueno I, et al. (2018). Comparison between direct contact and extract exposure methods for PFO cytotoxicity evaluation. Sci Rep 8:1425. doi:10.1038/s41598-018-19428-5.
- Srivastava GK, Kalaiselvan V, Andrés-Iglesias C, et al. (2022). Acute intraocular toxicity caused by perfluorocarbon liquids: safety control systems of medical devices. Graefes Arch Clin Exp Ophthalmol 260:2103–10. doi:10.1007/s00417-022-05578-w.
- Strohm E, Rui M, Gorelikov I, et al. (2011). Vaporization of perfluorocarbon droplets using optical irradiation. Biomed Opt Express 2:1432–42. doi:10.1364/BOE.2.001432.
- Sun L, Gao W, Wang J, et al. (2023). A new sono-chemo sensitizer overcoming tumor hypoxia for augmented sono/chemo-dynamic therapy and robust immune-activating response. Small 19:e2206078. doi:10.1002/smll.202206078.
- Sun T, Zhang Y, Zhang C, et al. (2020). Cyanobacteria-based bio-oxygen pump promoting hypoxia-resistant photodynamic therapy. Front Bioeng Biotechnol 8:237. doi:10.3389/fbioe.2020.00237.
- Tang H, Guo Y, Peng L, et al. (2018). In vivo targeted, responsive, and synergistic cancer nanotheranostics by magnetic resonance imaging-guided synergistic high-intensity focused ultrasound ablation and chemotherapy. ACS Appl Mater Interfaces 10:15428–41. doi:10.1021/acsami.8b01967.
- Tang R, He H, Lin X, et al. (2023). Novel combination strategy of high intensity focused ultrasound (HIFU) and checkpoint blockade boosted by bioinspired and oxygen-supplied nanoprobe for multimodal imaging-guided cancer therapy. J Immunother Cancer 11:e006226. doi:10.1136/jitc-2022-006226.
- Tharkar P, Varanasi R, Wong WSF, et al. (2019). Nano-enhanced drug delivery and therapeutic ultrasound for cancer treatment and beyond. Front Bioeng Biotechnol 7:324. doi:10.3389/fbioe.2019.00324.
- Tsang SH, Ma KW, She WH, et al. (2021). High-intensity focused ultrasound ablation of liver tumors in difficult locations. Int J Hyperthermia 38:56–64. doi:10.1080/02656736.2021.1933217.
- Wang B, Zhou L, Guo Y, et al. (2022). Cyanobacteria-based self-oxygenated photodynamic therapy for anaerobic infection treatment and tissue repair. Bioact Mater 12:314–26. doi:10.1016/j.bioactmat.2021.10.032.
- Wang C, Li Z, Bai J. (2022). Bubble-assisted HIFU ablation enabled by calcium peroxide. J Mater Chem B 10:4442–51. doi:10.1039/d2tb00587e.
- Wang D, Jiang F, Wang L, et al. (2021). Polyethylenimine (PEI)-modified poly (lactic-co-glycolic) acid (PLGA) nanoparticles conjugated with tumor-homing bacteria facilitate high intensity focused ultrasound-mediated tumor ablation. Biochem Biophys Res Commun 571:104–9. doi:10.1016/j.bbrc.2021.07.061.
- Wang P, Min D, Chen G, et al. (2021). Inorganic nanozymes: prospects for disease treatments and detection applications. Front Chem 9:773285. doi:10.3389/fchem.2021.773285.
- Wang S, Zhao J, Hu F, et al. (2016). Phase-changeable and bubble-releasing implants for highly efficient HIFU-responsive tumor surgery and chemotherapy. J Mater Chem B 4:7368–78. doi:10.1039/c6tb01861k.
- Waxman K. (1986). Perfluorocarbons as blood substitutes. Ann Emerg Med 15:1423–4. doi:10.1016/s0196-0644(86)80933-7.
- Wen S, Ovais M, Li X, et al. (2022). Tailoring bismuth-based nanoparticles for enhanced radiosensitivity in cancer therapy. Nanoscale 14:8245–54. doi:10.1039/d2nr01500e.
- Wu H, Zhou H, Zhang W, et al. (2022). Three birds with one stone: co-encapsulation of diclofenac and DL-menthol for realizing enhanced energy deposition, glycolysis inhibition and anti-inflammation in HIFU surgery. J Nanobiotechnol 20:215. doi:10.1186/s12951-022-01437-2.
- Wu M, Niu X, Zhang R, Ping Xu Z. (2022). Two-dimensional nanomaterials for tumor microenvironment modulation and anticancer therapy. Adv Drug Deliv Rev 187:114360. doi:10.1016/j.addr.2022.114360.
- Xu Z, Liu HM, Tian H, Yan F. (2020). Real-time imaging tracking of engineered macrophages as ultrasound-triggered cell bombs for cancer treatment. Adv Funct Mater 30:1910304. doi:10.1002/adfm.201910304.
- Yang H, Jiang F, Ji X, et al. (2021). Genetically engineered bacterial protein nanoparticles for targeted cancer therapy. Int J Nanomed 16:105–17. doi:10.2147/IJN.S292432.
- Yang H, Jiang F, Zhang L, et al. (2021). Multifunctional l-arginine-based magnetic nanoparticles for multiple-synergistic tumor therapy. Biomater Sci 9:2230–43. doi:10.1039/d0bm01932a.
- Yetisgin AA, Cetinel S, Zuvin M, et al. (2020). Therapeutic nanoparticles and their targeted delivery applications. Molecules 25:2193. doi:10.3390/molecules25092193.
- Yildirim A, Chattaraj R, Blum NT, et al. (2017). Phospholipid capped mesoporous nanoparticles for targeted high intensity focused ultrasound ablation. Adv Healthc Mater 6:10. doi:10.1002/adhm.201700514.
- You Y, Wang Z, Ran H, et al. (2016). Nanoparticle-enhanced synergistic HIFU ablation and transarterial chemoembolization for efficient cancer therapy. Nanoscale 8:4324–39. doi:10.1039/c5nr08292g.
- Yu JJ, Lee HA, Kim JH, et al. (2007). Bio-distribution and anti-tumor efficacy of PEG/PLA nano particles loaded doxorubicin. J Drug Target 15:279–84. doi:10.1080/10611860701357235.
- Yu Q, Huang T, Liu C, et al. (2019). Oxygen self-sufficient NIR-activatable liposomes for tumor hypoxia regulation and photodynamic therapy. Chem Sci 10:9091–8. doi:10.1039/c9sc03161h.
- Yu Q, Liu K, Su L, et al. (2014). Perfluorocarbon liquid: its application in vitreoretinal surgery and related ocular inflammation. Biomed Res Int 2014:250323–6. doi:10.1155/2014/250323.
- Zeng Z, Liu JB, Peng CZ. (2022). Phase-changeable nanoparticle-mediated energy conversion promotes highly efficient high-intensity focused ultrasound ablation. Curr Med Chem 29:1369–78. doi:10.2174/0929867328666210708085110.
- Zhang C, Liu J, Guo H, et al. (2019). Theranostic nanomedicine carrying L-menthol and near-infrared dye for multimodal imaging-guided photothermal therapy of cancer. Adv Healthc Mater 8:e1900409. doi: 10.1002/adhm.201900409.
- Zhang K, Chen H, Li F, et al. (2014). A continuous tri-phase transition effect for HIFU-mediated intravenous drug delivery. Biomaterials 35:5875–85. doi:10.1016/j.biomaterials.2014.03.043.
- Zhang X, Zheng Y, Wang Z, et al. (2014). Methotrexate-loaded PLGA nanobubbles for ultrasound imaging and synergistic targeted therapy of residual tumor during HIFU ablation. Biomaterials 35:5148–61. doi:10.1016/j.biomaterials.2014.02.036.
- Zhang Y, Yong L, Luo Y, et al. (2019). Enhancement of HIFU ablation by sonosensitizer-loading liquid fluorocarbon nanoparticles with pre-targeting in a mouse model. Sci Rep 9:6982. doi:10.1038/s41598-019-43416-y.
- Zhou Y, Wang Z, Chen Y, et al. (2013). Microbubbles from gas-generating perfluorohexane nanoemulsions for targeted temperature-sensitive ultrasonography and synergistic HIFU ablation of tumors. Adv Mater 25:4123–30. doi:10.1002/adma.201301655.
- Zhou YF. (2011). High intensity focused ultrasound in clinical tumor ablation. World J Clin Oncol 2:8–27. doi:10.5306/wjco.v2.i1.8.
- Zhu J, Li Z, Zhang C, et al. (2019). Single enzyme loaded nanoparticles for combinational ultrasound-guided focused ultrasound ablation and hypoxia-relieved chemotherapy. Theranostics 9:8048–60. doi:10.7150/thno.37054.
- Zhu T, Huang Z, Shu X, et al. (2022). Functional nanomaterials and their potentials in antibacterial treatment of dental caries. Colloids Surf B Biointerfaces 218:112761. doi:10.1016/j.colsurfb.2022.112761.
- Zi Y, Yang K, He J, et al. (2022). Strategies to enhance drug delivery to solid tumors by harnessing the EPR effects and alternative targeting mechanisms. Adv Drug Deliv Rev. 188:114449. doi: 10.1016/j.addr.2022.114449.