Abstract
Natural acellular polymeric hemoglobins (Hb) provide oxygen transport and delivery within many terrestrial and marine invertebrate organisms. It has been our premise that these natural acellular Hbs may serve as models of therapeutic hemoglobin-based oxygen carriers (HBOC). Our attention has focused on the acellular Hb from the terrestrial invertebrate, Lumbricus terrestris (Lt), which possesses a unique hierarchical structure and a unique ability to function extracellularly without oxidative damage. Lumbricus Hb and Arenicola Hb are resistant to autoxidation, chemical oxidation by potassium ferricyanide, and have little or no capacity to transfer electrons to Fe+3-complexes at 37°C. An understanding of how these invertebrate acellular oxygen carriers maintain their structural integrity and redox stability in vivo is vital for the design of a safe and effective red cell substitute. We report here a positive redox potential for these giant hemoglobins that may lie at the basis for its resistance to oxidation.
INTRODUCTION
For the past two decades, most research efforts directed towards the design of an alternate therapeutic oxygen carrier and delivery system have focused on the development of acellular hemoglobin-based oxygen carriers (HBOC) for a wide range of clinical applications. Although many of these HBOC substitutes have been demonstrated to be effective in the delivery of oxygen in specific clinical situations, continued observations of oxidative and cellular damage associated with their use have prevented full FDA regulatory approval for their clinical use [Citation[1-3]].
A fundamental physiological requirement for all therapeutic HBOCs is the maintenance of the heme-iron in the reduced state (Fe+2). This is necessary for the reversible binding-release of molecular oxygen in vivo. Oxidation leading to methemoglobin formation (heme-Fe+3) results in lowering of the oxygen carrying capacity of the HBOC. Without an adequate reducing environment, methemoglobin is converted to hemichromes, associated with the formation of oxidative free radicals in vivo [Citation[4], Citation[5]].
A number of invertebrate hemoglobins are acellular, and circulate and function as oxygen carriers without giving rise to oxidative stress. It has been our premise that these natural acellular hemoglobins may serve as models of therapeutic HBOCs [Citation[6-10]]. Efforts are directed towards analyzing the biochemical and biophysical properties of these unique oxygen transport/delivery proteins both in vivo and in vitro that contribute to their unique stability.
The particular focus of our work was initially on the acellular hemoglobin from the terrestrial invertebrate, Lumbricus terrestris (Lt), testing the hypothesis that its unique structure and functional properties are significant in its success as a natural oxygen carrier. Lumbricus hemoglobin possesses a unique hierarchical quaternary structure in the form of a naturally cross-linked dodecamer that meets the requirements of 1) molecular size [MW ∼ 3600 kDa], composed of 144 heme containing chains; 2) oxygen binding and Bohr effect similar to human hemoglobin; 3) resistance to subunit dissociation necessary to maintain normal oncotic pressure; 4) thermal stability; and 5) oxidative stability [Citation[11-15]]. As early as 1993 [Citation[9], Citation[10]], our laboratories demonstrated, in preliminary animal studies, the potential of this acellular hemoglobin (LtHb) as an oxygen carrier. In a series of biological evaluation studies, mice and a rat model were partially exchanged with purified LtHb, exhibiting no apparent behavioral and physical changes. To assess phosphocreatine levels as an indication of the ability of LtHb to serve as an oxygen carrier to the heart, 31P NMR spectroscopy of perfused guinea pig hearts demonstrated that LtHb provided oxygen to the tissue and maintained energy metabolism significantly better than the control non-Hb perfusion media. Exchanged mice rechallenged with LtHb showed no overt immunogenic response or deaths [Citation[10]].
The aim of this current work is to focus upon the structural integrity and redox stability of the terrestrial LtHb and the marine invertebrate, Arenicola marina acellular HB from the (ArHb). ArHb possesses a quaternary structure similar to the dodecameric structure of LtHb [Citation[12]]. ArHb proposed by Zal and co-workers [Citation[16]] as a potential therapeutic oxygen carrier, is presently undergoing review in pre-clinical studies. The studies presented here illuminate the chemical-physical basis for the unique structural and redox stability of LtHb and ArHb that could be useful in the design of an acellular HBOC.
MATERIALS AND METHODS
Isolation and Purification of Acellular Hemoglobins
Lumbricus hemoglobin. Live earthworms (10–20 cm) were obtained from Ward's Biological Campany in Rochester, NY. ArHb hemoglobin was isolated from live lugworms obtained from the Marine Resource Center at the Marine Biological Laboratory in Woods Hole, MA. A similar method of isolation and purification was employed for each hemoglobin. The coelomic fluid of each worm was drained into a cold (∼ 1°C) 50 mM Hepes buffer, pH 7.0. After the initial centrifugation at 10,000 rpm for 30 minutes at 4°C to remove any cellular debris, the supernatant was removed and centrifuged at 160,000 g for 2 hours at 4°C. The red pellet of each preparation was redissolved in buffer and the ultracentrifugation step was repeated. The red pellet was redissolved in a minimum amount of buffer to maintain a high stock concentration of each hemoglobin. The purified hemoglobins were used immediately according to the experimental protocol or stored under liquid nitrogen until needed. Storage under liquid nitrogen prevents autoxidation. No changes in the properties of the stored hemoglobins were evident for at least one year as determined by spectroscopic and light scattering measurements [Citation[17]].
Oxidation Studies
a) Autoxidation studies. Continuous spectra were recorded from 700 to 500 nm on a Shimadzu 160 recording spectrophotometer at specified time intervals. All spectral determinations were carried out at 37°C. The rate and extent of methemoglobin formation was established by the changes in the A576/A540 ratio. This method of analysis eliminates light scattering effects. b) Chemical oxidation. The traditional mode of chemical oxidation for hemoglobins is potassium ferricyanide. Rates of oxidation of Lumbricus and Arenicola hemoglobins were deterimined at precise time intervals (described for the autoxidation studies) using fixed concentrations of potassium ferricyanide. c) Electron transfer kinetics. The preparation of the Fe+3-complexes of adenosine triphosphate (ATP), ethylenediamine-tetracetate (EDTA), nitrilo triacetate (NTA), and citrate were carried out according to Harrington and Hicks [Citation[18]]. A series of kinetic measurements to determine the effective capacity of each of these hemoglobins to reduce the ferric complexes to the ferrous form were carried out at 37°C out as previously described [Citation[18]].
Structural Stability: Isothermal Unfolding Studies
The isothermal unfolding of each of the invertebrate hemoglobins was obtained at 25°C. Urea solutions of 1 to 9.5 M were prepared by volumetric dilution using a 10 M stock solution. For absorbance determinations, hemoglobin solutions were prepared by dilution in 5 ml volumetric flasks from appropriate stock solutions of each invertebrate hemoglobin. All protein solutions contained 50 mM Hepes, pH 7.0. Spectral measurements in the Soret region (450–350 nm) were recorded on a Shimadzu 160 recording spectrophotometer after 30 minutes of equilibration. The unfolding of each hemoglobin as a function of increasing urea concentration was followed by changes in absorbance at 413 nm, assuming a molar extinction of 1.25 × 105 [Citation[19]]. Hemoglobin concentrations varied between 10−5 to 10−6 M in heme.
Reduction Studies
The rate and extent of each invertebrate hemoglobin reduction reaction was carried out at 20°C in 50 mM Hepes, pH 7.0. Each hemoglobin was completely oxidized (100% metHb) by reacting each sample with an excess of K3Fe(CN)6. Each oxidized sample was eluted on a Sephadex G-25 column to remove the unreacted potassium ferricyanide. The reduction reactions were initiated by the direct addition of either glutathione or ascorbic acid to give the required concentration of reducing agent. Using direct absorbance analysis, spectra of the reduction process were recorded in overlay mode within the visible region (700–490 nm) as a function of time. The rate and extent of each hemoglobin reduction was determined either by the increase in the A576/A540 ratio or by the decrease in the charge-transfer band at 630 nm [Citation[20]].
Redox Potential Determined by Spectroelectrochemical Analysis
Determination of the formal reduction potentials (E°') for each acellular hemoglobin was carried out using a modified, optically transparent thin-layer electrochemical cell based upon the design of Faulkner and co-workers [Citation[21-23]]. Although heme proteins can react directly with the surfaces of the electrodes, the reaction is typically slow and often irreversible. For hemoglobins, the heme-iron center is not accessible for direct electron transfer from the working electrode due to the unique secondary and tertiary structure of the hemoglobin molecule, hence a mediated electron transfer process must be utilized for these reactions. The smaller mediator molecule is reduced or oxidized at the electrode surface and undergoes diffusion to the heme site where it in turn reduces or oxidizes the heme-iron center. Upon re-diffusion back to the electrode surface, the mediator can be re-oxidized or re-reduced and the cycle is continued.
A distinct advantage in using thin-layer electrochemistry is that the electroactive species conform to the Nernst Equation (Eq. 1) and allows for rapid analysis:
The log [Ox]/[Red] is plotted as a function of the potential using Eq. (1) where E is the potential applied to the Pt working electrode, E°′ is the formal reduction potential, and n is the electron stoichiometry. The formal reduction potential is defined as the applied potential required to bring the log [Ox]/[Red] to equal zero. The data analysis can be carried out using the absorbance values at either of the Soret peaks. For LtHb, the fully oxidized Soret has a λmax at 396 nm and the deoxy (reduced form) has a λmax at 430 nm. All spectroelectrochemical determinations were carried out in triplicate at 20°C [Citation[8]].
RESULTS
The uniqueness of LtHb and ArHb is the homogeneity of their molecular size and the hierarchical quaternary structure (two tiered hexameric bilayer) with 144 and 156 heme-containing chains, respectively [Citation[24], Citation[25]]. Linker chains crosslink the subunits into a stable dodecamer [Citation[26]]. Intrinsic molecular stability of acellular hemoglobins may be probed by comparative isothermal unfolding behavior as induced by increasing concentrations of urea (). The unfolding profiles evident for both LtHb and ArHb are in contrast to the unfolding behavior exhibited by two other acellular hemoglobins isolated from the clams, Astarte and Cardita. Interestingly, the clam hemoglobins are more resistant to unfolding than either the LtHb or the ArHb. This increased resistance may be associated with a significant difference in the quaternary structures of these clam hemoglobins which are rodlike in shape and possess a much higher molecular weight (MW ∼ 12 × 106 Da) [Citation[28], Citation[29]] (). According to Terwilliger and co-workers [Citation[29]], the structure of the acellular Cardita hemoglobin revealed by electron microscopy exhibits a structural similarity with molluscan hemocyanin. The unfolding profiles of LtHb and ArHb, similar to other globular shaped hemoglobin molecules [Citation[30], Citation[31]], have unfolding midpoints ranging from 4.5 to 5.4 M urea at 37°C. Unfolding is followed by changes in the Soret wavelength maximum wherein the decrease in absorbance is directly associated with greater heme exposure to the solvent as the urea concentration is increased. Earlier light scattering studies indicated that LtHb does not undergo subunit dissociation as a function of decreasing concentration in contrast to human and bovine hemoglobins, which undergo subunit dissociation to dimers as their concentrations are decreased [Citation[17]]. Hemoglobin dimers are toxic to the kidneys and was the rationale for the development of a variety of cross-linked hemoglobins to ensure low molecular weight hemoglobin forms (32 kDa or less) do not circulate.
Figure 1 Isothermal unfolding of invertebrate acellular hemoglobins in the presence of urea at 37°C, 50 mM Hepes, 100 mM KCl, pH 7.0. Astarte Hb (▪), Cardita Hb (♦), Lumbricus Hb (•), and Arenicola Hb (▴).
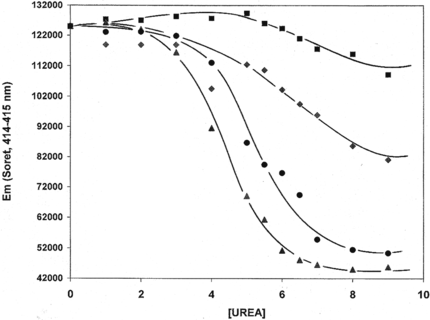
Table 1. Formal redox potentials of hemoglobin-base oxygen carriers by thin-layer spectroelectrochemistry (T = 20°C)
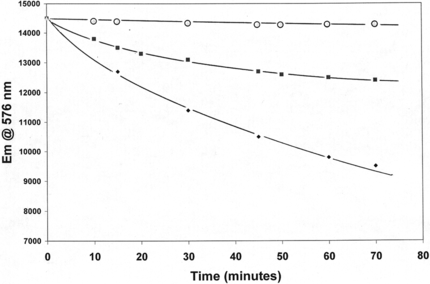
One of the most important observations about the chemical behavior of LtHb and ArHb is their overall resistance to oxidation (heme-Fe+2 → heme-Fe+3). The inherent resistance of these acellular hemoglobins to autoxidation at 37°C under normal atmospheric conditions is evident in the negligible change in the Abs576/Abs540 ratio up to 3 hours for LtHb and ArHb compared to the extensive decrease in this ratio for the Cardida hemoglobin (different quaternary structure) (). LtHb and ArHb resistance to chemical oxidation is observed with a strong oxidizing agent such as, K3Fe(CN)6, traditionally used to convert hemoglobin samples to metHb (heme-Fe+3). A 3 to 5 molar excess of the oxidizing agent is required to completely convert these acellular hemoglobins to the metHb form [Citation[32]]. The third example demonstrating their significant resistance to oxidation is the decrease in the kinetics and extent of electron transfer to different Fe+3-complexes associated with the reduction of different Fe+3-complexes (i.e., ATP, citrate, NTA, EDTA). The coupled release of Fe+2 ions to the solution is also monitored. The decreased capacity of LtHb and ArHb to donate an electron to the Fe+3-NTA complex compared to HbA is demonstrated in .
Figure 2 Autoxidaion of Lumbricus Hb (♦), Arenicola Hb (▪), and Cardita Hb (○) at 37°C. Decreases in the Abs576/Abs540 ratio reflect the conversion of oxyHb to metHb. All solutions contain 50 mM Hepes, 100 mM KCl, pH 7.0.
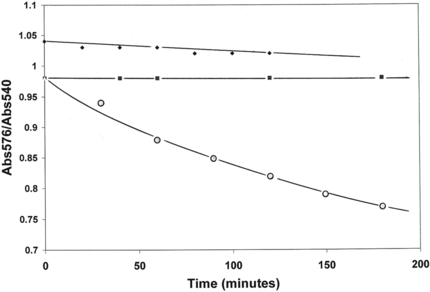
Figure 3 Comparison of oxidation of Arenicola Hb (○), Lumbricus Hb (▪), and HbA (♦) by Fe+3-ATP complex at 37°C. The HbA data (T = 25°C) is from Harrington and Hicks (14).
The unusual stability associated with the resistance of LtHb and ArHb to autoxidation, chemical oxidation by potassium ferricyanide, and decreased rate of electron transfer to Fe+3-complexes, can now be correlated with those Hbs exhibiting positive redox potentials (). presents the absorbance spectra of ArHb at differing applied potentials (+ 400 mV, oxidized to − 600 mV, reduced). The fully oxidized form (heme-Fe+3) λmax of ArHb occurs at 402 nm, while the fully reduced deoxy form (heme-Fe+2) exhibits λmax at 425 nm. Unlike HbA, HbS, HbXLα99, and oxyglobin (), which exhibit negative reduction potentials (− 52 to − 23 mV), the acellular LtHb and ArHb hemoglobins possess positive reduction potentials (+ 112 to + 63 mV) as determined by thin-layer spectroelectrochemistry. This redox characteristic is consistent with their resistance to oxidation [Citation[8], Citation[22], Citation[23]].
Figure 4 Absorbance spectra of Arenicola hemoglobin at differing potentials in the absence of oxygen (+ 400 mV to − 600 mV). The fully oxidized ArHb is indicated by λmax = 402 nm and the fully reduced ArHb is indicated by λmax = 425 nm. All potentials were measured against Ag/AgCl reference electrode at 20°C.
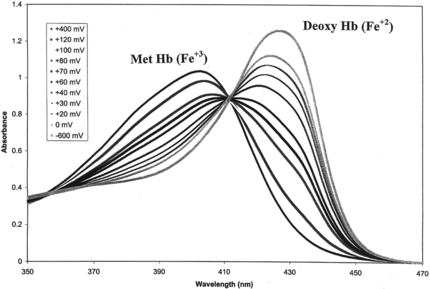
Table 2. Reduction (heme-Fe+3 → heme-Fe+2) of terrestrial and marine acellular Hbs by ascorbic acid and glutathione at T = 20°C, pH 7.0
The formal positive reduction potentials of LtHb and ArHb also have significant implications in their inherent ability to be maintained in the reduced (heme-Fe+2) state [necessary to maintain functionality (i.e., the reversible binding of oxygen in vivo)]. Each of these hemoglobins undergoes reduction in the presence of low concentrations of endogenous human plasma reducing agents (ascorbic acid and glutathione) from the fully oxidized metHb state to the reduced state (). An earlier study demonstrated that LtHb was stable in human plasma at 37°C for up to six hours and remained stable in the plasma for several days at room temperature [Citation[20]]. This same study showed that glutathione inhibited the rate of autoxidation of LtHb in contrast to its pro-oxidative effect on human HbA [Citation[20], Citation[33]].
DISCUSSION
In contrast to the tetrameric quaternary structure of human and bovine hemoglobins and crosslinked tetramers to create high molecular weight Hb polymers, which serve as the source of many of the first and second generation of HBOCs, the invertebrate LtHb and ArHb are composed of naturally cross-linked intra and inter disulfide bonds that lead to a unique hierachical array of heme-containing subunits. The high resolution structural details of LtHb have recently been defined [Citation[34], Citation[35]]. The unusual stability of the dodecameric structure was shown in earlier studies using high pressure fluorescence techniques [Citation[36]]. In addition to the linker chains, ions play a key role in stabilizing the molecule. Calcium is significant for the stabilization of LtHb [Citation[37]], while magnesium is more important for ArHb than calcium [Citation[38]]. As demonstrated by the present studies, it appears that the positive redox potentials of LtHb and ArHb serve as the basis for their extraordinary resistance to heme oxidation.
In contrast to the negative reduction potentials exhibited by human and bovine hemoglobins (− 155 to − 23 mV), the acellular LtHb and ArHb exhibit positive reduction potentials (+ 74 mV, + 63 mV) correlated and consistent with their observed resistance to several oxidation pathways (). The well-defined isosbestic points at 356 nm and 412 nm () clearly indicate the presence of only two discrete molecular species in equilibrium during the redox events. This behavior has permitted a reasonable estimate of the formal reduction potential for each of these acellular hemoglobins under these solution conditions.
Each of these acellular invertebrate hemoglobins undergoes rapid and extensive reduction (heme-Fe+3 → heme-Fe+2) from the methemoglobin state in the presence of low concentrations of human plasma reducing components [Citation[7]]. The effectiveness of ascorbic acid () in reducing LtHb and ArHb, in contrast to other HBOC, is significant in light of previous studies indicating that ascorbic acid converts to a pro-oxidative agent for other hemoglobins, such as HbA and HbXL99α, within a short period of time [Citation[33]]. This capacity for reduction, so important in the binding and release of oxygen within the circulation, is linked to a thermodynamically favorable reduction potential difference that must exist between the plasma reducing agent and the HBOC.
It is concluded that this positive reduction potential exhibited by both LtHb and ArHb, resulting from significant differences in the nature of the heme environment and/or the extent of the heme moiety's solvent-surface exposure, provides these acellular hemoglobins with oxidative protection. In a systematic study of several heme proteins, Stellwagen [Citation[39]] determined that the exposure of the heme moiety at the solvent-surface, rather than the apolarity or nature of the heme environment, was the principal factor in determining the magnitude of the reduction potential of a particular heme protein. Other studies suggest that multiple factors must be considered in any mechanistic explanation of a heme protein's intrinsic redox potential. Consideration must be given to: a) the nature of the heme environment; b) the orientation of the heme moiety within the globin fold; and c) the degree of non-planarity exhibited by the heme moiety [Citation[40]]. A recent investigation seeking to determine how the redox potentials of various cytochromes with different folding motifs are controlled was carried out by Gunner and co-workers [Citation[41]]. Their analysis indicated that each folded motif differentially altered the free energy of the buried charges. Factors shown to affect the free energy included, the axial ligands, the heme solvation energy, the surface and buried charged groups, the dipoles of the protein backbone and side chains, as well as any change in protein conformation. Considering all of the above, molecular alterations that give rise to a positive redox potential might be considered in the engineering of improved designs to heighten HBOC stability.
The molecular evolutionary development of highly stable circulating acellular hemoglobins, with greater resistance to oxidation, is a vital adaptation considering their normal ecological and physiological behavior. First of all, the acellular Hb lacks the red cell compartment housing the enzymatic reducing system for methemoglobin reduction. Secondly, the normal environment (terrestrial and aquatic) of these organisms with natural acellular hemoglobins undergo dramatic continual physical and chemical changes including significant alterations in temperature, soil and water compositions, and an array of toxic substances that directly affect their normal physiology [Citation[42]]. We cannot rule out that the composition of the carrier fluid or vehicle in which LtHb and ArHb are transported contain unusual levels of antioxidants or antioxidant enzymes. This will be the subject of another study. Nevertheless, it is agreed that LtHb and ArHb experience greater oxidative stress than vertebrate hemoglobins. Hence, the intrinsic oxidative resistance of LtHb is an adaptive molecular property.
In summary, the unusually highly positive redox potential exhibited by LtHb and ArHb, coupled with the natural crosslinking, impart a high stability allowing it to circulate as an acellular HBOC. Hence, there is a need for continued studies of these intrinsically stable natural acellular hemoglobins as viable models in the design of an efficient and safe HBOC.
REFERENCES
- Lee, R., Neya, K., Svizzero, T.A., Vlahahas, G.T. (1995). Limitations of the efficacy of hemoglobin-based oxygen carrying solutions. J. Appl. Physiol. 78: 236–246.
- Alayash, A.I., Cashon, R.E. (1995). Hemoglobin and free radicals: Implications for the development of a saf blood substitute. Mol. Med. Today. 1: 122–127.
- Alayash, A.I. (1999). Hemoglobin-based blood substitutes: Oxygen carriers, pressor agents, or oxidants. Nature Biotechnol. 17: 545–549.
- Alayash, A.I. (2000). Hemoglobin-based blood substitutes and the hazards of blood radicals. Free Radic. Res. 33: 341–348.
- D'Agnello, F., Alayash, A.I. (2000). Interactions of hemoglobin with hydrogen peroxide alters thiol levels and course of endothelial cell death. Adv. Drug Deliv. Res. 40: 199–212.
- Hirsch, R.E., Harrington, J.P. (2000). Blood substitutes: An overview and perspective. Einstein Quart. J. Biol. Med. 17: 113–123.
- Harrington, J.P., Hirsch, R.E. (2003). XIIIth Intern. Conference on Invertebrate Dioxygen Binding Proteins, Mainz, Germany September,.
- Dorman, S.C., Kenny, C.F., Miller, L., Hirsch, R.E., Harrington, J.P. (2002). Role of redo potential of hemoglobin-based oxygen cariers on methemoglobin reducton by plasma components. Art. Cells, Blood Subst. and Immob. Biotech. 30: 43–56.
- Harrington, J.P., Shear, H.L., Hirsch, R.E. (1993). Blood, 589a.
- Hirsch, R.E., Jelicks, L.A., Wittenberg, B.A., Kaul, D.K., Shear, H.L., Harrington, J.P. (1997). A first evaluation of the high molecular weight polymeric Lumbricus terrestris hemoglobin as an oxygen carrier. Art. Cells, Blood Subst. and Immob. Biotech. 25: 429–444.
- Kuchumov, A.R., Taveau, J.C., Lamy, J.N., Wall, J.S., Weber, R.E., Vinogradov, S.N. (1999). The role of linkers in the reassembly of the 3.6 Mda hexagonal bilayer hemoglobin from Lumbricus terrestris. J. Mol. Biol. 289: 1361–1374.
- Green, B.N., Gotoh, T., Suzuki, T., Zal, F., Lallier, F.H., Toulmond, A., Vinogradov, S.N. (2001). Observation of large, non-covalent globin subassemblies in the approximately 3600 kDa hexagonal bilayer hemoglobin by electrospray ionization time-of-flight mass spectrometry. J. Mol. Biol. 309: 553–560.
- Fushitani, K., Imai, K., Riggs, A.F. (1986). Oxygenation properties of hemoglobin from the earthworm, Lumbricus terrestris. Effects of pH, salt, and temperature. J. Biol. Chem. 261: 8414–8423.
- Zhu, H., Ownby, D.W., Riggs, C.K., Nolasco, N.J., Stoops, J.K., Riggs, A.F. (1996). Assembly of the gigantic hemoglobin of the earthworm Lumbricus terrestris. Roles of subunit equilibria, non-globin linker chains, and valence of the heme iron. J. Biol. Chem. 271: 30007–30021.
- Harrington, J.P. (1994). Multimeric Lumbricus hemoglobin stabilization by alkali and alkaline earth cations. Comp. Biochem. Physiol. 109A: 799–803.
- Chabasse, C., Rousselot, M., Bailly, X., Harnois, T., Zal, F. (2005). Novel dissociation mechanism of a polychaetous annid extracellular hemoglobin. Xth Intern. Symp. on Blood Subst., Providence, RI, p. 55a.
- Harrington, J.P., Pandolfelli, E., Herskovits, T.T. (1973). Solution studies on heme proteins: Circular dichroism and opical rotation of Lumbricus terrestris and Glycera diranchiata hemoglobin. Biochim. Biophys. Acta. 328: 71–83.
- Harrington, J.P., Hicks, R. (1994). Spectral analysis of Fe(III)-complex reduction by hemoglobin: Possible mechanisms of interaction. Intern. J. Biochem. 26: 1111–1117.
- DiIorio, E. (1981). Methods in Enzymology 96: 47–72.
- Harrington, J.P., Gonzalez, Y., Hirsch, R.E. (2000). Redox concerns in the use of acellular hemoglobin-based oxygen carriers: The role of plasma components. Art. Cells, Blood Subst. and Immob. Biotech. 28: 477–492.
- Dorman, S.C., Harrington, J.P., Martin, M.S., Johnson, T.V. (2004). Determination of the formal reduction potential of Lumbricus terrestris hemoglobin using thin-layer spectroelectrochemistry. J. Inorg. Biochem. 98: 195–198.
- Faulkner, K.M., Bonaventura, C., Crumbliss, A.L. (1994). A spectroelectrochemical method for evaluating factors which regulate the redox potential of hemoglobins. Inorg. Chim. Acta. 226: 187–194.
- Faulkner, K.M., Bonaventura, C., Crumbliss, A.L. (1995). A spectroelectrochemical method for differentiation of steric and electronic effects in hemoglobins and myoglobins. J. Biol. Chem. 270: 13604–13612.
- Vinogradov, S.N., Lugo, S., Mainwaring, M.G., Kapp, O.H., Crewe, A.V. (1986). Bracelet protein: A quaternary structure proposed for the giant extracellular hemoglobin of Lumbricus terrestris. Proc. Nat. Acad. Sci. USA 83: 8034–8038.
- Zal, F., Green, B.N., Lallier, F.H., Vinogradov, S.N., Toulmond, A. (1997). Quaternary structure of the extracellular hemoglobin of the lugworm Arenicola marina: A multi-angle-laser-light-scattering and electrospray-ionization-mass-spectrometry analysis. Eur. J. Biochem. 243: 85–92.
- Fushitani, K., Matsuura, M.S.A., Riggs, A.F. (1988). The amino acid sequences of chains a, b, and c that form the trimer of the extracellular hemoglobin from. J. Biol. Chem. 263: 6502–6517.
- Yager, T.D., Terwilliger, N.B., Terwilliger, R.C., Schabtach, E., Van Holde, K.E. (1892). Organization and physical properties of the giant extracellular hemoglobin of the clam Astarte castanea. Biochim. Biophys. Acta. 709: 194–203.
- Terwilliger, N.B. (1992). Adv. Comp. Environ.Physiol. 13: 193–229.
- Terwilliger, R.C., Terwilliger, N.B., Schabtach, E. (1978). Extracellular hemoglobin of the clam, Cardita borealis: An unusual polymeric hemoglobin. Comp. Biochem. Physiol. 59B: 9–14.
- Herskovits, T.T., Behrens, C.F., Siuta, P.B, Pandofelli, E.R. (1977). Solvent denaturation of globular proteins: Unfolding by the monalkyl- and dialkyl-substituted formamides and ureas. Biochim. Biophys. Acta. 490: 192–199.
- Regis, W.C.B., Fattori, J., Santoro, M.M., Jamin, M., Ramos, C.H.I. (2005). Of the differences in stability between horse and sperm whale myoglobins. Arch. Biochem Biophys. 436: 168–177.
- Kobayashi, S., Harrington, J.P. (2005). Unpublished data.
- Sampeth, V., Caughey, W.S. (1985). Prooxidant effects of glutathione in aerobic hemoglobin solutions. Superoxide generation from uncoordinated dioxygen. J. Am. Chem. Soc. 107: 4076–4078.
- Royer, W.E., Strand, K., Van Heel, M., Hendrickson, W.A. (2000). Structural hierarchy in erythrocruorin, the giant respiratory assemblage of annelids. Proc. Nat. Acad. Sci. USA 97: 7107–7111.
- Strand, K., Knapp, J.E., Bhyravbhatla, B, Royer, W.E. (2004). Crystal structure of the hemoglobin docecamer from Lumbricus terrestris erythrocruorin: Allosteric core of giant annelid respiratory complexes. J. Mol. Biol. 344: 119–134.
- Hirsch, R.E., Harrington, J.P., Scarlata, S. (1993). The differential effects of carbon monoxide and oxygen on the pressure dissociation of Lumbricus terrestris hemoglobin. Biochim. Biophys. Acta. 1161: 285–290.
- Harrington, J.P., Friedman, J.M., Hirsch, R.E. (1997). The effect of alkaline earth cations and of ionic strength on the dissociation of earthworm hemoglobin at alkaline pH. Biophys. J. 72(2): A86.
- Ochiai, T., Weber, R.E. (2002). Effects of Magnesium and calcium on the oxygenation reaction of erythrocruorin from the marine polychaete Arenicola marina and the terrestrial oligochaete Lumbricus terrestris. Zoolog. Sci.>19: 995–1000.
- Stellwagen, E. (1978). Heme exposure as the determinate of oxidation-reduction potential of heme proteins. Nature 275: 73–74.
- Chen, Z., Ost, T.W.B., Schelvis, J.P.M. (2004). Phe393 mutants of cytochrome P450 BM3 with modified heme redox potentials have altered heme vinyl and proprionate conformations. Biochemistry 43: 1798–1808.
- Mao, J., Hauser, K., Gunner, M.R. (2003). How cytochromes with different folds control heme redox potentials. Biochemistry 42: 9829–9840.
- Bispo, J.A.C., Landini, G.F., Santos, J.L.R., Norberto, D.R., Bonafe, C.F.S. (2005). Tendency for oxidation of annelid hemoglobin at alkaline pH and dissociated states probed by redox titration. Comp. Biochem. Physiol. 141B: 498–504.
