Abstract
Objective: To observe the changes during the process of modificating collagen with Arg-Gly-Asp (RGD) peptide, a series experiments were designed.
Methods: The 3D porous collagen matrices modified with RGD peptide were constructed by lyophilization. The modified and unmodified matrices were characterized by Scanning Electron Microscopy (SEM) and Electron Spectroscopy for Chemical Analysis (ESCA). Fibroblasts were used to evaluate the cell compatibility of the matrices.
Results: In terms of cell growth, the cells attached much better on the modified matrix than on the unmodified one. Compare to the unmodified matrices, the polar groups on the modified matrix increased.
Conclusions: The introducing of specific RGD receptor-mediated adhesion site on matrices obviously enhanced the cells adhesion on collagen matrices.
INTRODUCTION
Numerous biomaterials have been investigated as a matrix for Guided Tissue Regeneration (GTR) scaffold. To improve their histocompatibility and shorten the healing time, surface design is commonly used in modifying the biomaterials [Citation[1], Citation[2]]. The addition of biologically active molecules, which accelerate the processes of tissue regeneration, is expected to increase the probability and speed of wound closure, and simultaneously decrease the risk of infection. Specific amino acid sequences present in extracellular matrix proteins are efficient bioligands for cell adhesion on biomaterials surfaces. The most widely used cell-binding domain are RGD peptides, which is an integrin-mediated cell adhesion domain found in extracellular matrix proteins. It can promote integrin receptor-mediated cell to attach on various tissue surfaces [Citation[3-5]]. Collagen is a widely used biomaterial. The fibrous structure of the collagen is favorable to cell growth. With this understanding, three-dimensional collagen matrices coupled with RGD peptide have been studied extensively in vitro as templates for tissue regeneration. In this report, we attached RGD to the matrices by periodate oxidation to evaluate the effect of RGD modification on collagen matrices.
MATERIALS AND METHODS
Preparation of Matrices
80 ml collagen swelling solution was poured into 60 mm culture dishes and solidified in a refrigerator at −30°C. Porous collagen matrix was gained by lyophilization. Collagen matrix was marinated in 0.25% (v/v) pH 5.5 glutaraldehyde solution containing 2% CS (w/v) for 24 h at 4°C. After cross-linking, the collagen matrices were washed four times, respectively, and lyophilized again to form the collagen (Col) matrix [Citation[6], Citation[7]].
RGD peptide (Sigma Co. Ltd) was covalently coupled to Col matrices by periodate activation method. Col matrices (6 cm2: approximately 6 mg±10%) were incubated with 120 mM sodium periodate (NaIO4 Sigma Co. Ltd) in 0.3 M sodium acetate buffer (pH 5.6) containing 6 mg/ml RGD peptide and 150 mM sodium cyanoborohydrate (NaBH3CN) for 4 h at room temperature. The matrix was washed twice with 1% acetic acid /1 M NaCl for 10 min to remove ionically associated peptide, then washed twice with water, and lyophilized again to form the collagen-RGD (Col-RGD) matrix [Citation[8-10]].
SEM
Morphology of the matrices was observed by HI-TACHI X-650 model SEM.
Mechanical Property
The tensile mechanical property was tested with a mechanical tester (HE HOUNSFIELD). The samples (n = 3) were rectangular disks, at a dimension of 6 cm × 1 cm × t, where t is the thickness of the matrix. The crosshead speed was 5 mm/min. The fraction stress elongation and the rupture length at break were measured (statistical analyzed by STATA 8.0).
Amino Acid Analysis (AAA)
Reversed-phase HPLC (high-performance liquid chromatography) analysis was performed to determine the change of aminophenols in the collagen molecules of Col and Col-RGD. Samples containing 50 mg collagen were transferred into 20 ml vial. Add 10 ml 6 N HCl, seal the vials, and act at 110°C for 20 hours. Filter sample quantitatively into 25 ml volumetric flask. Bring to volume. Remove 1 ml sample into 10 ml volumetric flask. 1 ml 0.5 M NaHCO3 and 0.05 ml 1% 2, 4-Dinitro-1-fluorobenzene were added into the volumetric flask, and act at 60°C for an hour. Bring to volume with 0.01 mol/L KH2PO4. Transfer 1 ml sample to a centrifugal vial. Centrifugalize at 5000 r/min for 10 min. Inject 20 µl of sample automatically into the HPLC system. At the same time, 18 kinds of standard aminophenols were checked for the quantitative analysis [Citation[11]].
ESCA
The chemical character on the surface of the Col and Col-RGD was investigated by ESCA. The ESCA spectrum was obtained on PHI5300 ESCA system with a magnesium anode (MgK = 1253.6 eV), and the survey scan range was 0 ∼ 1000 eV.
Water Absorbency
Samples of Col and Col-RGD were weighed by analytical balance (Wd), and then immersed in PBS solution (pH = 7.4) for 2 h at room temperature. After the additional water was removed, they were weighed again (Wh). The water absorbency (Wa) was calculated according to the following formula [Citation[12]] (statistical analyzed by STATA 8.0).
Cells Culture
Fibroblasts were isolated from neonate prepuce. Prepuce was cut into 1 × 1 cm2 in asepsis, and washed in the D-Hanks solution containing penicillin (100 µ/ml) streptomycin (100 µg/ml), and amphotericin B (2.5 µg/ml), then incubated in 10 ml dispase (0.25%) at 4°C for 1–2 h. Epidermis was removed; the dermal layer was cut to pieces, and 10 ml tape I collagenase (0.25%) was added in. After digesting 0.5–1 h at 37°C, the suspension was collected, filtered by cell filter (×200), and centrifuged 1500 rpm for 5 min. Cells were collected, resuspended in culture medium (DMEM) with 10% fetal calf serum, and incubated at 37°C 5% carbon dioxide. After 3–4 generations, cells were harvested and suspended in DMEM at density of 1 × 105 cells/ml for seeding. 1 ml cell suspension (at 1 × 105 cells/ml) was added onto the matrices, which were sterilized with γ-irradiation, and then cultured at 37°C, 5% carbon dioxide, 85% relative humidity. The culture medium was changed every other day [Citation[13]].
Cell Adhesion Assay
To observe the cell adhesion on matrices, cells were allowed to attach to the matrices undisturbed for 2, 4, 6, and 8 hours, respectively. At each time interval, three specimens of each matrix were rinsed with PBS and the average cell number of each matrix was counted (statistical analyzed by STATA 8.0).
H&E Staining
After 8 hours, the samples were fixed by 4% paraformaldehyde, dehydrated by graded alcohol, embedded in paraffin, and sliced into section (the sections were 7 µm thick).The sections were stained with H&E.
RESULTS
SEM
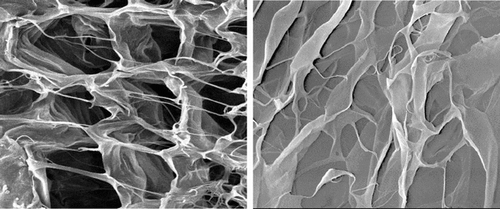
It is generally accepted that the matrix with symmetrical and regularly pores is most suitable for cell growth [Citation[14], Citation[15]]. Porosity provides space not only for the cells infiltrating into the matrix but also for the ECM formation. It also allows the inflow of nutrients and the elution of metabolic waste and biodegradational by-products. SEM result shows that the collagen matrices are porous with three-dimension interconnected fiber microstructure; aperture in matrices are around 100 ∼ 200 µm (). But the structure became irregular after RGD was introduced into collagen matrix through periodate oxidation. The interconnections between pores become much fewer; many pores have disappeared.
Mechanical Property
Matrices for tissue plerosis should be mechanically stable to keep their structural integrity during the tissue regeneration. The mechanical strength of matrices is decided by the strength of covalent bond in collagen fiber, and will affect the 3D structure of matrices. Compared with Col matrix (0.75 MPa), the tension strength of Col-RGD matrix decreases significantly (0.61 MPa). The rupture length also decreases from 3.4% to 2.9%. It indicates that the matrix's elasticity was also affected by the oxidizing process.
AAA
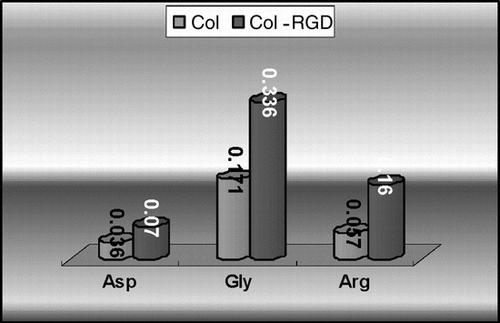
In this study, the RGD peptide was introduced into collagen matrix through periodate oxidation, and the content of Arg, Gly, and Asp in Col-RGD matrix was increased obviously. From , it is found that the content of Arg, Gly, and Asp per mg sample increased from 0.036 mg, 0.171 mg, and 0.057 mg to 0.07 mg, 0.336 mg, and 0.16 mg. The level of most other amino acids still keeps at the original level after modification. This indicates that the RGD peptide is attached into the matrix successfully.
ESCA
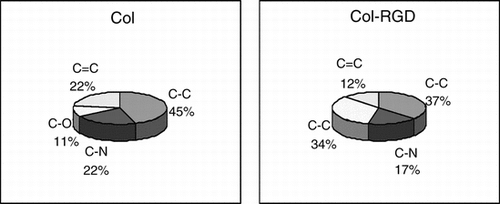
Surface characteristics of biomaterials, such as surface energy, play an essential part in cells adhesion on biomaterials. Polar groups could increase the water binding capability, and improve the cells attachment. It is generally known that the histocompatibility of biomaterials is closely related to the cell behavior on contact with them, especially to the cell adhesion [Citation[16-18]]. gives the proportion of several specific functional groups in both matrices. Through the process of periodate oxidation, the aldehyde groups, covalently couple site for RGD, increased from 11% to 34%. After RGD is attached into the collagen matrix, the total amount of polar groups increased from 55% in Col matrix to 63% in Col-RGD matrix, and the percentages of non-polar groups, C–C groups, decreased from 45% to 37%.
Figure 4 Adhesion percent of cell on both matrices over 2, 4, 6, and 8 hours (the initial seeding density was 1 × 105 cells/cm2).
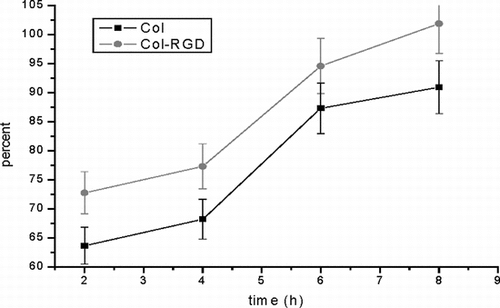
Table 1. The tension strength and the break elongation of the matrices (n = 3 p < 0.05)
Water Absorbency
In , the water absorbency properties of both kinds of matrices are compared. There is no significant difference (p > 0.05) between two groups at room temperate. Water absorbency is used as the token of biomaterials' hydrophilic capability [Citation[18]]. It is affected by two basic factors: the polar groups on the surface of the collagen fiber and the 3D structure of the matrix.
Table 2. The result of matrices' water absorbency (mg/mg, n = 6)
Cell Adhesion Assay
It has been demonstrated previously that the collagen matrix supports cell adhesion, especially after suitable modification. Recent studies have demonstrated that RGD is the primary cell adhesion domain for many integrin receptor-mediated cells. It could improve the cell adhesion to the surface of biomaterial matrix [Citation[19]]. After the RGD is introduced into the collagen matrix, the cell adhesion ratio on Col-RGD matrix can be observed to increase significantly.
H&E Staining
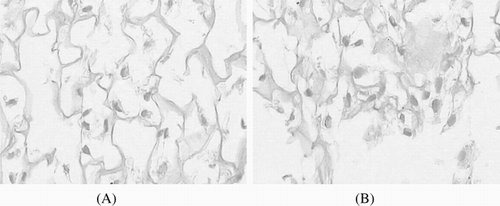
After implanted 8 hours, the cells on the scaffold surface were dispersed among the matrix. The cells on the scaffold surface were rounding, oval and fusiform in the matrix. But the number of the cells is very small. The cells on the collagen scaffold were lying between the fibers of scaffold, and the cells on the collagen-RGD have attached to the scaffold ().
DISCUSSION
Collagen is one of the main components of ECM proteins, which can be fabricated into porous matrix for tissue regeneration and tissue reconstruction. The collagen material has great potential in tissue plerosis because of its low antigenicity and excellent biodegradability. As has been discussed previously, some kinds of biomodification could improve the properties of collagen in many aspects [Citation[20], Citation[21]]. Exactitude and overall judgments about the modification are very important for biomodifications.
Experiments have shown that the cell behavior on matrix is influenced by the physicochemical properties of material surfaces [Citation[22]]. Cells attached onto the surface of matrices, then proliferated and spread at the space of matrices for tissue plerosis. Cell adhesion to the surface of the biomaterial is very important in its applications to support and stimulate tissue integration, tissue reconstruction, or cellular colonization.
We evaluated the cell adhesion and proliferation on both matrices by fibroblasts. In cell adhesion assays, cells attached onto the surfaces of the matrices, but the cell adhesion ratio was significantly different. We think that cell adhesion domain is the key factor that affects the cell adhesion. RGD is the primary adhesion site for many kinds of cells [Citation[23], Citation[24]]. Its addition could improve the cell adhesion to the surface of the biomaterial matrix. From the result of AAA, we found that RGD peptide was introduced into the matrices by periodate oxidation successfully. Other then adhesion domain, Arg and Asp are both polar amino acids. Attachment of RGD to collagen material can increase the polar groups on the surface of collagen matrix. As discussed, the polar group could improve the surface energy of biomaterials [Citation[25]]. High-energy surfaces will promote cell adhesion, as opposed to low-energy surfaces [Citation[22]]. In the result of ESCA, the total amount of polar groups on the surface of Col increased sharply after RGD modification. So the cell adhesion rate on Col-RGD matrix is higher then on Col matrix.
As reported previously, the high-energy surface matrix could absorb more water than the low-energy surface matrix [Citation[26]]. But, in this study it was found that there is no significant difference between two groups in water absorbency (p = 0.8005). We think that other than surface energy, there are other factors that affect the water absorbency. As has been shown in mechanical property, this oxidation process decreased the intensity of covalent bonds in the matrix. We think the oxidant affects the covalent bonds in collagen matrix during process of attaching RGD, and destroys the structure of matrix. Together with the result of SEM, we think that irregular matrix and deformational aperture will decrease the water absorbency in Col-RGD matrix.
The data presented above show that high cell adhesion ratio and enough cell adhesion domains can accelerate the cell proliferation on the surface of Col-RGD matrix. But the bond intensity was weakened by the process of periodate oxidation, and resulted in the failure under shear stress and the destruction of structure requiring by revision surgery.
REFERENCES
- Hubbell, J.A. (1995). Biomaterials in tissue engineering. Bio/Technology 13: 565–76, [INFOTRIEVE], [CSA]
- Cai, K.Y., Yao, K.D., Hou, X., Wan, Y.Q., Hou, Y.J., Yang, Z.M., Li, X.Q., Xie, H.Q. (2002). Improvement of the functions of osteoblasts seeded on modified poly(D, L-lactic acid) with poly(aspartic acid). J. Biomed. Mater. Res. 62: 283–291, [INFOTRIEVE], [CROSSREF], [CSA]
- Ruoslahti, E., Pierschbacher, M.D. (1987). New perspectives in cell adhesion: RGD and integrins. Science 238: 491–497, [INFOTRIEVE], [CSA]
- Rezania, A., Healy, K.E. (2000). The effect of peptide surface density on mineralization of a matrix deposited by osteogenic cells. J. Biomed. Mater. Res. 15: 52(4): 595–600, [CROSSREF], [CSA]
- Sakata, N., Sasatomi, Y., Meng, J., Ando, S., Uesugi, N., Takebayashi, S., Nagai, R., Horiuchi, S. (2000). Possible involvement of altered RGD sequence in reduced adhesive and spreading activities of advanced glycation end product-modified fibronectin to vascular smooth muscle cells. Connect Tissue Res. 41(3): 213–28, [INFOTRIEVE], [CSA]
- Yannas, I.V., Burke, J.F. (1980). Design of artificial skin. Basic design principles. J. Biomed. Mater. Res. 14: 65–81, [INFOTRIEVE], [CROSSREF], [CSA]
- Yannas, I.V., Burke, J.F., Gordeon, P.L., Huang, C., Rubenstein, R.H. (1980). Design of artificial skin. Control of chemical composition. J. Biomed. Mater. Res. 14: 107–131, [INFOTRIEVE], [CROSSREF], [CSA]
- Glass, J.R., Dickerson, K.T., Stecker, K., Polarek, J.W. (1996). Characterization of a hyaluronic acid Arg-Gly-Asp peptide cell attachment matrix. Biomaterials 17: 1101–1108, [INFOTRIEVE], [CROSSREF], [CSA]
- Matsumoto, T., Numata, M., Anada, T., Mizu, M., Koumoto, K., Sakurai, K., Nagasaki, T., Shinkai, S. (2004). Chemically modified polysaccharide schizophyllan for antisense oligonucleotides delivery to enhance the cellular uptake efficiency. Biochim. Biophys. Acta 1670(2): 91–104, [INFOTRIEVE], [CSA]
- Grzesiak, J.J., Pierschbacher, M.D., Amodeo, M.F., Malaney, T.I., Glass, J.R. (1997). Enhancement of cell interactions with collagen/glycosaminoglycan matrices by RGD derivatization. Biomaterials 18: 1625–1632, [INFOTRIEVE], [CROSSREF], [CSA]
- Wheeler, T.L., Shackelford, S.D., Koohmaraie, M. (2000). Variation in proteolysis, sarcomere length, collagen content, and tenderness among major pork muscles. J. Anim. Sci. 78: 958–965, [INFOTRIEVE], [CSA]
- Mao, J.S., Zhao, L.G., Yin, Y.J., Yao, K.D. (2003). Structure and properties of bilayer chitosan-gelatin scaffolds. Biomaterials 24(6): 1067–1074, [INFOTRIEVE], [CROSSREF], [CSA]
- Zhang, L.H., Ma, D.R., Wang, F.J., Zhang, Q.Q. (2002). The modification of scaffold material in building artificial dermis. Artif. Cells Blood Substit. Immobil. Biotechnol. 30(4): 319–32, [INFOTRIEVE], [CROSSREF], [CSA]
- Natsume, T., Ike, O., Okada, T., Takimoto, N., Shimizu, Y., Ikada, Y. (1993). Porous collagen sponge for esophageal replacement. J. Biomed. Mater. Res. 27(7): 867–875, [INFOTRIEVE], [CROSSREF], [CSA]
- Dagalakis, N., Flink, J., Stasikelis, P., Burke, J.F., Yannas, I.V. (1980). Design of artificial skin. Part III. Control of pore structure. J. Biomed. Mater. Res. 14: 511–528, [INFOTRIEVE], [CROSSREF], [CSA]
- Zhang, Q.Q., Ren, L., Liu, L.R. (1997). Structure investigation of poly(vinyl alcohol)-collagen composite. J. Mater. Sci. Technol. 13: 179–183, [CSA]
- Curtis, A.S.G., Forrester, J.V., Clark, P. (1986). Substratum hydroxylation and cell adhesion. J. Cell Sci. 86: 9–24, [CSA]
- Altankov, G., Groth, T. (1997). Fibronectin matrix formation by human fibroblasts on surfaces varying in wettability. J. Biomater. Sci. Polym. Edn. 8: 299–310, [CSA]
- Rickard, D.J., Sullivan, T.A., Shenker, B.J., Leboy, P.S., Kazhdan, I. (1994). Induction of rapid osteoblast differentiation in rat bone marrow stromal cell cultures by dexamethasone and BMP-2. Dev. Biol. 161(1): 218–228, [INFOTRIEVE], [CROSSREF], [CSA]
- Natsume, T., Ike, O., Okada, T., Takimoto, N., Shimizu, Y., Ikada, Y. (1993). Porous collagen sponge for esophageal replacement. J. Biomed. Mater. Res. 27(7): 867–875, [INFOTRIEVE], [CROSSREF], [CSA]
- Dagalakis, N., Flink, J., Stasikelis, P., Burke, J.F., Yannas, I.V. (1980). Design of artificial skin. Part III. Control of pore structure. J. Biomed. Mater. Res. 14: 511–528, [INFOTRIEVE], [CROSSREF], [CSA]
- Mizuno, K., Hayashi, T., Bachinger, H.P. (2003). Hydroxylation-induced stabilization of the collagen triple helix. Further characterization of peptides with 4(R)-hydroxyproline in the Xaa position. J. Biol. Chem. 278(34): 32373–32379, [INFOTRIEVE], [CROSSREF], [CSA]
- Lauer-Fields, J.L., Tuzinski, K.A., Shimokawa, K., Nagase, H., Fields, G.B. (2000). Hydrolysis of triple-helical collagen peptide models by matrix metalloproteinases. J. Biol. Chem. 275(18): 13282–13290, [INFOTRIEVE], [CROSSREF], [CSA]
- Curtis, A.S.G., Forrester, J.V., Clark, P. (1986). Substratum hydroxylation and cell adhesion. J. Cell Sci. 86: 9–24, [CSA]
- Altankov, G., Groth, T. (1997). Fibronectin matrix formation by human fibroblasts on surfaces varying in wettability. J. Biomater. Sci. Polym. Edn. 8: 299–310, [CSA]
- Pieper, J.S., Hafmans, T., Veerkamp, J.H., van Kuppevelt, T.H. (1999). Development of tailor-made collagen-glycosaminoglycan matrices: EDC/NHS crosslinking, and ultrastructural aspects. Biomaterials 20: 847–858, [INFOTRIEVE], [CROSSREF], [CSA]