Abstract
In order to prepare a biosensor for the determination of uric acid, electropolymerization of pyrrole on Pt surface was carried out with an electrochemical cell containing pyrrole, ferrocene (as a electron mediator) and tetrabutylammonium tetrafluoroborat in acetonitrile by cyclic voltammetry between 0.0 and 1.0 V (vs. Ag/AgCl) at a scan rate of 50 mV/s upon Pt electrode. Uricase was immobilized by a glutaraldehyde/gelatine croslinking procedure on to polypyrrole film after the electropolymerization processes. The response of the biosensor against uric acid was measured after 330 seconds following the application of a constant potential of +0.7 V (vs. Ag/AgCl). The resulting biosensor exhibits excellent electrocatalysis for the uric acid. The amperometric determination is based on the electrochemical detection of H2O2, which is generated in enzymatic reaction of uric acid. The sensor responds to uric acid with a detection limit of 5.0 × 10−7 M. The sensor remains relatively stable for 5 weeks. Interference effect were investigated on the amperometric response of the biosensor. Determination of uric acid was carried out in the biological fluids by biosensor.
INTRODUCTION
Uric acid is an end product from purine derivates in human metabolism. The assay of uric acid in body fluids (e.g., serum and urine) is a clinically valuable diagnostic indicator [Citation[1]].
The presence of elevated uric acid levels is a sign of gout, hyperuricemia, or Lesch-Nyhan syndrome [Citation[2]]. Similary, elevated uric acid levels are related to other conditions including increased alcohol consumption, obesity, diabetes, high cholesterol, kidney disease, and heart diseases. Many epidemiological studies have suggested that serum uric acid is also a risk factor for cardiovascular disease [Citation[3]].
Uricase, which is denoted as Uox, catalyzer in vivo oxidation of uric acid in the presence of oxygen as an oxidizing agent, producing allantoin and CO2 as oxidation products of uric acid and hydrogen peroxide as a reduction of O2.
The amperometric detection of uric acid can be made by electrochemical oxidizing the produced H2O2.
However, there is a serious problem to be solved in such sensors that uric acid itself is oxidized at conventional electrodes such as Pt, Au and carbon at the potentials positive enough to oxidize H2O2 [Citation[4]]. Use of highly sensitive assays would overcome this problem just by simple dilution of the sample [Citation[5]]. Also, it has been suggested that one function of uric acid in human body fluids is to act as an antioxidant [Citation[6]].
The thought for the construction of a biosensor stemmed from the reaction of uric acid to give hydrogen peroxide as given above with the presense of uricase [Citation[7]].
In recent years, mediators and conducting polymers have been used as a matrix for immobilizing the enzymes [Citation[8], Citation[9]]. The mediators are also known to have an important role in biosensors [Citation[10]]. Although the working principles of the biosensor constructed by the immobilization of uricase enzyme in a polypyrrolle film containing ferrocene are based upon the same reactions, there is no study found in literature where ferrocene was used as a mediator for uric acid biosensors.
Therefore, the purpose of this study was to develop a biosensor incorporating immobilized uricase and ferrocene for the determination of uric acid. The optimum working conditions with respect to the substrate concentrations, the pH and temperature were investigated.
MATERIAL AND METHODS
Equipment and Reagents
The electrochemical studies were carried out using Epsilon EC electrochemical analyzer using a three-electrode cell. The working electrode was a Pt plate (0.5 cm2). The auxiliary and the reference electrodes were a Pt wire and Ag/AgCl, respectively. The pH values of the buffer solutions are measured with ORION Model 720 A pH/ionmeter. Temperature control was achieved with Grant W14 thermostat.
Uricase (EC 1.7.3.3, purified from the microorganism and with an activity of 10 unit.ml−1) and uric acid were purchased from Sigma. Pyrrole and ferrocene was supplied from Fluka, tetrabutylammonium tetraflouroborate from Aldrich, and acetonitrile from Merck. All other chemicals were obtained from Sigma. All the solutions were prepared by the use of distilled water.
Preparation of Uric Acid Biosensor
The surface of the Pt plate electrode cleaned according to [Citation[11]] was covered with polypyrrole by the electropolymerization of pyrrole [Citation[9], Citation[12], Citation[9]]. The electrode was immersed in a 5 mL solution of 0.2 M pyrrole, 0.1 M tetrabutylammonium tetraflouroborate, 10 mM ferrocene in acetonitrile [Citation[13]]. The solution was purged with nitrogen in order to remove the oxygen. The electropolymerization of pyrrole upon the electrode surface was achieved by the cyclic voltammetric scans between 0.0 and +1.0 V at a scan rate of 50 mV/s. The electrode was washed with acetonitrile and then buffer solution after the coating process.
50 µL uricase enzyme (10 unit/mL) was dropped upon each other surface of platinium/polypyrrole-ferrocenium (Pt/PPy-Fc) electrode. The film was taken out of the enzyme solution, was completely wiped off from the film and was dried under ambient conditions. Then, 100 µL/cm2 of 5 mg/ml gelatin solution (prepared in water and incubated 30 min at 37°C before use) was uniformly spread over the enzyme adsorbed Pt/PPy-Fc electrode dried at room temperature. This electrode was then dipped into a glutaraldehyde solution (1.25% in water) for 30 s for crosslinking and washed immediately with plenty of borate buffer and dried at room temperature for several hours. The electrode was kept in refrigerator at the in borate buffer when it was not in use [Citation[14]].
Amperometric Measurements
In order to determine whether the enzyme electrode was sensitive to uric acid, an aqueous buffer solution containing 0.05 M borate at a pH of 8.0 was added to the cell. The solution, a steady-state background current, was allowed to decay at a constant value at +0.7 V. Then the aliquots uric acid stock solution was added to the cell using a micropipette, and stirred for 10 min. The response of the biosensor against uric acid was measured after 400 s following the application of a constant potential of +0.7 V (vs. Ag/AgCl). The current values against uric acid concentration were plotted in order to determine the working range of the electrode.
RESULTS AND DISCUSSION
In this study a new uric acid sensitive amperometric enzyme electrode was prepared by the use of ferrocene as mediator. The parameters effecting to the performance of the biosensor were investigated.
Response Time
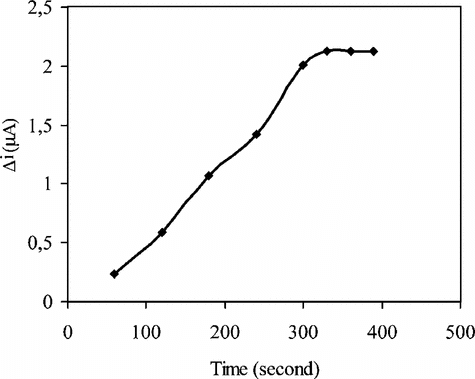
The amperometric response time of the enzyme electrode to uric acid was determined at constant uric acid concentration (5.0 × 10−5 M). The current for 5.0 × 10−5 M uric acid against time was plotted and given in . The response time can be taken as 330 seconds where the currents are approximately constant. This time is highly suitable for biosensor response [Citation[15]].
The Effect of pH
The effect of solution pH on the uric acid response at the proposed biosensor is shown in . The amperometric response is enhanced by increasing the solution pH in the range of pH 6.5 to 8.0 at a constant uric acid concentration of 5.0 × 10−5 M. The graph shows that the best pH for uricase is 8.0. This value is similar to those of solution phase enzyme [Citation[7], Citation[16], Citation[17]]. In uric acid determination with biosensors, pH values other than 8.0 were employed in literature (pH 7.5, 8.5, and 9.0). This was attributed to the fact that the used polymer and the type of immobilization were different [Citation[18]].
Figure 2 The effect of solution pH on the response to 5.0 × 10−5 M uric acid in 0.05 M borate buffer solution at 25°C. The operating potential is +0.7 V vs. Ag/AgCl.
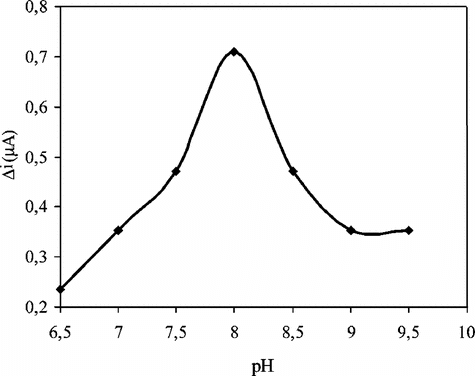
Therefore, pH 8.0 is selected by considering both the sensitivity and the stability of the biosensor and consequently further studies were performed at pH 8.0.
The Effect of Temperature
The amperometric response of the biosensor prepared was investigated at different temperatures using constant uric acid concentration of 5.0 × 10−5 M ().
Figure 3 The effect of temperature upon the sensitivity of biosensor against uric acid (0.05 M pH 8.0 borate buffer).
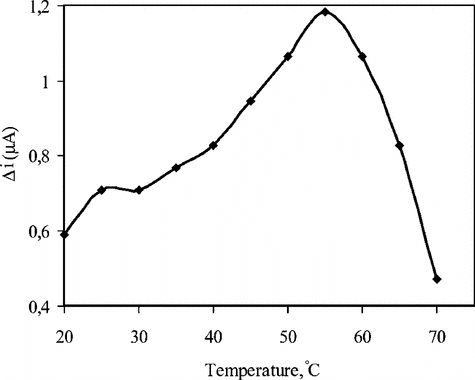
As seen from the figure, the current difference increases with temperature up to 55°C and decreases afterwards. The highest current difference was obtained at 55°C. The enzyme is thought to be denaturated after this temperature. The study was carried out at 25°C due to the difficulties involved in working at 55°C. Similar situations were encountered with other enzyme electrodes in literature [Citation[19]]. Using the data obtained between 20–55°C, 1/T − lnΔi graph was plotted and the slope of the curve gave the activation energy of the uricase catalyzed reaction of uric acid into allantoin as 22.9 kJ/mol. In addition, activation energy of the free uricase catalyzed reaction of uric acid into allantoin calculated as 11.42 kJ/mol.
Substrate Concentration and Calibration Curves
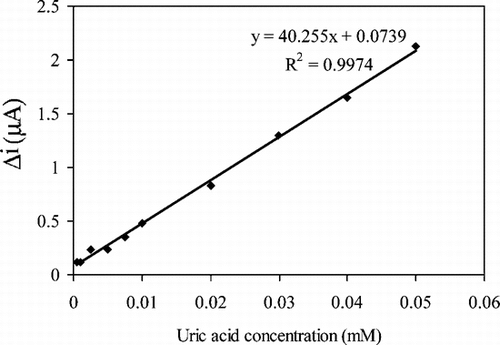
In order to determine the linear working range of the uric acid biosensor prepared, the current values obtained for solutions containing 5.0 × 10−7 − 1.0 × 10−3 M uric acid were recorded ().
Figure 4 The effect of uric acid concentration upon the amperometric response of the biosensor (0.05 M pH 8.0 borate buffer, 25°C).
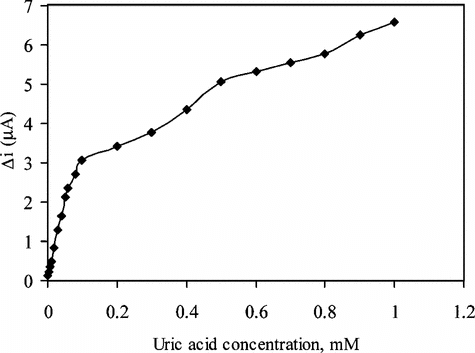
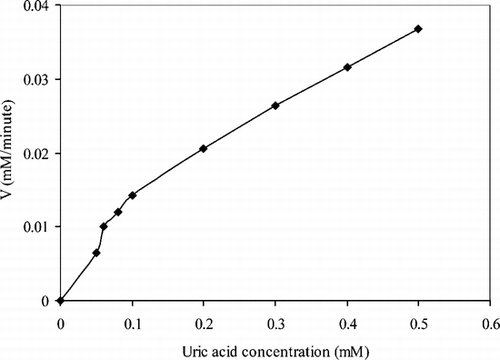
There are linear parts ranging between 1.0 × 10−6 − 5.0 × 10−5 (R2 = 0.9973). The graph for the calibration curve is given in . It has been shown that the linearity of this graph is highly satisfactory and it can be used for the quantitative determination of uric acid. The low detection limit of the biosensor prepared was found to be 5.0 × 10−7 M. The KM constant, a specific parameter of enzymes, was computed by the use of 1/[uric acid]) −1/V graph (Lineweaver–Burke plot). KM and Vmax values were found as 0.44 mM and 7.1 × 10−2 mM/min, respectively. The KM value, which shows the affinity of the biosensor, was 0.44 mM and the values cited in literature were 0.088 mM, 0.170 mM and 0.90 mM, 3.80 mM) [Citation[17], Citation[20], Citation[21]]. Since the enzymatic reactions take place through a carrier medium, the affinity of the enzyme towards the substrate is not the same in every carrier media. Therefore it is normal that KM values differ. Using , uric acid (mM)-V (mM/minute) graph was plotted ().
The Reproducibility of the Enzyme Electrode
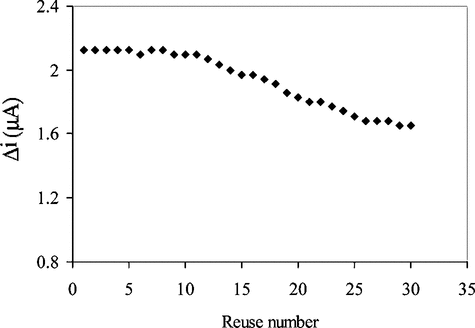
In order to test the reproducibility of the enzyme electrode prepared, the current changes obtained after subsequent usage were plotted against the number of measurements. The amperometric current obtained after 30 measurements at a constant uric acid concentration of 5.0 × 10−5 M was found to be 77.7% of initial activity (). This shows that the reproducibility of the uric acid electrode was highly satisfactory.
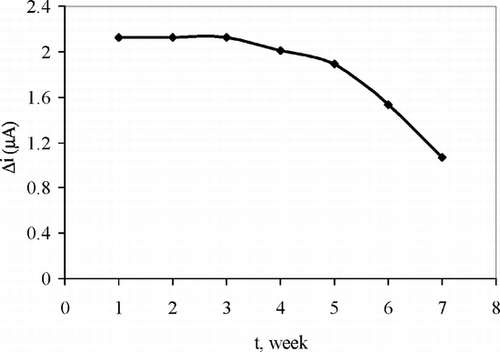
The Storage Stabilization of the Enzyme Electrode
The response of the enzyme electrode prepared under optimum conditions was measured for a period of 7 weeks at constant uric acid concentration (5.0 × 10−5 M). The result of 7 measurements during this period is plotted in .
There was no noticeable decrease in the response during the first five weeks. There was a rapid decrease in current values between 5th and 7th weeks. The electrode showed 49% of the initial amperometric response at the end of the 7th week. This indicates that the biosensor prepared can be used for quite a long time.
The properties and the optimum working conditions of the biosensor prepared are summarized in .
Table 1. The properties and the optimum working conditions of the biosensor
Interference Effect
A few common substances found in serum or urine were studied for any interfering effect on the analysis of uric acid. Known concentration of uric acid, ascorbic acid, glucose, bilirubin, paracetamol, (acetoaminophen), creatinine, urea, glutamic acid, aspartic acid, methionine and adenine were added to 5.0 × 10−5 M ürate solutions and are shown in . It has been shown that glucose, urea, bilirubin, creatinine, glutamic acid, adenine and aspartic acid have been no interfering effects on the analysis of uric acid. But the interfering effect of ascorbic acid, uric acid, paracetamol and methionine have been shown on the analysis of uric acid. Interfering effects of these substances and how to remove this interfering effect were investigated.
Table 2. Interfering substances (constant uric acid concentration of 5.0 × 10−5 M) on the amperometric response of the biosensor
Effect of Ascorbic Acid
It is well known that ascorbic acid co-exists with uric acid in many samples; therefore its interference was investigated in more detail. Ascorbic acid is the major interferent in the voltametric determination of uric acid and is oxidized almost at the same potential as uric acid at the base electrodes. It is known that in clinical samples uric acid is not the only component that can interact with the sensor electrodes.
The influence of ascorbic acid on uric acid amperometric signals was examined. Five different concentrations of ascorbic acid were added to cell media in the presence of 5.0 × 10−5 M uric acid. An amperometric response current measured + 0.7 V constant potential. Response currents of ascorbic acid have calculated. Ascorbic acid concentration of 5.0 × 10−5 M and at the lower than interfering effect of ascorbic acid haven't been shown.
It is mentioned that to remove the interfering effect of ascorbic acid dilution of samples, treatment with the cucumber, treatment with ascorbat oxidase in the publication [Citation[5]]. The concentration of ascorbic acid in the serum of a healthy subject is between 10 and 100 µM. Higher levels of ascorbic acid (up to 250 µM) are easily encountered on individuals under medical treatment and special diets [Citation[5]]. shows how the presence of ascorbic acid in a uric acid solution causes a positive error in the uric acid determination. Ascorbate oxidase is commonly incorporated to preserve the chromophore from reduction by the elimination of the ascorbic acid. However, the enzyme is rather extensive and its stability is low. also shows how with highly sensitive assay all these features are overcome just by sample dilution. These results demonstrate the reliability on the biosensor in the determination of uric acid concentration in the presence of ascorbic acid by sample dilution.
Interfering Effect of Uric Acid
At 1.0 × 10−4 M and at lower than 1.0 × 10−4 M uric acid, there was no interfering effect. It is mentioned that interfering effect has been removed by dilution of samples. In this study, interfering effect of uric acid was prevented by sample dilution.
Interfering Effect of Paracetamol and Methionine
The influences of methionine and paracetamol on uric acid amperometric signals were examined. Three different concentrations of paracetamol and methionine were respectively added to cell media in the presence of 5.0 × 10−5 M uric acid. An amperometric response current measured +0.7 V constant potential. Response currents of paracetamol and methionine have been calculated. It is mentioned that to remove interfering effect of paracetamol and methionine dilution of samples in the publication. also shows how with highly sensitive assay all these features are overcome just by sample dilution. These results demonstrate the reliability on the biosensor in the determination of uric acid concentration in the presence of paracetamol and methionine by sample dilution.
Determination of Uric Acid in Human Serum and Urine
The biosensor was applied for determination of uric acid in the serum and urine of healthy humans and results are shown in . The samples were vortex-mixed for 15 s and filtered through a filter. Urine samples for analysis were diluted 50–fold with 50 mM borate buffer (pH = 8.0). Aliquots of serum (0.2 ml) were mixed with 0.2 ml of 10% trichloroacetic acid (TCA) for deproteinization. The solution was vortexed for 30 s. After centrifugation for 5 min (3000 g), the supernatants were filtrated and diluted 100–fold with the borate buffer. Using , uric acid content has been determined in blood and urine.
Table 3. Determination of uric acid in blood and urine
In conclusion, the uric acid biosensor prepared in this study:
is usable in a large concentration range (1.0 × 10−6 − 5.0 × 10−5 M).
has a very low detection limit (5.0 × 10−7 M).
has an acceptable response time for a biosensor (330 second).
gives highly reproducible results (the electrode maintains 77.7% of the initial amperometric response at the end of the 30th measurement.
has a satisfactory storage stabilization (the electrode maintains 49% of the initial amperometric response at the end of the 7th week).
the KM and Vmax values of uricase enzyme immobilized in polypyrrole–ferrocene film were 0.44 mM and 7.1·10−2 mM/minute, respectively.
it is easy to prepare and highly cost–effective.
We acknowledge the support of this project by Gazi University Research Fund (FEF 05/2002-28).
REFERENCES
- Bhargava, A.K., Lai, H., Pundir, C.S. (1999). Discrete analysis of serum uric acid with immobilized uricase and peroxidase. J. Biochem. Biophys. Methods. 39: 125–136, [INFOTRIEVE], [CSA]
- Zen, J.M., Hsu, C.T. (1997). A selective voltammetric method for uric acid detection at Nafion-coated carbon paste electrodes. Talanta. 46: 1363–1369, [CROSSREF], [CSA]
- Inoue, K., Namiki, T., Iwasaki, Y., Yoshimura, Y., Nakazawa, H. (2003). Determination of uric acid in human saliva by high-performance liquid chromatography with amperometric electrochemical detection. Journal of Chromatography 785: 57–63, [INFOTRIEVE], [CROSSREF], [CSA]
- Miland, E., Ordieres, A.J.M., Blanco, P.T., Smyth, M.R., Fágáin, C.Ó. (1996). Poly(o-aminophenol)-modified bienzyme carbon past electrode for the detection of uric acid. Talanta. 43: 785–796, [CROSSREF], [CSA]
- Perez, D.M., Ferrer, M.L., Mateo, C.R. (2003). A reagent less fluorescent sol-gel biosensor for uric acid detection in biological fluids. Analytical Biochemistry 322: 238–242, [CROSSREF], [CSA]
- Whiteman, M., Halliwell, B. (1996). Protection against peroxynitrite dependent tyrosine nitration and alpha I-antiproteinase inactivation by ascorbic acid. A comparision with other biological antioxidants Free Radical Research 25: 275–283, [INFOTRIEVE], [CSA]
- Akyilmaz, E., Sezgintürk, M.K., Dinçkaya, E. (2003). A biosensor based on urate oxidase-peroxidase coupled enzyme system for üric acid determination in urine. Talanta. 61: 73–79, [CROSSREF], [CSA]
- Gerard, M., Chavbev, A., Malhotra, B.D. (2002). Application of conducting polymers to biosensors. Biosens. Bioelectron 17: 345–359, [INFOTRIEVE], [CROSSREF], [CSA]
- Reiter, S., Habermüller, K., Shuhman, W. (2001). A reagentless glucosebiosensor based on glucose entrapped into osmium–complex modified polypyrrole films. Sensors and Actuators. B. 79: 150–156, [CROSSREF], [CSA]
- Chavbey, A., Malhotra, B.D. (2002). Mediated biosensors. Biosens. Bioelectron 17: 441–456, [CROSSREF], [CSA]
- Gros, P., Durliat, H., Comtat, M. (2000). Use of polypyrrole film containing Fe(CN)63− as pseudo-reference electrode: Application for amperometric biosensors. Electrochim. Acta. 46: 643–650, [CROSSREF], [CSA]
- Chaubey, A., Gerard, M., Singhal, R., Singh, V.S., Malhotra, B.D. (2000). Immobilization of lactate dehydrogenase on electrochemically prepared polypyrrole-polyvinylsulphonate composite films for application to loctate biosensors. Electrochim. Acta. 46: 723–729, [CROSSREF], [CSA]
- Arslan, F., Yaşar, A., Kιlιç, F. (2006). Preparation of Pt/Polypyrrole-Ferrocene Hydrogen Peroxide Sensitive Electrode for the Use as a Biosensors, Gazi University Institute of Science and Technology, Ph.D Thesis.
- Khan, G.F., Wernet, W. (1997). A highly sensitive amperometric creatinine sensor. Analytica Chimica Acta. 351: 151–158, [CROSSREF], [CSA]
- Telefoncu, A. (1999). Biyosensörler, Biyokimya Lisansüstü Yaz Okulu, Haziran Ege Üniversitesi: Kuşadasi, pp. 136–137.
- Uchiyama, S., Sakamoto, H. (1997). İmmobilization of uricase to gas diffusion carbon felt by electropolymerization of aniline and its application as an enzyme reactor for uric acid sensor. Talanta. 44: 1435–1439, [CROSSREF], [CSA]
- Nakamura, N., Murayama, K., Kinoshita, T. (1986). Immobilization of uricase on protamine bound to glass beads and its application to determination of uric acid. Analytical Biochemistry 152: 386–390, [INFOTRIEVE], [CROSSREF], [CSA]
- Telefoncu, A. (1997). Immobilize enzimler, Enzimoloji, Biyokimya Lisans Üstü Yazokulu: Kuşadasi, pp. 193–243.
- Hu, S., Liu, C.-C. (1997). Development of a hypoxanthine biosensor based on immobilized xanthine oxidase chemically modified electrode. Electroanal. 9: 372–377, [CROSSREF], [CSA]
- Kuwabata, S., Nakaminami, T., Ito, S., Yoneyama, H. (1998). Preparation and properties of amperometric uric acid sensors. Sensors and Actuators B52: 72–77, [CSA]
- Gasper, S., Habermüller, K., Csöregi, E., Schuhmann, W. (2001). Hydrogen peroxide sensitive biosensors based on plant peroxidases entrapped in Os-modified polypyrrole films. Sensors Actuat. B72: 63–68, [CROSSREF], [CSA]