Abstract
New potentiometric and amperometric biosensors were developed for the determination of creatine. The potentiometric creatine biosensor was prepared by immobilizing urease and creatinase on poly(vinylchloride) (PVC) ammonium membrane electrode containing palmitic acid prepared by using nonactine as an ammonium-ionophore. The linear working range of the biosensor was 1.0 × 10−5–1.0 × 10−3 M and the response time was about 60 s. The optimum pH, temperature, and buffer concentration were found to be 7.0, 20°C, and 5 mM, respectively. The slope of the electrode was 49.2 mV/p[creatine]. The storage stabilization of the biosensor was investigated and 40–45% decrease in the response was detected after 2 months. The amperometric creatine biosensor was prepared by immobilizing creatinase (CI) and sarcosine oxidase (SO) in a poly(vinylferrocenium) matrix onto the surface of a platinum working electrode by crosslinking with glutaraldehyde (GA) and bovine serum albumine (BSA). Determination of creatine was performed by the oxidation of enzymatically generated H2O2 at +0.7 V vs. Ag/AgCl. The linear working range of the biosensor was 2.0 × 10−5–3.2 × 10−4 M and the response time was about 50 s. The effects of pH, temperature, enzyme ratio and buffer concentration were investigated and optimum parameters were found to be 7.5, 37°C, 2.5:1 (CI:SO) and 0.05 M, respectively. The determination of creatine in commercial creatine powder was successfully carried out with these creatine biosensors by using the standard addition and calibration curve methods. The results were in good agreement with those obtained from Jaffé method at 95% confidence level.
INTRODUCTION
Creatine is an amino acid that is synthesized from L-arginine, glycine and S-adenosylmethionine. Creatine is converted to phosphocreatine in muscle tissue in a reversible reaction with adenosine triphosphate (ATP), and this reaction is catalyzed by the enzyme creatine kinase. Phosphocreatine is a major energy storage form in the body and plays an important role in muscle energy metabolism. Creatine is excreted from the body as creatinine, thus the amount of creatinine in the urine is proportional to the amount of creatine and is used to predict the total muscle mass [Citation[1]]. Creatine is also popular as ergonoic aid [Citation[2]]. The creatine level in blood serum and urine is clinically used as a parameter of muscle damage. The physiological normal concentration range for creatine is below 140 µmol/L in serum, but pathological values due to muscle disorder may rise to concentrations higher than 1000 µmol/L [Citation[3]].
For creatine determination, the most commonly used method is the spectrophotometric one based on the Jaffé reaction, in which the active methylene group reacts with alkaline sodium picrate to give a yellow-red complex [Citation[4]]. However, this reaction is not specific for creatine, because many substances could interfere in this assay [Citation[5]]. Therefore, other various instrumental methods such as HPLC [Citation[6], Citation[7]], capillary electrophoresis [Citation[8]], HPLC-mass spectrophotometry [Citation[9], Citation[10]], and IR spectrophotometry [Citation[11]] were also proposed for the determination of creatine. An alternative method for creatine determination is the use of enzyme biosensors, which allow direct measurement of the creatine in the samples [Citation[12-16]]. A bienzymatic potentiometric enzyme electrode containing urease and creatinase enzymes for creatine has been developed by Koncki et al. by using carboxylated PVC [Citation[12]].
In this work, new potentiometric and amperometric biosensors were prepared; then the creatine in commercial creatine powder were determined by using these biosensors. The results obtained from the new biosensors were compared with those of classical spectrophotometric Jaffé method.
MATERIALS AND METHODS
Equipments and Reagents
Potential and pH measurements were carried out with an ORION 720A model pH-ionmeter. The potential values were given against the saturated Ag/AgCl double junction reference electrode (ORION 90-02). Amperometric measurements were carried out using BAS 100 B/W electrochemical analyzer using a three-electrode cell. The working electrode was the Pt plate (0.5 cm2) coated with PVF. The counter and the reference electrodes were Pt wire (MW 1034) and Ag/AgCl (MF 2052) electrode, respectively. Temperature control was achieved with Grant LTD GG thermostat. Spectrophotometric measurements for Jaffé method were carried out by using Jenway 6105 model UV/VIS spectrophotometer.
Creatinase (E.C.3.5.3.3.; from Flavobacterium sp.) and sarcosin oxidase (E.C.1.5.3.1.; from Arthrobacter sp.), 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDC) were purchased from Sigma Chem. Co. Dichloromethane, sodium monohydrogenphosphate and sodium dihydrogenphosphate were supplied from Riedel-de Haën. Urease (E.C.3.5.1.5), hydrogen peroxide and sodium hydroxide were obtained from Merck (Darmstadt, FRG). Nonactin, palmitic acid, bis-(2ethyl)hexyl sebacate (DOS), poly(vinylchloride) (PVC) were obtained from Fluka. Vinylferrocen was from Aldrich. The commercial creatine powder was purchased from a vitamin store. All other chemicals were obtained from Fluka. The standard solutions of creatine were prepared every 2–3 days and stored at 4°C. All other chemicals used were of analytical reagent grade. Standard solutions and buffer solutions were prepared with deionized water.
Preparation of Potentiometric Creatine Biosensor
The membrane compositions of ammonium-ion selective electrodes were: 30% PVC, 3% palmitic acid, 3% nonactin, 64% bis-(2-ethyl)hexyl sebacate (DOS). The membrane was prepared by dissolving about 400 mg membrane components in 5 mL of tetrahydrofurane (THF). The solutions were poured on a glass plate inside a glass ring (42 mm diameter). After 24 hours drying at room temperature, the membrane was formed. Disks of 5 mm diameter were cut out and attached to the glass electrode body. The inner electrode solution was 1.0 × 10−2 M ammonium chloride. Enzyme was chemically immobilized on membrane electrode surface according to the same procedures given by Karakuş et al. [Citation[17]] and Koncki et al. [Citation[18]].
A combination of 20 mg creatinase, 30 mg urease, and 5 mg 1-ethyl-3-(3-dimethyl amino propyl) carbodiimide was dissolved in 1 mL deionized water. 50 µL of this solution was dropped on the membrane of ammonium-selective electrode and left overnight then, 50 µL of 2.5% glutaraldehyde solution was deposited on the surface of electrode and the electrode was left for a half hour. Then the electrode surface was washed with deionized water to remove the excess of glutaraldehyde. Again 30 mg urease and 20 mg creatinase were dissolved in 1 mL deionized water, then 50 µL of this solution was deposited on the electrode surface. The electrode was left overnight. To remove the excess of unbounded enzymes, biosensor was left for 1 hour in vigorously stirred phosphate buffer (pH 7.0, 10 mM). When not in use, biosensor was stored in a refrigerator at 4°C.
Potentiometric Measurements
Potential measurements for creatine biosensor were carried out by varying creatine concentration in steady-state condition. 5 mM of TRIS buffer was used as a working buffer solution (pH 7.0). The following electrochemical cell was formed with the proposed creatine biosensor by using a Ag/AgCl double junction reference electrode.
Measurements were made with the proposed creatine biosensor and reference electrode. Biosensor was immersed to a depth of 1.5 cm in creatine solution that was stirred by a magnetic stirrer. The pH values were determined using an Orion combinated glass-pH electrode. All the experimental works were carried out at 20 ± 1°C.
The calibration curve was obtained by plotting the potential values of a series of standard creatine solution against the logarithm of creatine concentration.
Preparation of Amperometric Creatine Biosensors
The surface of the Pt plate electrode (0.5 cm2) was cleaned according to Gros et al. [Citation[19]]. PVF was prepared by the chemical polymerization of vinylferrocene [Citation[20]]. PVF is an electroactive redox polymer and the oxidized form of the polymer, , can be electroprecipatated on Pt electrode [Citation[21]]. Before the electroprecipitation the solution was purged with nitrogen for 10 minutes in order to remove the oxygen.
modified electrode was prepared by electro-oxidizing the polymer (2 mg/mL PVF) at +0.8 V vs. Ag/AgNO3 in a 5 mL solution of 0.1 M tetrabutylammonium perchlorate (TBAP) in dichloromethane. The electrode was washed with phosphate buffer solution (0.05 M pH 7.5) after the coating process.
The enzymes were immobilized by using the following procedure: The mixture of 100 µL sarcosine oxidase (26 U/mL), 300 µL creatinase (41.6 U/mL), 1 mg BSA, 100 µL 0.05 M phosphate buffer at a pH of 7.5 and 30 µL 5% glutaraldehyde was dropped onto modified electrode. The electrode was dried at room temperature.
This electrode was washed with buffer solution several times in order to remove the excess enzyme and glutaraldehyde and stored in refrigerator at 4°C in phosphate buffer when not in use [Citation[22]].
Amperometric Measurements
All amperometric measurements were performed in phosphate buffer solutions (0.05 M pH 7.5) with constant stirring. In order to determine whether the biosensor was sensitive to creatine, 5 mL buffer solution was added to the cell. The solution was purged with oxygen for 10 minutes and after stabilization of the background current at +0.7 V, the aliquots of creatine stock solution were added to the cell. The response of the biosensor against creatine was measured at +0.7 V vs. Ag/AgCl after 5 minutes constant stirring and calibration curve was plotted. The cell was purged with oxygen for one minute prior to each addition of creatine to the cell.
Procedure for Determination of Creatine in Commercial Creatine Powder with Potentiometric Creatine Biosensor
The creatine amount in the commercial creatine powder was determined by using the standard addition method. The creatine biosensor was immersed in the 5 mM TRIS buffer (pH 7.0) containing a certain amount of commercial creatine powder solution. Then, stock standard creatine solution was added to this solution. Potential values against standard creatine concentrations were plotted and total creatine amount was determined.
Procedure for Determination of Creatine in Commercial Creatine Powder with Amperometric Creatine Biosensor
The proposed creatine biosensor was used to determine the creatine in commercial creatine powder by using the calibration curve method. The creatine enzyme electrode was placed in the cell containing 5 mL phosphate buffer (pH 7.5) and after the stabilization of background current, 0.5 mL of the creatine sample was added and the response of the electrode against creatine was determined by using the calibration curve plotted before.
To check our results obtained from proposed both potentiometric and amperometric biosensors, the Jaffé method [Citation[4]] was also used for the determination of creatine in commercial creatine powder.
RESULTS AND DISCUSSION
The principle of the potentiometric measurements is to determine ammonium ions produced after enzymatic reaction of creatinase and urease in PVC membrane surface of biosensor. First of all, creatinase enzyme catalyses the creatine hydrolysis:
A nonelectroactive intermediate product, urea, is decomposed by urease to electroactive species (ammonium ion) according to the reaction:
In amperometric creatine biosensors, creatine is converted to electroactive H2O2 by two enzymatic reactions with creatinase and sarcosine oxidase:
The idea for the construction of an amperometric biosensor stemmed from the reaction of sarcosine to give H2O2 as given above with the presence of sarcosine oxidase. It is a well-known fact that H2O2 is oxidized as follows on Pt electrode [Citation[23]].
If a sufficient anodic potential to create the above reaction is applied to the electrode, the anodic current passing from the circuit is proportional to the amount of H2O2 formed and thus to the concentration of creatine [Citation[24]].
Creatine Response of the Potentiometric Creatine Biosensor
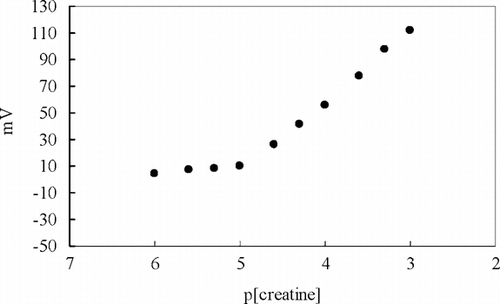
A series of creatine solutions was prepared to determine the sensitivity of creatine biosensor towards creatine and the pH values of these solutions were adjusted to pH 7.0 with trizma-base. The calibration curves were obtained and the working ranges and the slopes for the creatine biosensors were determined from these curves. The linear range of the biosensor extends from 1.0 × 10−3 to 1.0 × 10−5 M and it showed an apparent Nernstian response (49.2 ± 0.3 mV/p[creatine]) within this concentration range. The calibration curve of the creatine biosensor for creatine is shown in . Therefore we have decided that the creatine biosensor can be used for creatine determination in biological fluids and pharmaceutical preparations containing creatine.
We studied the parameters that affect the electrode response for the potentiometric creatine biosensor and performed all the measurements under optimum conditions [Citation[25]]. The optimum pH, temperature, and buffer concentration were found to be 7.0, 20°C, and 5 mM, respectively. The response time of the biosensor was about 60 s. The storage stabilization of the biosensor was also investigated and 40–45% decrease in the response was detected after 2 months. The relative standard deviation obtained from the calibration curves plotted during one working day was less than 1%.
Creatine Response of the Amperometric Biosensor
In order to determine the linear working range and sensitivity of the creatine biosensor, the current values obtained for solutions containing creatine were recorded (). The linear working range of the creatine biosensor prepared by crosslinking with GA and BSA was 2 × 10−5–3.2 × 10−4 M. The current remained constant at creatine concentrations above 3.2 × 10−4 M, which showed that CI and SO enzymes were saturated with creatine.
Figure 2 The calibration curve of the creatine biosensor for creatine (0.05 M pH 7.5 phosphate buffer).
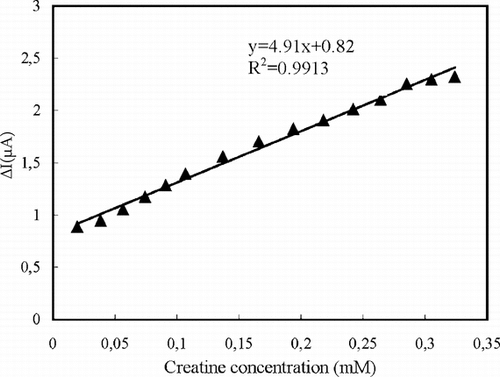
We studied the parameters that affect the electrode response for the enzyme electrode prepared by crosslinking with GA-BSA and we performed all the measurements under optimum conditions. The optimum pH, temperature, enzyme ratio and buffer concentration were found to be 7.5, 37°C, 2.5:1 (CI:SO) and 0.05 M, respectively. The response time of the biosensor was about 50 s. The relative standard deviation computed from the slopes of the calibration curves plotted during one working day was very low and the storage stabilization was about 2 weeks.
It can be concluded that the presented redox polymer, PVF+ is a suitable immobilization matrix for creatinase and sarcosine oxidase. This matrix catalyzes the oxidation of hydrogene peroxide and provides the development of sensitive amperometric creatine biosensors:
Determination of Creatine in Commercial Creatine Powder with Potentiometric and Amperometric Creatine Biosensors
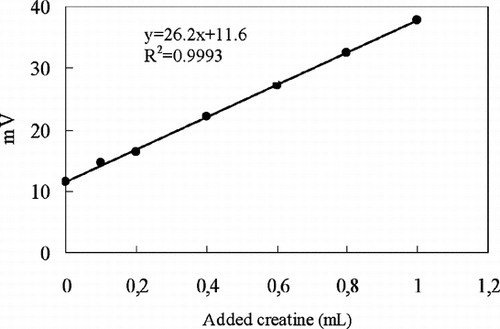
The proposed creatine biosensors were used to determine the creatine in commercial creatine powder. The amount of creatine in the commercial creatine powder was determined by using the standard addition method with potentiometric biosensor and the calibration curve method with amperometric biosensor. Creatine concentration in commercial creatine powder was calculated by using the calibration curve () amperometrically and standard addition curve () potentiometrically.
Figure 3 The calibration curve obtained by standard addition method used for determining creatine in commercial creatine powder.
For the evalution of the new creatine biosensors, the measurement results were compared to those obtained by Jaffé method. The results obtained from both our new biosensors and the standard spectrophotometric method (Jaffé) are presented in . There was a good correlation of the new potentiometric and amperometric creatine biosensor methods to the Jaffé method and there was no systemic error at 95% confidence level. As a result, it can be said that the proposed creatine biosensors allowed the determination of creatine with comparable accuracy to the Jaffé method in commercial creatine powders.
Table 1. The results of creatine content (% m/m) obtained from the proposed creatine biosensors and Jaffé method in a commercial creatine powder
In this study, the determination of creatine in commercial product was successfully performed by using the proposed amperometric and potentiometric biosensors with good repeatability and accuracy for the measurement of creatine in commercial product.
We gratefully acknowledge the financial support of T.R. Prime Ministry State Planning Organization (Project No: 98-K-120830) and a scholarship by Scientific and Technical Research Council of Turkey for P. E. Erden.
References
- Champe, P., Harvey, R. (1997). Biyokimya, Nobel Tιp Kitap Evleri, İstanbul, 264–265.
- Stefan, R.-I., Bokretsion, R.G., Van Standen, J.F., Aboul-Enein, H.Y. (2003). Talanta 60: 1223–1228, [CSA]
- Sena, S.F., Syed, D., McComb, R.B. (1988). Clin. Chem. 34: 594–595, [INFOTRIEVE], [CSA]
- Jaffé, M.Z. (1886). Hoppe-Seyler's Z. Physiol. Chem. 10: 391–400, [CSA]
- Weber, J.A., Van Zanten, A.P. (1991). Clin. Chem. 37: 695–700, [INFOTRIEVE], [CSA]
- Persky, A.M., Hochhaus, G., Brazeau, G.A. (2003). J. Chromatography B. 794: 157–165, [CROSSREF], [CSA]
- Karatzaferi, C., De Haan, A., Sargeant, A.J. (1999). J. Chromatography B. 730: 183, [CSA]
- Smith-Palmer, T. (2002). J. Chromatography B. Review 781: 93–106, [CROSSREF], [CSA]
- Yasuda, M., Sugahara, K., Zhang, J., Ageta, T., Nakayama, K., Schuin, T. (1997). Anal. Biochem. 253: 231–235, [INFOTRIEVE], [CROSSREF], [CSA]
- Schwedhelm, I., Tsikas, D., Durand, T., Gutzki, F.M., Guy, A., Rossi, J.C., Froelich, J.C. (2000). J. Chromatography B: Biomed. Sci. Appl. 744: 99–112, [CROSSREF], [CSA]
- Pezzanti, J.L., Jeng, T.W., Mcdowell, L., Oosta, G.M. (2001). Clin. Biomed. 34: 239–246, [CSA]
- Koncki, R., Walcerz, I., Ruckruh, F., Glab, S. (1996). Anal. Chim. Acta. 333: 215–222, [CROSSREF], [CSA]
- Yomato, H., Ohwa, M., Wernet, W. (1995). Anal. Chem. 67: 2776–2780, [CROSSREF], [CSA]
- Madaras, M.B., Buck, R.P. (1996). Anal. Chem. 68(21): 3832–3839, [INFOTRIEVE], [CROSSREF], [CSA]
- Shin, J.H., Choi, Y.S., Lee, H.J., Choi, S.H., Ha, J., Yoon, I.J., Nam, H., Cha, G.S. (2001). Anal. Chem. 73: 5965–5971, [INFOTRIEVE], [CROSSREF], [CSA]
- Tsuchida, T., Yoda, K. (1983). Clin. Chem. 29: 51–55, [INFOTRIEVE], [CSA]
- Karakuş, E., Pekyardιmcι, Ş., Kιlιç, E. (2005). Artificial Cells, Blood Substitutes and Biotechnology 33: 329–341, [CSA]
- Koncki, R., Kopczewska, E., Glab, S. (1994). Anal. Lett. 27(3): 475–486, [CSA]
- Gros, P., Durliat, H., Comtat, M. (2000). Electrochim. Acta. 46: 643–650, [CROSSREF], [CSA]
- Smith, W.T., Kuder, J., Wychick, E. (1976). J. Polym. Sci. 14: 2433–2448, [CSA]
- Gülce, H., Çelebi, S.S., Özyörük, H., Yιldιz, A. (1995). J. Electroanal. Chem. 397: 217–223, [CROSSREF], [CSA]
- Erden, P.E., Arslan, F., Pekyardιmcι, Ş., Kιlιç, E. (2005). Artif. Cells Blood Subst. Biotech. (in press), , [CSA]
- Umana, M., Waller, J. (1986). Anal. Chem. 58: 2979–2983, [CROSSREF], [CSA]
- Schneider, J., Gründig, B., Renneberg, R., Cammann, K., Madaras, M.B., Buck, R.P., Vorlop, K.-D. (1996). Anal. Chim. Acta. 325: 161–167, [CROSSREF], [CSA]
- Karakuş, E. (2005). Development of Bienzymatic Potentiometric Biosensor for Determination of Creatine. Ph.D. Thesis, Ankara University Graduate School of Natural and Applied Science Department of Chemistry.