Abstract
Problems of displacement, poor healing, degradation of the polymers and corrosion of the metallic frame in endovascular devices still require in–depth investigations. As the tissues and the foreign materials are in close contact, it is of paramount importance to efficiently investigate the interfaces between them. Inclusion in polymethymethacrylate (PMMA) permits us to obtain thin slides and preserve the capacity to perform the appropriate stainings.
An AneuRx prosthesis was harvested in bloc with the surrounding tissues at the autopsy of a patient 25 months post deployment in a 5.7 cm diameter AAA and sectioned in the direction of the blood flow in two halves. A cross-section of the encapsulated distal segment together with the surrounding aneuryshmal sac was embedded in polymethylmethacrylate (PMMA). Further to complete polymerization, slices of the specimen were cut on a precision banding saw under coolant. They were affixed onto methacrylate slides with a UV cured adhesive. Binding and polishing were done on a numeric grinder and slices 25 to 30 µm in thickness were stained with toluidine blue prior to observation in light microscopy. Additional slices were prepared for scanning electron microscopy and X-ray energy dispersive spectrometry for determination of the elemental composition of the Nitinol stent.
The aortic wall did not demonstrate complete integrity along with its circumference. Some areas of rupture were noted. The content of the sac was heavily shrunk and was mostly acellular. The walls of the device were very well encapsulated. The PMMA embedding permitted the polyester wall, the Nitinol wire and the collagen to keep in close contact. Scanning electron microscopy involved backscattered electrons and confirmed the corrosion the Nitinol wire at the boundary with living tissues.
Based upon the results obtained, we believe that PMMA embedding is the most appropriate method to process endovascular devices for histological and material investigation. Needless to say, that paraffin embedding would have not been feasible for such a big size specimen involving different materials.
INTRODUCTION
When implanted in humans, textiles and microporous prostheses fail to reach complete healing. The encapsulations, both internal and external, are mostly irregular and the luminal surface is usually free of endothelial cells [Citation[1], Citation[2]]. Sauvage was the only one to convincingly demonstrate the presence of endothelial cells on the flow surface of synthetic blood conduits, mainly in texturized polyester knitted grafts [Citation[3]]. The situation with endovascular devices appears to be even more challenging. The polyester tube is made of woven polyester whose construction is much tighter than the construction of knitted grafts. Consequently, these woven grafts are unlikely to heal properly with tissue incorporation of the wall and endothelialization of the luminal surface in animals [Citation[4], Citation[5]] and in humans [Citation[6]]. Healing in humans is rare [Citation[7]]. Experimental healing was reported in animals in the absence of aneurysm but this is very different from human healing patterns [Citation[8]]. The real problem is the healing of the prosthetic wall deployed in the aneurysmal sac. Should healing occur, strong tissue anchoring to woven polyester structures is therefore mandatory. Too frequently both internal and external capsules lay on both sides of the wall conduit but are not encroached together as in any woven textile tube [Citation[9]]. Most expanded PTFE grafts behave the same way. Thrombotic deposits accumulate on the flow surface and external capsules are usually very thin [Citation[10]]. Most of polyurethane grafts cannot withstand the exposure to living tissues: they degrade at different rates [Citation[11]] and the degradation of aromatic polyurethanes products includes TDI, a carcinogenic chemical [Citation[12]]. In the absence of open porosity in the wall of any synthetic blood conduit, healing is very unlikely. Following old paradigms of vascular graft technology may not be sufficient or applicable for endografts healing [Citation[13]].
On top of that, endovascular devices incorporate a metallic wire or metallic stents sewn to the synthetic tube either inside or outside [Citation[14]]. This metallic frame is preferred outside by most of the manufacturers to obtain an adequate recanalization of the blood flow within the aneurysmal sac; as no suture is employed to securely anchor the device to the host artery, sliding must be prevented by another approach. Many implantations were performed worldwide, raising a lot of question marks as endovascular surgery was still in its infancy [Citation[15-22]]. Fortunately, those were classified and endoleaks classification proved to be the way to bring everyone on the same wave length of comparison [Citation[23-25]]. In addition, endograft registries such as the Eurostar chaired by Harris proved to be instrumental in the validation process of endovascular surgery [Citation[26]]. However, up to date attention paid to the device itself remained limited: such a device can shorten or flatten [Citation[27]], the polymer structure degrade [Citation[9]], and the metallic frame corrode [Citation[28]]. Should adequate healing with solidification of the content of the aneurysmal sac, foreign wall encapsulation and luminal surface endothelialization occur, the device would probably be stabilized less with likelyhood progressive complications [Citation[29]]. It is therefore of paramount importance to pay attention to the interfaces between the different foreign materials and the living tissues, despite an improvement in the clinical results [Citation[30-32]]. Indications for endovascular surgery were also refined and they complement rather than compete with open surgery [Citation[33]].
Specific details of the histological investigations are important. Routine histology performed by paraffin embedding of specimen is not suitable for endovascular devices because it is not feasible to cut thin slides a few microns in thickness [Citation[34]]. We must take advantage of the protocols recommended, orthopedic devices and/or dental implants [Citation[35]] to investigate, and stents [Citation[36]]. Embedding in polymethylmethacrylate (PMMA) permits one to preserve the integrity of the structure during the polymerizing process. The specimen can be trimmed, slides are cut and stained. Further investigations involving SEM observation in a backscattered mode, together with microanalysis and immuno-histology, can be carried out [Citation[37], Citation[38]]. We therefore hereby propose to highlight the potential benefits of PMMA embedding to better validate the in vivo behavior of endovascular devices.
MATERIALS AND METHODS
1. The Explanted Device
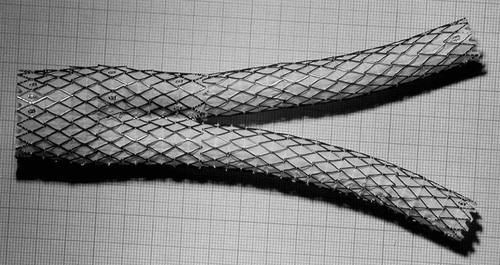
An explanted AneuRx stent-graft manufactured by Medtronic AVE, Santa Rosa, USA, was selected amongst the devices harvested at autopsy or reoperation based upon an outstanding encapsulation of the wall of the device. The AneuRx device is made of a thin wall woven polyester graft externally supported by self expanding stents made of Nitinol, a shape memory alloy [Citation[39]]. The stent-graft of the specimen investigated had a bifurcated configuration together with an ipsilateral straight graft ().
Figure 1 Deployed AneuRx stent-graft manufactured by Medtronic-AVE. The device is made by assembling a bifurcation with asymmetrical limbs with an ipsilateral limb. The thin wall of the woven polyester tubes is externally supported by self–expanding stents made of Nitinol, a shape memory alloy, and sewn by means of a polyester suture. The cross-section investigated is given by the transversal double-headed arrow.
2. Clinical Report
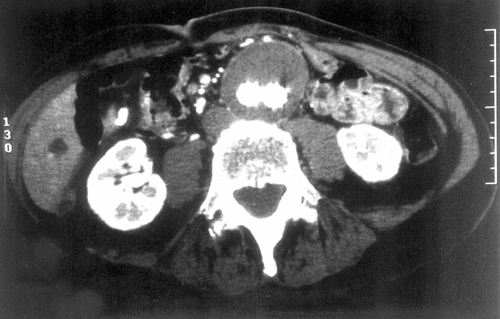
A 75 y.o. man with a symptomatic 5.7 cm AAA was treated with 24 × 14 × 16.5 AneuRx bifurcated module (early stiff device with pre-RPM material) placed through the femoral artery and a 14 × 8.5 iliac limb placed through the left femoral artery. The device was placed below the accessory left renal artery. And both iliac limbs were dilated with 14 mm balloons. Completion angiography revealed a “transgraft leak” ().
Figure 2 CT Scan of the aneurysm of the abdominal aorta fitted with the AneuRx endovascular prosthesis. The limbs of the device within the aneurysmal sac are well delineated and the content of the sac appears solidified.
The patient had complete resolution of his abdominal and back pain and no longer felt pulsation in his aneurysm immediately following the stent graft procedure. He gained weight and had no symptoms referable to his aneurysm. However, the patient was quite thin and follow-up clinical examinations revealed pulsation of his aneurysm. Twenty–five months post procedure, the patient developed back pain and azotemia. A diagnosis of multiple myeloma was made on bone marrow biopsy and the patient was begun on chemotherapy. Abdominal examination was normal. He developed bronchopneumonia and sepsis and died on 12/5/99. An autopsy confirmed the above diagnoses. The aorta was removed en bloc with the stent graft in place for explant analysis. A more detailed clinical data is reported elsewhere [Citation[40]].
3. Histology
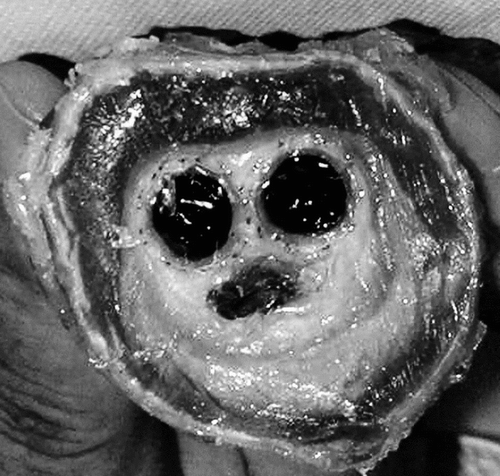
A slice of the cross-section, 1 cm thick, was cut from the as-received specimen immediately adjacent at the separation of the body with the limbs (). As the evaluation of the tissue reactions to the polyester structure (tube and suture) and to the shape memory alloy is the key issue of the investigation, it was of paramount importance to procede to embedding of the encapsulated prosthesis together with the surrounding aneurysmal sac in polymethylmethacrylate (PMMA).
Figure 3 Macroscopic cross-section of the specimen harvested at autopsy. The aortic wall is well delineated but fragmented in several locations. The aneurysmal sac is filled with a solidified substance with a void more or less filled with a gelatinous content below the two limbs of the graft that are well encapsulated.
3.1. Preparation of the Embedding Medium
Methylmethacrylate containing 200 ppm hydroquinone (a polymerization inhibitor) was purified by alkali washes and −20°C freezing according to a fast procedure previously described by Chappard [Citation[38]]. Benzoyl peroxide, a polymerization accelerator, was dissolved at a final concentration of 1%. The final accelerated medium was completed by adding dibutylphtalate to a final concentration of 10% and was stored in a −20°C environment. All reagents were of laboratory grade and purchased from Aldrich Chemical (Illkirsh, France).
3.2. Specimen Processing
The specimen, fixed in formaldehyde, was dehydrated in absolute acetone, cleared in xylene and infiltrated in the accelerated medium for 3 days at −20°C in large glass vials. The medium was changed 3 times to ensure complete infiltration (3 days each time, at −20°C). Then, the vial was transferred in a water bath at room temperature and allowed to polymerize during 3 weeks. The specimen was trimmed of excess polymer and sectioned on a precision banding saw under coolant (Exakt, Hanover, Germany). Slices of the specimen were 300 µm in thickness. They were affixed onto methacrylate slides with a UV-cured adhesive. Grinding and polishing were done on a numeric Exakt grinder system and sections (about 25–30 µm in thickness) were surface-stained with toluidine blue (0.5% in sodium borax) prior to observation in light microscopy.
An additional slice was prepared for scanning electron microscopy and X-ray energy dispersive spectrometry for determination of the elemental composition of the Nitinol stent. The slice, about 100 µm thick, was carbon coated by sputtering and observed under backscattered electrons using an emission field scanning electron microscope, Jeol 6301 F (Jeol Inc., Tokyo, Japan) coupled to an EDX detector (Link, Oxford, UK).
RESULTS
The specimen received consisted of the two halves of the explanted prosthesis that was removed en bloc from the patient: the prosthesis was clearly idientified within the aneurysmal sac whose content was almost completely solidified with thrombus. The gross observation gave the impression of an exceptional healing, with the exception of a hyalinized plug below the two limbs of the endovascular device. This might be related to the pulsation of the aneurysm observed clinically. As this explanted prosthesis together with its aneurysmal sac was sectioned transversally, lumens of both limbs were very well delineated. They were surrounded by an external fibrous encapsulation as any implanted porous textile vascular prosthesis.
The aortic wall did not demonstrate complete integrity all along its circumference. Some areas of rupture were noted. The content of the sac was heavily shrunk, leaving some void spaces as the result of the fixation of a highly hyaline substance. This filling proved to be mostly acellular with fibrinous layers of various densities, whose solidification was irregular and related to the age of the thrombotic deposition ().
Figure 4 Cross-section of the specimen investigated at low magnification; the aortic wall is poorly defined and presents an irregular and incomplete structure. The cross–sections of the limbs are well identified. The aneurysmal sac is filled with fibrinous deposits almost acellular, which shrank during the fixation process. The void at the bottom is an artifact resulting from shrinkage of the solid filling of the sac where water content was very high (A). Both limbs were surrounded with organized fibrin and collagen deposits (B). The foreign material was well encapsulated, both outside and inside. The external encapsulation was very adhesive to the Nitinol stents and the polyester wall, whereas this internal capsule, as the result of the hyaline content, also shrank.
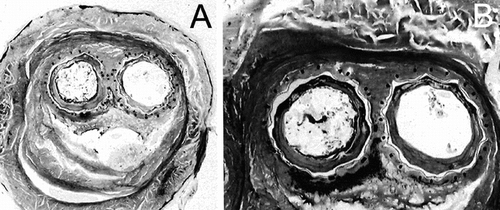
The walls of the device were very well encapsulated, both internally and externally. Internally, as the result of the high hyaline content of the fibrinous deposits left by the blood stream in absence of complete reorganization, the internal capsule was slightly detached from the polyester wall. Externally, the formation of collagen proved to keep in close contact the polyester wall, the Nitinol stents and the polyester sutures. No area of necrotic tissues and/or mineralized sections was evidenced ().
Figure 5 Illustration of the imperviousness of the polyester wall of the prostheses. The construction is very regular and the compaction of the yarns was very well preserved during the implantation. Such a structure does not permit blood percolating through the wall after a thin layer of fibrin is deposited all over the luminal surface.
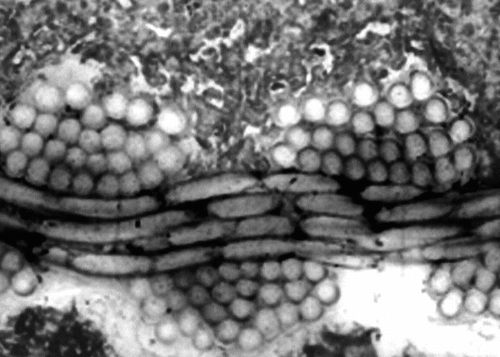
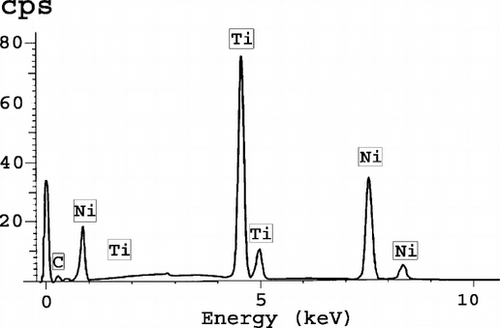
The prosthesis itself was observed in detail: the woven polyester sheath and the Nitinol stents were easily identified. Under transmitted and polarized light, the polymer sheath was made up of yarns of 26 micro-filaments between 10 and 20 µm in diameter. These yarns were found regularly interlaced in ends and picks to contribute a continuous sheath (). Cross sections of the Nitinol wires were well identified with square shapes whose angles were frequently dull. However, in some locations, the sections presented some asperities over the surface ( and ). No cellular differentiation was visible in the external capsule. Scanning electron microscopy, involving backscattered electrons, confirmed those irregularities in the Nitinol wires at the boundary with living tissues (). It was clearly the result of an undisputable ongoing corrosion process with some metallic particles detached from the stent. The real corrosion state was probably overemphasized as the result of the preparation procedure. The EDX study confirmed the presence of nickel and titanium in a 50/50 atomic percentage proportion, which is the composition of the Nitinol currently selected in implantology ().
Figure 6 Illustration of the regularity of the polyester wall. Such a structure is very compact and unlikely to be penetrated by cells and fibrin. The weakness is the lack of capacity to securely anchor any encapsulation of fibrous tissues.
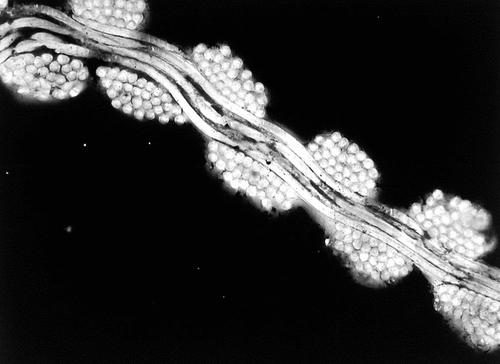
Figure 7 Cross-section at the contact between the ipsilateral limb and the bifurcation sutures are well identified. However, the surfaces of the Nitinol stents present some irregularities.

Figure 8 Absence of exacerbated inflammatory reaction caused by the Nitinol wire. Note that the shape of the wire is ill-defined: it is probably related to an early stage of corrosion.
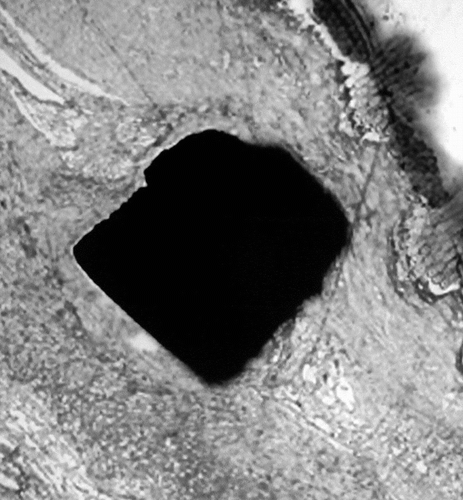
Figure 9 Backscattered observation of the Nitinol wires demonstrating an early stage of corrosion with fragmentation and particle dispersion. The surface of the Nitinol stents is eroded, the material in contact with the tissues appears fragile and some fragments can be dispersed in the surrounding tissues.
DISCUSSION
Recipient's organism reacts toward the endovascular prothesis deployed to restore a normal blood conduit though an aneurismal sac by a complex tissue response. The first purpose from a surgical point of view is to restore the normal blood flow and to prevent the aneurismal arterial wall from fatal rupture. As the healing process is going on, the prosthesis becomes isolated by the formation of surrounding capsules, both internally and externally. As a result, the implanted prosthesis is remodelled and is transformed from a mechanical body alien for the patient to a composite device incorporating various tissue components. The luminal surface ensures conditions for a normal blood flow; ideally the flow surface would be endothelialized. Such a healed internal capsule is only obtained with great difficulty in conventional vascular grafts. The challenge is even more important in endovascular prostheses because they incorporate a second component of metallic nature.
The entire length of the endovascular device is in contact with the host artery at the sites of adhesion to a relatively normal aortic wall and goes through the aneurismal sac, whose content is made of thrombi more or less solidified. The goals to be attained can be defined as follows: stable attachment of the prosthesis to the arterial wall above and below the aneurysm with a minimal risk of arterial wall remodelling, safe contact between the endovascular graft and the aneurismal sac with unperviousness of the prosthetic wall, and solidification of the thrombotic content of the aneurysmal sac to prevent fatal rupture. A perfect healing of the blood conduit, i.e. textile and metal frame, would ensure a good biocompatibility, biofunctionality and biodurability to guarantee the long-term fullfilment of the therapeutic purpose.
Therefore the histological examination of any explanted endovascular device is of utmost importance. The absence of artefacts in the analysis of explanted devices is a highly challenging undertaking as most of the specimens are not harvested for the purpose of in-depth pathological investigation [Citation[2]]. The key issue is to preserve the intimate contact between foreign materials and tissues in thin slides to be able to carefully observe the interface [Citation[41-43]]. As the contact between a foreign surface and living tissues is still poorly understood, the interface between them must be kept intact for detailed investigations. Paraffin sections frequently result in filament fraying of the textile yarns; elimination of the metallic wire is mandatory. SEM observations involving secondary electron emission are of great interest to study the flow surface [Citation[44]]. As section PMMA embedding enables the preservation of the encroachment between foreign materials and tissues, it sounds like the method of choice to permit the best assessment of the interactions between the device and the blood.
The technique reflects the method that proved to be very efficient in the pathology of explanted devices from dentistry and from orthopedics. We, however, must recognize that some artefacts are associated with this protocol in cardiovascular devices because of the highly hyaline content of the materials deposited at the contact of the blood flow with the foreign surface. There is some separation of the internal capsule, which is probably related to the initial fixation. The formaline solution acts as a cross-linking chemical and causes some shrinkage of the tissues as the fixation process is in progress [Citation[45]]. With higher hyaline content, more significant shrinkage is demonstrated into specimen. The voids that are thus created can possibly reduce the anticipated benefits of PMMA embedding.
Staining and immunochemistry are possible with the thin and the semi-thin slides, which is the way to go to better understand the behavior of multiple biomaterials in contact with living tissues. On top of that, SEM observations in a backscattered mode can be performed. It is therefore feasible to also perform X-ray analysis of the metallic stents or wires. Corrosion of any metal or alloy is a key issue and must be addressed very cautiously [Citation[28]]. Unfortunately, this is only part of the problem, as mechanical failures were and still are too frequently associated with endovascular devices [Citation[46]].
Despite weaknesses that can bring some pessimistic views [Citation[47]], endovascular surgery represents a step forward that can benefit many patients. Endovascular therapy represents an additional approach to address the treatment of aneurysms [Citation[48]]. Careful patient follow-up is mandatory [Citation[49]]. In case of complications, open surgery is still available, with, however, a higher mortality rate [Citation[50]]. New vision of patients' treatments involve undisputably minimally invasive surgery [Citation[51]]. Follow-up of the devices to validate their 3Bs, i.e. biocompatibility, biofunctionality and bioresilience, will necessitate not only the current techniques but also the development of virtual biopsies [Citation[40]].
CONCLUSION
PMMA embedding of the explanted devices used in cardiovascular surgery is the way to go to investigate the healing characteristics and the material integrity. Further investigations are required to solve the problem of tissue shrinkage but its superiority over paraffin embedding is not disputable, in terms of quality of the results and in terms of possibility of additional investigations.
Special thanks are due to Suzanne Bonnet and Michel Letort at Medtronic AVE, Santa Rosa, Ca, USA, for taking care of the retrieval program.
REFERENCES
- Berger, K., Sauvage, L.R., Rao, A.M., Wood, S.J. (1972). Healing of arterial prostheses in man: Its incompleteness. Ann. Surg. 175: 118–127, [INFOTRIEVE], [CSA]
- Guidoin, R., King, M., Blais, P., Marois, M., Gosselin, C., Roy, P., Courbier, R., David, M., Noël, H.P. (1981). A biological and structural evaluation of retrieved Dacron® arterial prostheses, in Implant Retrieval: Material and Biological Analysis, A. Weinstein, D. Gibbons, S. Brown, W. Ruff, Eds., US Dept. of Commerce, NBS Special Publication: 601, pp. 29–129.
- Sauvage, L.R., Berger, K., Beilin, L.B., Smith, J.C., Wood, S.J., Mansfield, P.B. (1975). Presence of endothelium in an axillary-femoral graft of knitted Dacron with an external velour surface. Ann. Surg. 182: 749–753, [INFOTRIEVE], [CSA]
- Krievins, D., Bey, Gin, R., Fogarty, T.J., Zarins, C.K. (1998). Healing characteristics of endovascular stent-grafts repair of experimental aortic aneurysm. Proc. Latvian Acad. Sci. 52(section B): 164–168, [CSA]
- Formichi, M., Marois, Y., Roby, P., Marinov, G., Stroman, P., King, M.W., Douville, Y., Guidoin, R. (2000). Endovascular repair of thoracic aortic aneurysm in dogs: Evaluation of a Nitinol-polyester self-expanding stent-graft. J. Endovasc. Ther. 7: 47–67, [INFOTRIEVE], [CROSSREF], [CSA]
- Malina, M., Brunkwall, J., Ivancev, K., Jönsson, J., Malina, J., Lindblad, B. (2000). Endovascular healing is inadequate for fixation of Dacron stent-grafts in human aortoiliac vessels. Eur. J. Vasc. Endovasc. Surg. 19: 5–11, [INFOTRIEVE], [CROSSREF], [CSA]
- Shin, C.K., Rodino, W., Kirwin, J.D., Ramirez, J.A., Wissenlink, W., Papierman, G., Panetta, T.F. (1999). Histology and electron microscopy of explanted bifurcated endovascular aortic grafts: Evidence of early incorporation and healing. J. Endovasc. Surg. 6: 246–250, [INFOTRIEVE], [CROSSREF], [CSA]
- Lambert, A.W., Budd, J.S., Fox, A.D., Potter, U., Rooney, N., Horrocks, W. (1999). The incorporation of a stent-graft into the porcine aorta and the inflammatory response to the endoprosthesis. Cardiovasc. Surg. 7: 710–714, [INFOTRIEVE], [CROSSREF], [CSA]
- Guidoin, R., Marois, Y., Douville, Y., King, M.W., Castonguay, M., Traoré, A., Formichi, M., Staxrud, L.E., Norgren, L., Bergeron, P., Becquemin, J.P., Egana, J.M., Harris, P.L. (2000). First generation aortic endografts: Analysis of explanted Stentor devices from the Eurostar registry. J. Endovasc. Ther. 7: 105–122, [INFOTRIEVE], [CROSSREF], [CSA]
- Formichi, M., Guidoin, R., Jausseran, J.M., Awad, J.A., Johnston, K.W., King, M.W., Courbier, R., Marois, M., Rouleau, C., Batt, M., Girard, J.F., Gosselin, C. (1988). Expanded PTFE prostheses as arterial substitutes in humans: Late pathological findings in 73 excised grafts. Ann. Vasc. Surg. 2: 14–27, [INFOTRIEVE], [CSA]
- Bull, P.G., Denck, H., Guidoin, R., Gruber, W. (1992). Preliminary clinical experience with polyurethane vascular prostheses in femoro-popliteal reconstruction. Eur. J. Vasc. Surg. 6: 217–224, [INFOTRIEVE], [CROSSREF], [CSA]
- Guidoin, R., Therrien, M., Rolland, C., King, M., Grandmaison, J.L., Kaliaguine, S., Blais, P., Pakdel, M., Roy, C. (1992). The polyurethane foam covering the Même breast prosthesis: A biomedical breakthrough or a biomaterial tar baby? Ann. Plast. Surg. 28: 342–353, [PUBMED], [INFOTRIEVE], [CSA]
- Deng, X., Guidoin, R. (2000). Alternative blood conduits: Assessment of whether the porosity of synthetic prostheses is the key to long-term biofunctionality. Med. Biol. Eng. Comput. 38: 219–225, [INFOTRIEVE], [CROSSREF], [CSA]
- AFSSAPS. (2001). Evaluation des endoprothèses aortiques utilisées pour le traitement endovasculaire des anévrismes de l'aorte abdominale sous-rénale. Paris, France, Février.
- Parodi, J.C., Palmaz, J.C., Barone, H.D. (1991). Transfemoral intraluminal graft implantation for abdominal aortic aneurysms. Ann. Vasc. Surg. 5: 491–499, [INFOTRIEVE], [CROSSREF], [CSA]
- May, J., White, G.H., Yu, W., Waugh, R.C., Stephen, M.S., McGahan, T.J., Harris, J.P. (1995). Early experience with the Sydney and EVT prostheses for endoluminal treatment of abdominal aortic aneurysms. J. Endovasc. Surg. 2: 240–247, [INFOTRIEVE], [CROSSREF], [CSA]
- Wilson, G.J., Klement, P., Kato, Y.P., Martin, J.B., Kahn, I.J., Alcine, R., Dereume, J.P., McGregor, D.C., Pinchuk, L. (1996). A self-expanding bifurcated endovascular graft for abdominal aortic aneurysm repair. An initial study in the canine model. ASAIO J. 42: M386–M393, [INFOTRIEVE], [CSA]
- White, R., Kopchok, G., Zalewski, M., Ayres, B., Wilson, W.E., De Virgilio, C., Donayre, C. (1996). Comparison of the deployment and healing of thin walled expanded PTFE stented grafts and covered stents. Ann. Vasc. Surg. 10: 336–346, [INFOTRIEVE], [CROSSREF], [CSA]
- Mialhe C., Amicabile C., Becquemin, J.P. (1997). Endovascular treatment of infra-renal abdominal aneurysms by the Stentor system: Preliminary results of 79 cases. Stentor retrospective study group. J. Vasc. Surg. 26: 199–209, [INFOTRIEVE], [CROSSREF], [CSA]
- Krohg-Sorensen, K., Brekke, M., Drolsum, A., Kvernebo, K. (1999). Periprosthetic leak and rupture after endovascular repair of abdominal aortic aneurysm: The significance of device design for long-term results. J. Vasc. Surg. 29: 1152–1158, [CROSSREF], [CSA]
- Koskas, F., Cluzel, P., Benhamou, A.C., Kieffer, E. (1999). Edovascular treatment of aortoiliac aneurysms: Made to measure stent-grafts increase feasability. Ann. Vasc. Surg. 13: 239–246, [INFOTRIEVE], [CROSSREF], [CSA]
- Zarins, C.K., White, R.A., Moll, F.L., Crabtree, T., Bloch, D.A., Hodgson, K.J., Fillinger, M.F., Fogarty, T.H. (2001). The AneuRx stent-graft: Four year experience and worldwide experience 2000. J. Vasc. Surg. 33 (Suppl. 2): S135–S145, [INFOTRIEVE], [CROSSREF], [CSA]
- White, G.H., May, J., Waugh, R.C., Yu, W. (1998a). Type I and II endoleaks: A more useful classification for reporting results of endoluminal AAA repair. J. Endovasc. Surg. 5: 189–191, [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- White, G.H., May, J., Waugh, R.C., Chaufour, X., Yu, W. (1998b). Type III and type IV endoleak: Toward a complete definition of blood flow in the sac after endoluminal AAA repair. J. Endovac. Surg. 5: 305–309, [CROSSREF], [CSA]
- Chaikof, E.L., Blankensteijn, J.D., Harris, P.L., White, G.H., Zarins, C.K., Bernhard, V.M., Matsumura, J.S., May, J., Veith, F.J., Fillinger, M.F., Rutherford, R.B., Kent, K.C. (2002). Ad Hoc committee for standardized reporting practices in vascular surgery of the society for vascular surgery/American association for vascular surgery. Reporting standards for endovascular aortic aneurysm repair. J. Vasc. Surg. 35: 1048–1061, [INFOTRIEVE], [CROSSREF], [CSA]
- Buth, H., Van Marrewijk, C.J., Harris, P.L., Hop, W.C., Riambau, V., Laheij, R.J., Eurostar collaborators. (2002). Outcome of endovascular abdominal aortic aneurysm repair in patients with conditions considered unfit for an open procedure: A report on the Eurostar experience. J. Vasc. Surg. 35: 211–221, [INFOTRIEVE], [CROSSREF], [CSA]
- Wolf, Y.G., Hill, B.B., Lee, W.A., Corcoran, C.M., Fogarty, T.J., Zarins, C.K. (2001). Eccentric stent-graft compression: An indicator of insecure proximal fixation of aortic stent graft. J. Vasc. Surg. 33: 481–487, [INFOTRIEVE], [CROSSREF], [CSA]
- Heintz, C., Riepe, G., Birken, L., Kaiser, E., Chakfe, N., Morlock, M., Delling, G., Imig, H. (2001). Corroded nitinol wires in explanted aortic endografts: An important mechanism of failure? J. Endovasc. Ther. 8: 248–253, [INFOTRIEVE], [CROSSREF], [CSA]
- May, J., White, G.H., Waugh, R., Ly, C.N., Stephen, M.S., Jones, M.A., Harris, J.P. (2001). Improved survival after endoluminal repair with second-generation prostheses compared with open repair in the treatment of abdominal aortic aneurysms: A 5-year concurrent comparison using life table method. J. Vasc. Surg. 33 (Suppl. 2): S21–S26, [INFOTRIEVE], [CROSSREF], [CSA]
- Zarins, C.K. (2000). The US AneuRx clinical trial: 6-year clinical update 2002. J. Vasc. Surg. 37: 904–908, [CSA]
- Zarins, C.K., Arko, F.R., Crabtree, T., Bloch, D.A., Duriel, K., Allen, R.C., White, R.A. (2004). Explant analysis of stent grafts: Relationship between structural finding and clinical outcome. J. Vasc. Surg. 40: 1–11, [INFOTRIEVE], [CROSSREF], [CSA]
- Chakfé, N., Dieval, F., Riepe, G., Mathieu, D., Zbali, I., Thaveau, F., Heintz, C., Kretz, J.G., Durand, B. (2004). Influence of the textile structure on the degradation of explanted aortic endoprotheses. Eur. J. Vasc. Endovasc. Surg. 27: 33–41, [CROSSREF], [CSA]
- Arko, F.R., Lee, W.A., Hill, B.B., Olcott, C., IV, Dalman, R.L., Harris, E.J., Jr., Cipriano, P., Fogarty, T.J., Zarins, C.K. (2002). Aneurysm related death: Primary endpoint analysis for comparison of open and endovascular repair. J. Vasc. Surg. 36: 297–304, [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Buijs, R., Dogterous, A.A. (1983). An improved method for embedding hard tissue in polymethylmethacrylate. Stain. Techn. 58: 135–141, [INFOTRIEVE], [CSA]
- Sanderson, C.A., Mantas, J.R. (1990). Histologic techniques for analysis of bone implants (a historic and artistic perspective). Histo-Logic 20: 169–174, [CSA]
- Malik, N., Gunn, J., Holt, C.M., Shepherd, L., Francis, S.E., Newman, C.M., Crossman, D.C., Cumberland, D.C. (1998). Intravascular stents: A new technique for tissue processing for histology, immunohistochemistry and transmission electron microscopy. Heart 80: 509–516, [INFOTRIEVE], [CSA]
- Berger-Gorbet, M., Broxup, B., Rivard, C., Yahia, L.H. (1996). Biocompatibility testing of NiTi screws using immunohistochemistry on sections containing metallic implants. J. Biomed. Mater. Res. 32: 243–248, [INFOTRIEVE], [CROSSREF], [CSA]
- Chappard, D., Gaborit-Retailleau, N., Montheard, J.P., Baslé, F. (1999). Photopolymerized 2. Hydroxyethylmethecrylate as a mounting medium preserving immunocytochemical reaction and nuclear counterstain. Biotech. Histochem. 74: 135–140, [INFOTRIEVE], [CSA]
- Allen, R.C., White, R.A., Zarins, C.K., Fogarty, T.J. (1997). What are the characteristics of the ideal endovascular graft for abdominal aortic aneurysm exclusion? J. Endovasc. Surg. 4: 195–202, [INFOTRIEVE], [CROSSREF], [CSA]
- Guidoin, R., Bonny, J.M., Renou, J.R., Baslé, M.F., Zarins, C.K., Legrand, A.P., Zhang, Z., Guzman, R., Douville, Y. (2006). MRI virtual biopsies: Analysis of an explanted endovascular device and perspectives for the future. Art. Cells, Blood Subst. Biotech 34: 241–261, [CSA]
- Chappard, D., Alexandre, M., Camps, M., Montheard, J.P., Riffat, G. (1983). Embedding iliac bone biopsies at low temperature using glycol and methyl methacrylates. Stain. Tech. 58: 299–308, [CSA]
- Lundeen, G.A., Shea, K.G., Sanderson, C., Bachus, K.N., Bloebaum, R.D. (1998). Technique for identification of submicron metal particulate from implants in histological specimens. J. Biomed. Mater. Res. 43: 168–174, [INFOTRIEVE], [CROSSREF], [CSA]
- Aparicio, S., Doty, S.B., Camacho, N.P., Paschalis, E.P., Spevak, L., Mendelsohn, R., Boskey, A.L. (2002). Optimal methods for processing mineralized tissues for Fourier transform infrared microspectroscopy. Calc. Tiss. Int. 70: 422–429, [CROSSREF], [CSA]
- Slizová, D., Krs, O., Pospisvilová, B. (2003). Alternative method of rapid drying vascular specimens for scanning electron microscopy. J. Endovasc. Thes. 10: 285–287, [CROSSREF], [CSA]
- Palmaz J.C., Windeler S.A., Garcia F., Tio F.O., Sibbitt R.R., Reuter S.R. (1986). Atherosclerotic rabbit aortas: Expandable intraluminal grafting. Radiology. 160: 723–726, [INFOTRIEVE], [CSA]
- Jacobs, T.S., Won, J., Gravereaux, E.C., Faries, P.L., Morrissey, N., Teodorescu, V.J., Hollier, L.H., Marin, M.L. (2003). Mechanical failure of prosthetic human implants: A 10 year experience with aortic stent graft devices. J. Vasc. Surg. 37: 16–26, [INFOTRIEVE], [CROSSREF], [CSA]
- Riepe, G., Heintz, C., Kaiser, E., Chakfé, N., Morlock, M., Delling, M., Iming, H. (2002). What can we learn from explanted endovascular devices? Eur. J. Vasc. Endovasc. Surg. 24: 117–122, [INFOTRIEVE], [CROSSREF], [CSA]
- Teufelsbauer, H., Prusa, A.M., Wolff, K., Polterauer, P., Nanobashuili, J., Prager, M., Holzenbein, T., Thurnher, S., Lammer, J., Schemper, M., Kretschmer, G., Huk, I. (2002). Endovascular stent grafting versus open surgical operation in patients with infrarenal aortic aneurysms: A propensity score-adjusted analysis. Circulation 106: 782–787, [INFOTRIEVE], [CROSSREF], [CSA]
- Rozenblit, A.M., Patlas, W., Rosenbaum, A.T., Okhi, T., Veith, F.J., Laks, M.P., Ricci, Z.J. (2003). Detection of endoleaks after endovascular repair of abdominal aortic aneurysms: Value of unenhanced and delayed helical CT acquisitions. Radiology 227: 426–433, [CSA]
- Bockler, D., Probst, T., Weber, H., Raithel, D. (2002). Surgical conversion after endovascular grafting for abdominal aortic aneurysms. J. Endovasc. Ther. 9: 111–118, [INFOTRIEVE], [CROSSREF], [CSA]
- Sala, F., Hassen-Khodja, R., Declemy, S., Bouillanne, P.J., Haudebourg, P., Batt, M. (2003). Laparascopic aortoiliac surgery for occlusive disease and/or aneurysm. Ann Chir. 128: 4–10, [INFOTRIEVE], [CROSSREF], [CSA]