Abstract
The development of hexaPEGylated Hb, (SP-PEG5K)6-Hb, using the newly designed thiolation-mediated maleimide chemistry based PEGylation, has validated the concept that engineering ‘plasma volume expander’ -like properties to Hb neutralizes its vasoactivity. The high O2 affinity of hexaPEGylated Hb has been attributed to the two PEG-5K chains on its two Cys-93(β) residues. In an attempt to map the influence of the additional four PEG-5K chains of HexaPEGylated Hb on the O2 affinity, we have now investigated the influence of PEGylation of the surface amino groups alone on the subunit interface interactions and O2 affinity of Hb using rHb(βC93A). The molecular radius of PEGylated rHb(βC93A) was slightly smaller than that of (SP-PEG5K)6-Hb, and the overall site-selectivity of PEGylation in the PEGylated rHb(βC93A) at Lys-residues was comparable to that of (SP-PEG5K)6-Hb. Proton NMR studies have shown that the conjugation of the protein with PEG-5K does not have any significant influence on its subunit interface interactions. Surprisingly, the influence of PEGylation on the O2 affinity and Bohr effect of HbA and rHb(βC93A) is also nearly the same. Apparently, conjugation of PEG-chains to Lys residues of Hb by the thiolation mediated PEGylation induces unique changes in the structure of the hydration shell of Hb (layer of tightly bound water molecules), which, in turn, induces constraints in its R to T conformational transition to favor the more hydrated R-state.
INTRODUCTION
Development of a hemoglobin-based blood substitute has been an active area of research for several decades. Although the nephrotoxicity aspect of Hb was overcome by the products generated by the early design strategies, vasoconstrictive activity has been a major toxicity issue with these preparations [Citation[1-4]]. Recent studies have shown that PEGylation of Hb can modulate its vasoactivity [Citation[5-8]].
Our laboratory has been developing new PEGylation protocols with a view to develop cost-effective PEGylation approaches to design and generate novel PEGylated Hbs [Citation[7-12]]. One of the approaches is PEGylation based on maleimide chemistry. This approach takes advantage of the high specificity of the maleimide for reaction with the –SH groups as well as the high reactivity of the Cys-93(β) of Hb under oxy conditions. Thus, under oxy conditions, the maleimide chemistry based PEGylation is targeted to the two Cys-93(β) residues of hemoglobin. Hence, this is a site-specific modification that results in the conjugation of two PEG chains/Hb. This reaction has an additional advantage in that the modification does not alter the charge of the site modified and thus is a conservative modification. Using PEG maleimides of varying PEG chain length, namely PEG5K, PEG10K and PEG20K maleimides, we have generated three diPEGylated Hbs, namely (SP-P5K)2-Hb, (SP-P10K)2-Hb, (SP-P20K)2-Hb [Citation[8]]. The three diPEGylated Hbs exhibited higher oxygen affinity and a lower cooperativity relative to HbA. However, both the increase in oxygen affinity and the decrease in cooperativity of the three diPEGylated Hbs were comparable and hence appeared to be a consequence of the modification of Cys-93(β) and independent of the length of the conjugated PEG chain.
We have subsequently developed another approach for the conjugation of more than two copies of PEG chains per Hb molecule by the maleimide chemistry based PEGylation that involves introduction of new thiol groups on the most reactive surface amino groups of the protein—thiolation mediated maleimide chemistry based PEGylation [Citation[7], Citation[8], Citation[10]]. Using this protocol and employing Mal-Phe-PEG5K as the PEGylating reagent, we have generated a hexaPEGylated Hb (SP-P5K6-HbA) in which two of the PEG5K chains are on the two Cys-93(β) residues and an average of four PEG5K chains are on the thiolated surface amino groups of the protein. A comparison of the oxygen affinities of the diPEGylated Hbs and the hexaPEGylated Hb revealed that the hexaPEGylated Hb exhibits a high oxygen affinity similar to the diPEGylated Hbs that carry only two PEG5K chains/Hb, one each on the two Cys-93(β) residues. These results suggested that the high oxygen affinity of the PEGylated Hbs is a consequence of the modification of Cys-93(β) and the additional four PEG chains of the hexaPEGylated Hb, conjugated via the amino groups, do not have a significant influence on the oxygen affinity of Hb.
Based on our thiolation mediated PEGylation chemistry [Citation[10]], another version of the hexaPEGylated Hb, known by the name MP4, has been reported by Vandegriff et al. [Citation[13]]. Our recent study [Citation[14]] on the propensity of the PEGylated Hbs for oxygen delivery to the tissues has revealed that a diPEGylated Hb, (SP-PEG5K)2-Hb, can deliver oxygen better than the hexaPEGylated Hb, MP4. Another diPEGylated Hb, (SP-PEG10K)2-Hb, which carries two copies of PEG10K chains, also delivered oxygen as efficiently as the (SP-PEG5K)2-Hb. These results suggest that the four copies of PEG5K chains conjugated on the ε-amino groups through the intermediary of γ-mercapto butyrimidyl chains (extension arms) influence the structure/conformation of Hb in such a way that reduces the efficiency of the molecule to deliver oxygen even though the oxygen affinity of the di- and the hexaPEGylated Hb are comparable [Citation[8]].
With a view to dissect out the role of PEGylation of Cys-93(β) and that of the PEG-chains conjugated at the ε-amino groups on the structure and oxygen affinity of hexaPEGylated Hb, we have now investigated the influence of PEGylation of the surface amino groups alone on the subunit interface interactions and O2 affinity of Hb using a recombinant Hb in which Cys-93(β) is mutated to Ala. This recombinant Hb – rHb(βC93A) has an oxygen affinity that is comparable to that of HbA [Citation[15]]. Since this rHb lacks Cys-93(β), we expect to conjugate an average of four PEG chains onto this protein by the above described thiolation mediated PEGylation protocol.
MATERIALS AND METHODS
Materials
Purification of HbA from human blood and expression and purification of rHb(βC93A) were carried out as previously described [Citation[15]]. 2-iminothiolane was a product of BioAffinity Systems, Rockford, IL. Maleimidophenyl-PEG5K was synthesized as previously described [9; unpublished results]. TetraPEGylated canine Hb was prepared by reaction of canine Hb with maleimidophenyl PEG5K according to the previously described procedures [9; unpublished results].
Thiolation Mediated PEGylation of Hemoglobin
The thiolation mediated PEGylation of Hb and rHb(βC93A) was carried out by the one-step procedure as previously described [Citation[7], Citation[8]]. Briefly, HbA or rHb(βC93A) at 0.5 mM in phosphate buffered saline (PBS), pH 7.4 was incubated with 5 mM 2-iminothilane and 10 mM maleimidophenyl-PEG5K at 4°C overnight. The reaction mixture was dialyzed extensively against PBS, pH 7.4, prior to analysis. Purification of PEGylated Hbs was carried out on a Superose 12 Prep Grade column (two 2.6 × 65 cm columns connected in series) in PBS, pH 7.4, using an AKTA Purifier 10 Protein Purification System (Amersham Biosciences).
Analytical Methods
Measurement of the hydrodynamic volume of PEGyalted Hbs by size exclusion chromatography on Superose 12 column, globin chain analysis of PEGylated Hbs by RPHPLC on Vydac C4 column, identification of the sites of PEGylation by tryptic peptide mapping, SDS-PAGE analysis and measurement of the molecular radius by dynamic light scattering were carried out as described previously [Citation[9]]. Oxygen-binding equilibrium measurements were carried out using a Hemox Analyzer as described by Cheng et al. [Citation[15]]. Structural characterization of the PEGylated rHb was carried out by proton NMR spectroscopy [Citation[16]]. 1H NMR spectra of Hb samples in the CO form were obtained from a Bruker AVANCE DRX-600 NMR spectrometer in 0.1 M sodium phosphate buffer at pH 7.0 and 29°C. Proton chemical shifts were referenced to the water resonance, which occurs at 4.76 ppm downfield from the methyl porton resonance of 2,2-dimethyl-2-silapentane-5-sulfonate (DSS) at 29°C.
RESULTS AND DISCUSSION
Characterization of PEGylated rHb(βC93A)
The hydrodynamic volume of the PEGylated rHb(βC93A) as determined by size exclusion chromatography is shown in , along with those of the hexaPEGylated HbA, (SP-PEG5K)6-Hb, and tetraPEGylated canine Hb. The hydrodynamic volume of unPEGylated rHb(βC93A) was essentially indistinguishable from that of HbA (data not shown). As can be seen from , the hydrodynamic volume of the PEGylated rHb(βC93A) is slightly smaller than that of (SP-PEG5K)6-Hb, but is comparable to that of tetraPEGylated canine Hb. The molecular radius of rHb(βC93A), as measured by dynamic light scattering, is shown in . Like the hydrodynamic volume, the molecular radius of unPEGylated rHb(βC93A) is comparable to that of unmodified HbA, and that of the PEGylated rHb(βC93A) is slightly smaller than that of the hexaPEGylated Hb, but is comparable to that of tetraPEGylated canine Hb.
Figure 1 Size exclusion chromatographic analysis of PEGylated Hb and rHb(βC93A). The size exclusion chromatography was carried out at room temperature on two HR10/30 Superose 12 columns (Amersham-Pharmacia Biotech) connected in series. The column was eluted at room temperature with PBS, pH 7.4 at a flow rate of 0.5 ml/min, and the effluent was monitored at 540 nm. The chromatography of PEGylated Hb and rHb(βC93A) and P5K4-canine-Hb were shown in a, b and c.
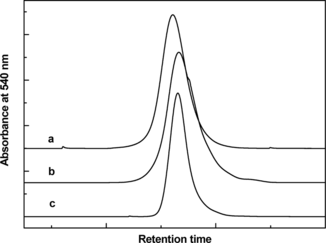
Table 1 Molecular radii of PEGylated proteins
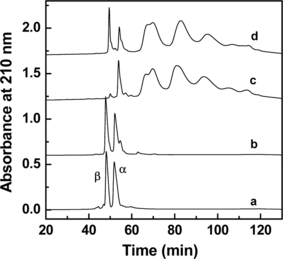
A comparison of the globin chain analysis of the PEGylated rHb(βC93A) with that of (SP-PEG5K)6-Hb is shown in . As can be seen, the β-globin of rHb(βC93A) exhibits the same retention time as the β-globin from HbA. Thus, the replacement of Cys-93(β) by Ala does not alter its retention time by RPHPLC. The data presented in also reveal that, with the exception of the presence of unmodified β-globin, the globin chain pattern of the PEGylated rHb(βC93A) is qualitatively comparable to that of (SP-PEG5K)6-Hb. The presence of unmodified β-globin in PEGylated rHb(βC93A) and its absence in the PEGylated HbA suggests that in absence of a Cys residue at position 93, the level of PEGylation in the β-chain is lower. In addition, the modification of the α-chain appears to be slightly higher in rHb(βC93A). These results indicate that the mutation of Cys-93(β) to Ala does not alter the overall pattern of PEGylation of the globin chains.
Figure 2 Characterization of PEGylated Hb and rHb(βC93A) by RPHPLC. RPHPLC analysis was carried out on a Vydac C4 column (4.6 × 250 mm), using a linear gradient of 35–50% acetonitrile containing 0.1% TFA in 100 min and then 50–70% acetonitrile containing 0.1% TFA in 30 min. The flow rate was 1 ml/min and the effluent was monitored at 210 nm. The chromatography of HbA and rHb(βC93A) control and PEGylated Hb and rHb(βC93A) were shown in a, b, c and d.
Identification of the Sites of PEGylation in rHb(βC93A)
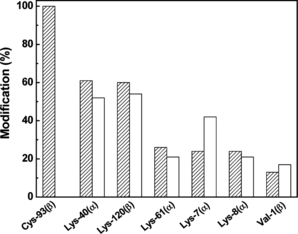
The sites of conjugation of PEG-chains in the PEGylated rHb(βC93A) were determined by tryptic peptide map analysis by methods previously described [Citation[8]]. A comparison of the results obtained for the PEGylated rHb(βC93A) with the data previously obtained for (SP-PEG5K)6-Hb [Citation[8]] is presented in . As can be seen, for the lack of modification at position β93, the overall site-selectivity of PEGylation in the PEGylated rHb(βC93A) is quite comparable to that of (SP-PEG5K)6-Hb. These results indicate that the mutation of Cys-93(β) to Ala does not influence the pattern of PEGylation of Hb. Based on the tryptic peptide map analysis, the number of residues PEGylated in rHb(βC93A) was calculated to be 4. The smaller hydrodynamic volume of the PEGylated rHb(βC93A) appears to be the result of the presence of two less PEG chains per tetramer as compared to the hexaPEGylated Hb.
Figure 3 Identification of the sites of PEGylatetion in PEGylated Hb and rHb(βC93A) by tryptic peptide mapping. The tryptic peptides were analyzed using a Vydac C18 column (10 × 250 mm) with a linear gradient of 5–50% acetonitrile containing 0.1% TFA in 160 min, followed by a linear gradient of 50–70% acetonitrile containing 0.1% TFA in 20 min at a flow rate of 1 ml/min and the effluent was monitored at 210 nm. PEGylated Hb and PEGylated rHb(βC93A) are shown in hatched bars and blank bars, respectively.
Functional properties of PEGylated rHb(βC93A)
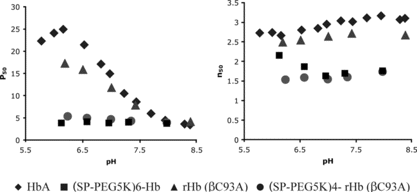
A comparison of the oxygen binding characteristics of PEGylated rHb(βC93A) with that of (SP-PEG5K)6-Hb, as a function of pH, is presented in . As can be seen, the oxygen affinity of rHb(βC93A) is quite comparable to that of HbA over the pH region from 7.0 to 8.5, whereas it is noticeably higher below pH 7.0. However, the Hill coefficient, a measure of cooperativity in oxygen binding, is quite comparable for the two proteins throughout the region of the pHs examined. More strikingly, as with HbA, PEGylation increases the oxygen affinity of rHb(βC93A), and the oxygen affinities of PEGylated rHb(βC93A) and hexaPEGylated Hb are comparable throughout the pH region examined. PEGylation also decreases the cooperativity in oxygen binding for both the proteins and the n values for the two PEGylated Hbs are comparable in the pH region of 7.0 to 8.5. These results demonstrate that despite the lack of modification at β93, the PEGylated rHb exhibits high oxygen affinity like the (SP-PEG5K)6-Hb in which the residue at this position [i.e., Cys-93(β)] is modified. Thus, surface decoration of Hb with PEG-5K via its reactive amino groups by thiolation-mediated PEGylation by itself can induce high O2 affinity to Hb, even in the absence of modification at β93.
STRUCTURAL CHARACTERIZATION OF PEGylated rHb(βC93A) BY NMR
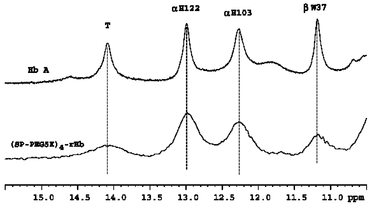
The structural characteristics of the PEGylated rHb(βC93A) was studied by 1H NMR spectroscopy, which has been shown to be a valuable technique in the study of the tertiary and quaternary structures of hemoglobins [Citation[17]]. shows the resonances of HbA and PEGylated rHb(βC93A) in the deoxy form. In general, the 1H NMR spectra of PEGylated Hb are much broader than those without PEGylation as expected due to an increase in the molecular weight of the PEGylated sample, resulting in a decrease in the tumbling of the protein molecule. However, as can be seen, the exchangeable resonances at 12.9 and 12.1 ppm are the same between Hb A and PEGylated rHb(βC93A). The resonances at 12.9 and 12.1 ppm have been assigned to the intersubunit H-bonds between α122His and β35Tyr and between α103His and β131Gln, respectively [Citation[16]]. In addition, the 14.1-ppm resonance has been assigned to the intersubunit H-bond between α42Tyr and β99Asp, a characteristic feature of the T-structure in the α1β2 subunit interface [Citation[17]] and the resonance at 11.2 ppm assigned to β37Trp, another T-structure marker in the α1β2 subunit interface [Citation[17]]. Previous proton NMR studies have shown that there are some minor changes in the heme pocket as a result of the substitution of Cys by Ala at β93, but there is no change in the α1β2 subunit interface [Citation[15]]. Studies on the hexaPEGylated (SP-PEG5K)6-Hb have demonstrated that PEGylation induces some minor changes in the environment of the β heme, but significant changes in the α1β2 subunit interface are absent (8). The T markers (i.e., the 14.1 and 11.2-ppm resonances) and the two resonances at 12.9 and 12.1 ppm are not altered.
CONCLUSION
The new paradigms for the design of Hb-based oxygen carriers are to increase the viscosity, colloidal osmotic pressure, molecular size, and oxygen affinity of the Hb solution. The first three properties can be engineered into Hb by conjugating either a limited number of large molecular size PEG chains or multiple copies of small PEG chains. The data to date appear to suggest that the surface decoration of Hb with multiple copies of small PEG chains is the optimum approach. The new PEGylation protocol developed by us, namely thiolation mediated maleimide chemistry based PEGylation (extension arm facilitated PEGylation), generated a non-hypertensive product with an average of six copies of PEG5K chains per Hb. This product has high oxygen affinity, a desired property for an oxygen carrier that delivers oxygen from the plasma. However, two PEGylated Hbs with different levels of PEGylation, but similar oxygen affinity differed in their ability to deliver oxygen to the tissues. Thus, the higher level of PEGylation needed to increase viscosity and colloidal osmotic pressure and molecular size influence the structure in such a way as to reduce tissue oxygenation. Although the increase in oxygen affinity of (SP-PEG5K)6-Hb appears to be a direct consequence of modification of Cys-93(β), the results of the present study with rHb(βC93A) that lacks Cys93(β), and hence modification at this site, demonstrate that the PEGylation of the ε-amino groups of Hb by thiolation mediated maleimide chemistry based PEGylation by itself induces a higher oxygen affinity even in the absence of modification of at β93. Since the PEGylated rHb(βC93A) has a lower level of PEGylation, it will be of interest to see whether the oxygen delivering properties of this product will be similar to the diPEGylated-Hb or the hexaPEGylated Hb.
In another recent study, we have conjugated PEG chains to hemoglobin using reductive alkylation chemistry [Citation[12]]. Using this protocol, we have generated a hexaPEGylated Hb – (Propyl-PEG5K)6-Hb, that is comparable to the hexaPEGylated Hb [(SP-PEG5K)6-Hb] generated by the thiolation mediated maleimide chemistry [Citation[8]]. The predominant sites of PEGylation in this molecule are the four amino terminal valines, which are completely modified, and a few lysine residues that are partially modified. (Propyl-PEG5K)6-Hb also exhibited an increased O2 affinity with decreased co-operativity and reduced modulation by allosteric effectors comparable to that of (SP-PEG5K)6-Hb, although its Cys-93(β) is not derivatized as in the latter. Taken together, these findings suggest that the functional properties of Hb-PEG conjugates may be a direct consequence of surface decoration of Hb with PEG, but independent of the site (pattern) and/or the chemistry of PEGylation. Apparently, in view of its high affinity for water, conjugation of PEG-chains to Hb results in a layer of tightly bound water molecules around the protein outside the normal hydration shell of Hb. This PEG-shell with its bound water in turn induces unique changes in the structure of the hydration shell of Hb and presumably increases constraints for its R to T conformational transition, thus favoring the higher hydration R-state.
This study was supported by grants from NIH (HL58247, HL71064) and US Army (PR023085).
REFERENCES
- Reiss, J.G. (2001). Chem. Rev. 101: 2797–2919.
- Klein, H.G. (2000). New Engl. J. Med. 342: 1666–1668.
- Chang, T.M.S. (1999). Trends Biotechnol. 17: 61–67.
- Winslow, R.M. (1999). Annu. Rev. Med. 50: 337–353.
- Winslow, R.M., Gonzales, A., Gonzales, M.L., Magde, M., McCarthy, M., Rohlfs, R.J., Vandegriff, K.D. (1998). J. Appl. Physiol. 85: 993–1003.
- Rohlfs, R.J., Bruner, E., Chiu, A., Gonzales, A., Gonzales, M. L., Magde, M.D., Vandegriff, K.D., Winslow, R.M. (1998). J. Biol. Chem. 273: 12128–12134.
- Acharya, A.S., Intaglietta, M., Tsai, A.G., Malavalli, A., Vandegriff, K., Winslow, R.M., Smith, P.K., Friedman, J.M., Manjula, B.N. (2005). Artificial Cells, Blood Subs. Biotechnol. 33: 239–258.
- Manjula, B.N., Tsai, A.G., Intaglietta, M., Tsai, C.-H., Ho, C., Smith, P.K., Perumalsamy, K., Kanika, N.D., Friedman, J.M., Acharya, A.S. (2005). Protein J. 24: 133–146.
- Manjula, B.N., Tsai, A., Upadhya, R., Perumalsamy, K., Smith, P.K., Malavalli, A., Vandegriff, K.D., Winslow, R.M., Intaglietta, M., Prabhakaran, M., Friedman, J.M., Acharya, A.S. (2003). Bioconjugate Chem. 14: 464–472.
- Acharya, A.S., Manjula, B.N., Smith, P.K. (1996). US Patent 5,585,484.
- Manjula, B.N., Malavalli, A., Smith, P.K., Chan, N.-L., Arnone, A., Friedman, J.M., Acharya, A.S. (2000). J. Biol. Chem. 275: 5527–5534.
- Hu, T., Manjula, B.N., Prabhakaran, M., Acharya, S.A. (2005). Biochem. J. 392: 555–564.
- Vandegriff, K.D., Malavali, A., Gonzales, M.L., Wooldrigde, J., Lohman, J., Winslow, R.M. (2003). Transfusion 43: 509–516.
- Cabrales, P., Kanika, N.D., Manjula, B.N., Tsai, A.G., Acharya, S.A., Intaglietta, M, 2004). Am. J. Physiol. Heart Circ. Physiol. 287: H1609–H1617.
- Cheng, Y., Shen T., Simplaceanu, V., Ho, C. (2002). Biochemistry 41: 11901–11913.
- Simplaceanu, V., Lukin, J.A., Fang T., Zou, M., Ho, N.T., Ho, C. (2000). Biophys. J. 79: 1146–1154.
- Ho, C. (1992). Adv. Protein Chem. 43: 153–312.