Abstract
PEG-hemoglobin SB1 (SB1) is a polyethylene glycol (PEG)-modified hemoglobin-based oxygen carrier, intended for use as resuscitation fluid for brain stroke and as a blood substitute. An intravenous pharmacokinetics (PK) studies with SB1 was investigated in male albino Sprague-Dawley (SD) rats and male beagle dogs at doses of 5 and 12.5 ml/kg for rats and 10 ml/kg for dogs. Total hemoglobin in plasma and whole blood was determined by gamma scintillation counter–detecting 125I-radiolabelled SB1. In the 5 ml/kg rats (n = 9), the Cmax, t1/2, AUCt and Tmax were 9.055 mg equivalents/ml, 9.6 hr, 79.6 mg equivalents.hr/ml and 0.20 hr in the plasma and 4.954 mg equivalents/ml, 9.7 hr, 37.6 mg equivalents.hr/ml and 0.11 hr in the whole blood, respectively. Those parameters in the 12.5 ml/Kg of rats (n = 9) were 19.00 mg equivalents/ml, 10.6 hr, 223.5 mg equivalents.hr/ml and 0.33 hr in the plasma and 10.58 mg equivalents/ml, 16.1 hr, 99.0 mg equivalents.hr/ml and 0.33 hr in the whole blood, respectively. An increase in the dose level from 5 to 12.5 ml/kg resulted in the increase in both Cmax and AUC24, and the increases in these parameters appeared to be in proportion to the dose increment. Thus, following the 2.5-fold increase in administered dose, Cmax was increased by a factor of 2.1 in both plasma and whole blood, while AUC24 was increased by a factor of 2.8 for plasma and 2.6 for whole blood. In the dogs receiving 10 ml/kg (n = 3), the Cmax, t1/2, AUC168 and Tmax were 12.70 mg equivalents/ml, 47.2 hr, 425.7 mg equivalents.hr/ml and 0.083 hr in the plasma and 8.372 mg equivalents/ml, 50.3 hr, 241.3 mg equivalents.hr/ml and 1.003 hr in the whole blood, respectively. The present work provides an insight into the pharmacological behavior of a PEG-modified hemoglobin.
INTRODUCTION
PEG-hemoglobin SB1 is a polyethylene glycol (PEG)-modified bovine hemoglobin, being developed for the emergency treatment of brain stroke and ischemia. The modification of hemoglobin with PEG has been known to be an effective means of overcoming problems of short half-life and antigenicity borne with native hemoglobin. The PEG modification (pegylation) of proteins has become increasingly common for pharmaceutical industry as a way of improving the pharmacological properties of the parent molecule [Citation[1-4]]. Significant improvements in clinical efficacy have been seen with several marketed pegylated drugs. Peptides and native proteins often undergo rapid clearance from the body, which can be triggered by a combination of events including proteolysis, renal filtration, hepatic uptake and by the immune system [Citation[5]]. Interaction and accumulation within tissues may represent an additional pathway for removal of peptides and proteins from blood. Pegylation is currently considered one of the most successful techniques by which to prolong the residence time of protein drugs in the bloodstream. Several different formulations of PEG-modified hemoglobins have been extensively studied for their pharmacokinetic behavior [Citation[6-9]]. Although there is no consensus or regulatory guideline regarding how long the intravascular residence time of hemoglobin products should be, this study offers an additional example of the pharmacokinetic profile of PEG-modified hemoglobin in male SD rats and beagle dogs after single intravenous infusion of 125I-PEG-hemoglobin SB1.
MATERIALS AND METHODS
Materials
PEG-hemoglobin SB1 was prepared under asceptic environment by aqueous reaction between hemoglobin and activated PEG. Bovine hemoglobin had been obtained from local cow herds in quarantine in South Korea. Bleeding of cows was conducted in an asceptic way using a trochar inserted into neck vein. Blood was citrated on site and then treated in the laboratory to obtain pure native hemoglobin. The pure hemoglobin was then reacted with activated methoxy PEG of molecular weight 5,000 to obtain PEG-hemoglobin in which each hemoglobin was pegylated with average of 8 PEG. Thereby, the average molecular weight of PEG-hemoglobin was 105,000.
PEG-hemoglobin SB1 was then radiolabelled with 125I by the lactoperoxidase reaction and purified. 125I-PEG-Hemoglobin SB1 was prepared using the PEG-Hemoglobin SB1 supplied by SunBio Inc. (Anyang City, South Korea) and 125Iodine (commercially obtained from Amersham) at Huntingdon Life Sciences as a part of preclinical trials. The radiochemical purity was >95%. 125I-PEG-Hemoglobin SB1 was formulated for dosing in 5 mM phosphate buffered saline (110 mM saline), pH 7.6 solution and stored at about +4°C (for a maximum of 7 days) until taken for use.
Studies with Animals
For rats, a total of 9 male albino rats (Charles River code crl:CD(SD)IGS BR) having no previous history of any test xenobiotic treatment were obtained from Charles River UK Ltd (Margate, England) for use in this study. Rats were aged approximately 6–8 weeks and bodyweight ranged 225–275 g on the day of dosing. During the experimental period, the ambient air temperature in the animal housing unit was generally maintained between 19°C and 23°C, and the relative humidity between 35 and 65%; both temperature and humidity were continuously monitored and automatically recorded. Lighting was controlled in a 12-hour light/darkcycle throughout the study, and the animal rooms were ventilated with frequent air changes.
For dogs, a total of 3 male beagle dogs were obtained from Harlan Interfauna UK Ltd for use in this study. The dogs were aged approximately 11 months and bodyweight ranged 13.4 to 15.1 kg on the day of dosing. Each dog was individually identified in the form of a colony number tattooed in one ear. The animals were offered a single ration of 500 g of a standard diet each day. The dogs were fasted for a period of about 16 hour prior to dose administration and for 4 hours afterwards. Drinking water from the local water supply was freely available at all times.
All animals were weighed before dose administration and prior to termination.
Dose Preparation and Administration
Intravenous doses of the test compound were prepared by mixing appropriate volumes of 125I-PEG-hemoglobin SB1 and non-radiolabelled PEG-hemoglobin SB1 saline solutions.
In SD rats, each 125I-PEG-hemoglobin SB1 formulation (5 or 12.5 ml/kg) was administered to rats by slow injection into a caudal vein at a rate of 5 ml/kg/10 minutes (for both dose levels). The actual radioactive dose administered to each animal was calculated from the volume of formulation given and its mean measured radioactivity concentration.
In beagle dogs, each 125I-PEG-hemoglobin SB1 formulation (10 ml/kg) was administered to dogs by slow injection into a forelimb vein (cephalic) over 50 minutes. The actual radioactive dose administered to each animal was calculated from the volume of formulation given and its mean measured radioactivity concentration.
Sample Collection and Measurement of Radioactivity
For rats, following single 5 and 12.5 ml/kg intravenous infusion doses of 125I-PEG-hemoglobin SB1 to 9 male rats (three sub-groups of three rats per group) blood samples (0.5 ml) were collected from a caudal vein (opposite to the vein used for dosing) into heparinized containers at the following times after completion of dosing. Blood samples (ca 8 ml) were obtained at pre-dose, and at 0.083, 0.17, 0.25, 0.75, 1, 2, 3, 4, 6, 8, and 12 hours after the end of infusion. From each serial (caudal) blood sample, two 50 µl aliquots were removed for radioactivity measurement, and the remainder was centrifuged at 4°C to separate plasma from the cells (the latter being discarded). For the collection of the final sample, each rat was anesthetized using isoflurane and a terminal blood sample (ca 2 ml) was collected by cardiac puncture into a heparinized container. The rats were then sacrificed by cervical dislocation. The terminal blood sample was stored at about +4°C and used for measurement of packed cell volume (PCV).
For dogs, following single l0 ml/kg intravenous infusion doses of 125I-PEG-Hemoglobin SB1 to three male dogs (1–3 M), blood samples (ca 8 ml) were obtained by venupuncture at pre-dose, mid-infusion (25 minutes) and at the end of infusion (50 minutes), and at 0.08, 0.17, 0.5, 0.75, 1, 2, 3, 4, 6, 8, 24, 48, 72, 96, 120 and 168 hours after the end of infusion. The blood samples were transferred to heparinized tubes, and a portion of each (ca 2 ml) was retained as whole blood for the measurement of radioactivity and hemoglobin concentration and packed cell volume (hematocrit) where specified.
Radioactivity was measured by gamma counting using a multiple crystal Cobra II Series Auto-Gamma™ counter (Model 5005; Packard Biosciences, Groningen, The Netherlands). Raw counts, expressed as cpm, were adjusted for decay by automatic back-calculation to a manually entered reference date (which was the date and time of dose administration for all test samples) using a 125I-half-life of 1440 hours (60 days). Dpm (ccpm) values were also automatically calculated by the counter by adjustment for the counting efficiency.
Pharmacokinetics Analysis
Plasma total radioactivity, TCA-precipitable radioactivity and whole blood total radioactivity concentration-time data were processed using the software, WinNonlin Pro version 3.1 (Pharsight Corp., CA, USA). Non-compartmental analysis was performed using a constant infusion model (Model no. 202). Maximum concentrations (Cmax) and the times of their occurrence (Tmax) were taken directly from the experimental data. Areas under the concentration-time curves to the last measurable sample point (AUCt) were calculated using the linear trapezoidal rule. Areas under the concentration-time curves to infinite time (AUC) were calculated using the expression:where Clast is the predicted concentration at the last measurable sample point and λz is the terminal rate constant, determined after log-linear regression analysis of those points that constituted the final, linear phase of the concentration-time curve. The terminal half-life (t1/2) was calculated as ln2/λz.
RESULTS
Clinical Signs
After single 5 or 12.5 ml/kg intravenous doses of 125I-PEG-hemoglobin SB1 infused at rates of 10 and 25 minutes/kg, respectively, to male rats, no adverse reactions to treatment were observed. After single intravenous doses of 125I-PEG-hemoglobin SB1 (10 ml/kg infused over 50 minutes) to male dogs, all three animals vomited on at least one occasion during the 6-hour post-dose period, and became subdued in their behavior.
Pharmacokinetics in the 5 ml/kg of Rats
Following single 5 ml/kg intravenous infusion doses of 125I-PEG-hemoglobin SB1 to three sub-groups of three rats per group from which serial blood samples were taken alternately, the maximum mean concentration of total radioactivity in plasma of 9.055 mg equivalents/ml occurred at 10 minutes post-dose (). Concentrations declined thereafter to a mean of 1.113 mg equivalents/ml at 24 hours post-dose (the final sampling time). The terminal rate constant (and hence the corresponding half-life and AUC) could not be calculated accurately, but the terminal phase half-life was estimated to be approximately 9.6 hours from the available data ().
Figure 1 Mean concentrations of total radioactivity in plasma and whole blood and of TCA-precipitable radioactivity in plasma following intravenous administration of 125I-PEG-hemoglobin SBl (nominal 5 ml/kg/10 minute infusion rate) to male rats.
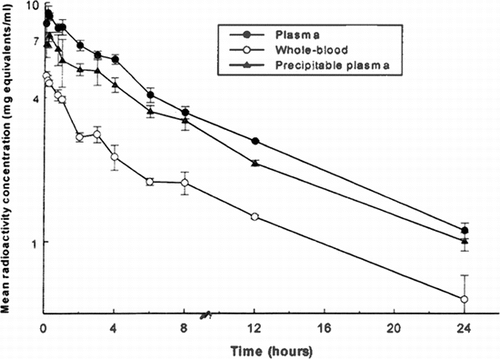
Table 1. Total radioactivity pharmacokinetic data following intravenous administration of 125I-PEG-hemoglobin SBl (nominal 5 ml/kg/10 minute and 12.5ml/kg/25 minute infusion rates) to male rats
Whole blood levels of radioactivity were less than those in plasma at all sampling times, indicating that association of drug-derived material with the blood cells was not extensive. Concentrations mirrored those in plasma (), with a terminal phase-half-life (ca 9.7 hours, ) which was similar to the corresponding plasma half-life.
Concentrations of radioactivity in the blood cells at the time of sacrifice (i.e., at 8, 12, and 24 hours post-dose) for these nine rats were calculated from the measured whole blood and plasma concentrations and the packed cell volumes (hematocrit). The results show that cell concentrations of radioactivity were below the limit of detection at each time ().
Table 2. Whole blood: plasma radioactivity concentration ratios, calculated concentrations of radioactivity in blood cells and distribution of systemic radioactivity into blood cells at the time of sacrifice following intravenous administration of 125I-PEG-hemoglobin SB1 (nominal 5 ml/kg/10 minute infusion rate) to male rats
In order to ascertain the proportion of the radioactivity in the systemic circulation that remained associated with protein, aliquots of plasma were treated with trichloroacetic acid (TCA) and radioactivity was measured in the precipitate. The results () showed that 62–93% of the plasma radioactivity was precipitable in this manner, and so represented test compound-related material.
Table 3. Proportions of the plasma radioactivity precipitated by 15% trichloroacetic acid solution and concentrations of radioactivity in the precipitate following intravenous administration of 125I-PEG-hemoglobin SB1 (nominal 5 ml/kg/10 minute infusion rate) to male rats
Pharmacokinetics in the 12.5 ml/kg of Rats
Following single 12.5 ml/kg intravenous infusion doses of 125I-PEG-hemoglobin SB1 to three other sub-groups of three rats per group from which serial blood samples were taken alternately, the maximum mean concentration of total radioactivity in plasma of 19.00 mg equivalents/mloccurred at 15 minutes post-dose (). Concentrations declined thereafter to a mean of 4.160 mg equivalents/ml at 24 hours post-dose (the final sampling time). Also in this case, the terminal rate constant (and hence the corresponding half-life and AUC) could not be calculated accurately, but the terminal phase half-life was estimated to be approximately 10.6 hours from the available data.
Figure 2 Mean concentrations of total radioactivity in plasma and whole blood and of TCA-precipitable radioactivity in plasma following intravenous administration of I25I-PEG-hemoglobin SBl (nominal 12.5 ml/kg/10 minute infusion rate) to male rats.
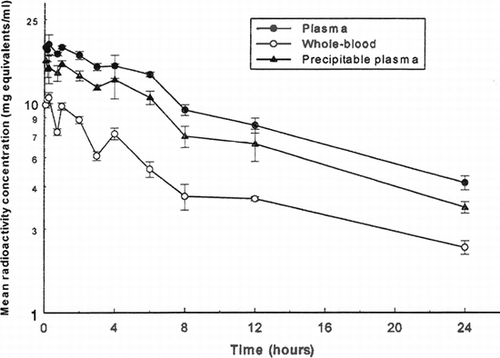
Whole blood levels of radioactivity were less than those in plasma at all sampling times, indicating that association of drug-derived material with the blood cells was not extensive. Concentrations mirrored those in plasma (), with a terminal phase-half-life (ca 16.1 hours, ) which was similar to the corresponding plasma half-life.
Concentrations of radioactivity in the blood cells at the time of sacrifice (i.e., at 8, 12, and 24 hours post-dose) for these nine rats were calculated from the measured whole blood and plasma concentrations and the packed cell volumes (hematocrit). The results show that cell concentrations of radioactivity were below the limit of detection at each time (). The proportion of the plasma radioactivity precipitated by TCA in these rats was in the range 70–90% at all sampling times ().
Table 4. Whole blood: plasma radioactivity concentration ratios, calculated concentrations of radioactivity in blood cells and distribution of systemic radioactivity into blood cells at the time of sacrifice following intravenous administration of I25I-PEG-hemoglobin SB1 (nominal 12.5 ml/kg/25 minute infusion rate) to male rats
Table 5. Proportions of the plasma radioactivity precipitated by 15% trichloroacetic acid solution and concentrations of radioactivity in the precipitate following intravenous administration of I25I-PEG-hemoglobin SB1 (nominal 12.5 ml/kg/25 minute infusion rate) to male rats
Pharmacokinetics in Dogs
Following single 10 ml/kg 50-minute infusion doses of 125I-PEG-hemoglobin SB1 to three male dogs, the maximum mean concentration of total radioactivity in plasma of 12.70 mg equivalents/ml occurred at the end of the 50-minute infusion period (). Plasma levels declined thereafter in an apparently triphasic manner to a mean of 0.388 mg equivalents/ml at 168 hours post-dose (the final sampling time). During the terminal phase, concentrations declined with a mean half-life of 47.2 hours ().
Figure 3 Mean concentrations of radioactivity in plasma and whole blood following a single intravenous administration of l25I-PEG-hemoglobin SBl (nominal 10 ml/kg infused over 50 minutes) to male dogs.
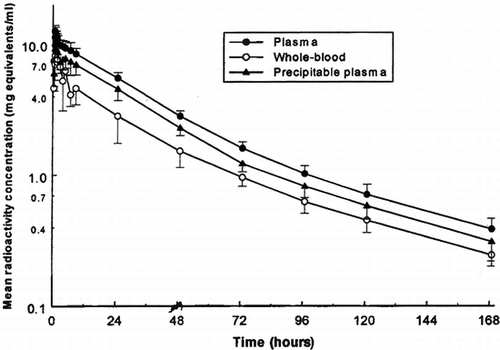
Table 6. Total radioactivity pharmacokinetic data following intravenous administration of 125I-PEG-hemoglobin SB1 (nominal 10 ml/kg infused over 50 minutes) to male dogs
Whole blood concentrations of radioactivity were less than those in plasma at all sampling times, indicating that association of drug-derived material with the blood cells was not extensive. Maximum levels occurred during 1 hour after the end of infusion, and concentrations generally mirrored those in plasma (), with a mean terminal phase-half-life (50.3 hours) which was similar to the corresponding plasma half-life.
Concentrations of radioactivity in blood cells were calculated from the measured whole blood and plasma concentrations and the packed cell volumes (hematocrit). The resulting blood cell levels exhibited marked inter-animal variation, but rarely exceeded 50% of the corresponding plasma concentration.
In order to ascertain the proportion of the radioactivity in the systemic circulation that remained associated with protein, aliquots of plasma were treated with trichloroacetic acid and radioactivity was measured in the precipitate. The results showed that 70–90% of the plasma radioactivity was precipitable in this manner, and so represented test compound-related material. The calculated concentrations of TCA-precipitable plasma radioactivity are presented in and illustrated in .
Table 7. Concentrations of radioactivity in plasma precipitated by 15% trichloroacetic acid solution during and after intravenous administration of 125I-PEG-hemoglobin SB1 (nominal 10 ml/kg infused over 50 minutes) to male dogs
CONCLUSION AND DISCUSSION
Male rats were administered single intravenous doses of I25I-PEG-hemoglobin SB1 by infusion over 2 minutes (nominal dose of 5 ml/kg body weight) or 5 minutes (nominal dose of 12.5 ml/kg body weight) and blood samples were collected from 3 animals at each time point at various times up to 24 hours post-dose. Total radioactivity concentrations in plasma and whole blood and plasma TCA precipitable radioactivity concentrations were available for pharmacokinetic analysis.
In male rats administered the lower dose (5 ml/kg), mean plasma total radioactivity concentrations reached a maximum at 10 minutes after the end of the infusion (12 minutes from the start of the infusion) and thereafter declined in an apparently biexponential manner. However, the time of the maximum mean plasma TGA precipitable radioactivity concentration was 15 minutes post-infusion although the decline in radioactivity concentrations was also apparently biexponential in function. Maximum mean total radioactivity concentrations in whole blood occurred slightly earlier, at 5 minutes post-infusion.
Plasma total and TCA precipitable radioactivity concentrations were higher than whole blood total radioactivity concentrations and the whole blood to plasma ratio was 0.48 total radioactivity. These results indicated that very little radioactivity distributed into red blood cells following intravenous administration of a 5 ml/kg dose of I25I-PEG-Hemoglobin SB1, to male rats. The terminal half-life of radioactivity varied little between the analytical matrices, from 9.6–9.8 hours. Comparison of plasma total and TCA precipitable radioactivity indicated that the latter accounted for ca 84% of the total plasma radioactivity, based on AUC values. This result suggested little loss of the I25I- label following dose administration.
Following administration of the higher dose of I25I-PEG-hemoglobin SB1 (12.5 ml/kg) to male rats, maximum mean radioactivity concentrations occurred at 5 (plasma TCA precipitable radioactivity) or 15 minutes (plasma and whole blood total radioactivity) post-infusion (10 and 20 minutes, respectively, from the start of the infusion). Thereafter, mean concentrations declined in an apparently monoexponential manner in plasma but in an apparently biexponential manner in whole blood.
Radioactivity concentrations were higher in plasma and the whole blood to plasma ratio, calculated using AUC, was 0.52 for total radioactivity. These values were similar to those observed following administration of the lower dose of 125I-PEG-hemoglobin SB1 (5 ml/kg). The terminal half-life of plasma radioactivity was similar for total and TCA precipitable radioactivity (10.6 and 10.2 hours, respectively) but was slightly shorter than the terminal half-life of total radioactivity in whole blood (16.1 hours). TCA precipitable radioactivity accounted for ca 78% of the total plasma radioactivity, based on AUC values.
Comparison of the results of the two dose levels administered indicated that, based on plasma total radioactivity data, the rate and extent of exposure of male rats to radioactivity increased approximately proportionately with the dose increase. Thus, for a dose ratio of 2.5:1, the parameter Cmax, which reflects the rate of exposure, increased in the ratio 2.1:1 while AUC, which reflects the extent of exposure increased in the ratio 3.0:1. These results indicate linear, dose-independent kinetics of radioactivity in male rats over the dose range 5–12.5 ml/kg I25I-PEG-hemoglobin SBl.
Radioactivity concentrations in the blood of 3 male dogs administered single intravenous doses of 10 ml 125I-PEG-hemoglobin SB1/kg body weight by infusion over 50 minutes were available for pharmacokinetic analysis. Total radioactivity and TCA precipitable radioactivity concentrations were determined in plasma and total radioactivity concentrations only in whole blood. Blood samples were collected during the infusion and for up to 168 hours post-infusion.
Examination of the radioactivity concentration-time curves indicated that, in each animal, radioactivity concentrations declined in an apparently triexponential manner for each analyte; i.e., plasma total, and TCA precipitable radioactivity, and whole blood radioactivity. There was also a suggestion of a second peak of radioactivity, which occurred during ca 3–9 hours from the start of the infusion. Mean pharmacokinetic parameters are presented in the following table:
The mean values for the parameters AUC, λz, and t1/2 must be viewed with some caution as they were calculated from data that did not meet all the acceptance criteria for all the animals. Where the acceptance criteria failed, the ratio of the measurement period to the estimated half-life was <2.
The mean parameters indicated that plasma radioactivity concentrations were greater than whole blood radioactivity concentrations and that the terminal half-life of radioactivity was slightly longer in whole blood. However, the difference in terminal half-life was not consistent in the individual animals and the longer mean half-life in whole blood may have merely reflected inter-animal variability in this parameter. Calculation of the whole blood to plasma ratio, using mean AUC values, yielded a ratio of 0.57 for total radioactivity. These results suggested that little radioactivity distributed into red blood cells. It was calculated that TCA precipitable radioactivity represented ca 80% of total plasma radioactivity (based on AUC).
Therefore there appeared to be very little loss of the radiolabel from the protein material over the period of the study.
REFERENCES
- Kompella, U.B., Lee, V.H.L. (1991). Pharmacokinetics of peptide and protein drugs, in Peptide and Protein Drug Delivery, V.H.L. Lee, Ed., Marcel Dekker: New York.
- Delgado, C., Francis, G.E., Fisher, D. (1992). The uses and properties of PEG-linked proteins. Crit. Rev.Ther. Drug Carrier Syst. 9: 249–304, [INFOTRIEVE]
- Kartre, N. (1993). The conjugation of proteins with polyethylene glycol and other polymers: Altering properties of proteins to enhance their therapeutic potential. Adv. Drug Deliv. Rev. 10: 91–114, [CROSSREF], [CSA]
- Rabkin, R., Dahl, D.C. (1993). Renal uptake and disposal of proteins and peptides, in Biological Barriers in Protein Delivery, K.L. Audus and T.J. Raub, Ed., Plenum Press: New York.
- Sweeney, M.I., Yager, J.Y., Walz, W., Juurlink, B.H. (1995). Cellular mechanisms involved in brain ischemia. Can J. Physiol. Pharmacol. 73: 1523, [CSA]
- Gilbert, C., Nho, K., Johnson, M., Linberg, R., Shorr, R. (1994). Hemoglobinuria in rats: a sensitive test of renal filtering and absorption of PEG-hemoglobin, a red blood cell substitute. Artif. Cells Blood Substit. Immobil. Biotechnol. 22: 535, [INFOTRIEVE], [CSA]
- Kim, E.J., Lee, R.K., Bak, J.Y., Choi, G.K. (1999). General pharmacology of PEG-hemoglobin SB1. J. Appl. Parmacol. 7: 170, [CSA]
- Conover, C.D., Gilbert, C., Shum, K., Shorr, R. (1997). The impact of polyethylene glycol conjugation on bovine hemoglobin's circulatory half-life and renal effects in a rabbit top-loaded transfusion model. Artif. Cells Blood Substit. Immobil. Biotechnol. 21: 907, [CSA]
- Conover, C.D., Linberg, R., Gilbert, C, Shum, K., Shorr, R. (1997). Effect of polyethylene glycol conjugated bovine hemoglobin in both top-loaded and exchange transfusion rat models. Artif. Organs 21: 1066, [INFOTRIEVE], [CSA]
