Abstract
To gain insight into the degree to which hemoglobin based oxygen carriers may replace the need for transfusion in surgical patients experiencing blood loss, a simple mathematical model was developed. This model predicts the amount of blood sparing resulting from a bolus infusion of different doses of hemoglobin solution as a function of circulating hemoglobin half-life and degree of erythropoesis enhancement subsequent to treatment. The results of this analysis are consistent with published clinical data and imply that blood sparing increases with increasing oxygen carrier dose and half-life, as well as increasing levels of erythropoesis enhancement. The analysis also predicts that the total circulating hemoglobin content in patients infused with HBOC solutions may reach a minimum value up to ten days after treatment.
INTRODUCTION
Reducing the need for allogeneic blood transfusion in surgical patients has been identified as a desirable clinical goal by the United States Food and Drug Administration and many clinical practitioners [Citation[1], Citation[2]]. Among the different approaches evaluated for this purpose, the use of alternative oxygen transporting formulations that have substantially reduced risk of pathogen transmission and, potentially, a lower propensity for untoward side effects such as immune suppression, immunologic mismatch, and transfusion associated lung injury, is of particular interest. Hemoglobin based oxygen carriers (HBOCs) have been clinically evaluated with respect to their ability to reduce or eliminate the need for red blood cell transfusions in human surgical patients who have experienced blood loss [Citation[3-6]]. While initial results suggest utility in this application, expectations vary widely with respect to the amount of blood sparing that can be achieved with this class of product given the relatively short circulating half-life of HBOCs relative to that of transfused red cells [Citation[7]]. In addition, the effect of HBOC infusion on the subsequent variation of hemoglobin levels appears to be complex and the interpretation of clinical data challenging. To gain insight into the amount of blood sparing that should be expected given the properties of current HBOCs, a simple mathematical model was developed to predict the degree of long–term blood transfusion avoidance that would be expected in an idealized cohort of surgical patients. This model considers the influence of the functional residence time of HBOC in the circulation, HBOC dose, and degree of enhanced erythropoesis that occurs immediately after treatment. From this analysis predictions are derived that may be compared to published clinical data, and expectations set with respect to the degree to which HBOCs may be useful in achieving this clinical endpoint.
METHODS
In this modeling exercise it is assumed that patients receive a single bolus infusion of HBOC solution, after which there is no additional blood loss. HBOC dosing is measured in blood unit equivalents assuming that 65 g of HBOC hemoglobin carries and delivers the same amount of oxygen as one unit of blood. The dose of HBOC is denoted as UI. The total blood units present immediately prior to HBOC dosing is denoted as U0. The HBOC is assumed to disappear from the circulation with a mono-exponential decay characterized by a half-life of t1/2 days and to retain functionality during the time of residence within the circulation. Thus, after HBOC infusion the amount of HBOC hemoglobin present in the circulation may be denoted as . Erythropoesis is also assumed to be increased after HBOC infusion with a constant rate of excess erythrocyte hemoglobin output of k units/day for four to ten days after treatment. This parameter is the amount of erythrocyte hemoglobin produced in excess of that required to replace the normal attrition of red cells due to senescence. Thus, the amount of blood units present at time t after treatment due to enhanced erythropoesis is kt. The amount of long-term blood sparing achieved after HBOC infusion is assumed to be the minimum difference between the sum of HBOC and erythrocyte hemoglobin after treatment and the starting total hemoglobin value immediately prior to HBOC infusion (). This degree of blood sparing is denoted as US. It is assumed that the parameter of greatest importance with respect to oxygen transport is the total absolute oxygen carrying capacity present in the circulation, i.e. the sum of erythrocyte and HBOC hemoglobin content, expressed as the total blood unit equivalents, U(t), present at time t (days) after infusion. This parameter is simply the sum of the preexisting erythrocyte hemoglobin, the HBOC hemoglobin, and the hemoglobin added as a result of enhanced erythropoesis. In terms of the parameters noted above, this relationship is expressed as:
Blood volume changes due to non oxygen carrying fluid shifts are not considered in this model.
Figure 1 Graphical illustration of the components contributing to blood sparing after treatment with HBOC solution. Parameters are plotted for an HBOC dose of two blood equivalent units, an HBOC plasma half-life of one day, and an excess erythropoesis rate of 0.2 blood units per day. Long–term blood sparing is assumed to be the minimum value for excess oxygen transport capacity after HBOC treatment relative to the capacity value immediately before HBOC infusion. Since the pretreatment value is arbitrary in this analysis, it has been set equal to zero in this graph for convenience.
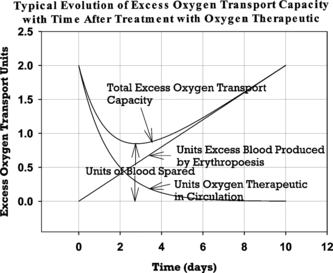
To calculate the degree of blood sparing, Equation (1) is differentiated with respect to time and set equal to 0 since the rate of change of U(t) will be zero when the minimum value for U(t) is achieved. Solving the resulting equation for t gives tmin, the time after HBOC infusion at which the total circulating hemoglobin content is a minimum. This yields:
Substituting tmin into Equation (1) and subtracting the pre-infusion hemoglobin content, U0, gives the number of blood units spared. These manipulations give:
Graphs of Equations (2) and (3) were generated using SigmaPlot® 9.0. Clinical data gleaned from published sources was added to plots with the assumptions noted in the Results and Discussion sections.
RESULTS
Blood sparing was evaluated as a function of variation in HBOC circulating half-life (t1/2) over the range of 0.5 to 2.0 days, enhanced erythropoesis varying from 0.1 to 0.3 units/day, and HBOC doses of one to ten units (). Blood sparing increases with longer HBOC circulating half-life and as the rate of enhanced erythropoesis increases. Blood sparing also increases as the dose of HBOC increases, although the incremental improvement in blood sparing diminishes as the dose of HBOC is raised. Due to the fact that half-life and rate of enhanced erythropoesis always occur as a product in the equation describing the number of blood units spared, the same degree of blood conservation may be achieved when these two parameters are varied reciprocally.
Figure 2 Comparison of data from clinical trials in surgical patients treated with HBOC solutions with mathematical model predictions. Model predictions for blood sparing are graphed as solid lines versus dose of HBOC for assumed half-lives and rates of erythropoesis enhancement noted to the right of each line. The first value in parentheses is the HBOC half-life in days. The second value is the rate of erythropoesis enhancement in blood units per day. Clinical data are plotted from published studies as noted in the text. Data from Biopure Corporation are plotted as dosing ranges with the data points placed in the range midpoint. The number of patients included in each trial data set is denoted by n.
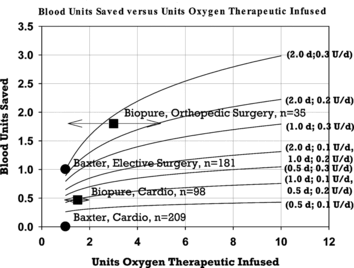
In order to compare the theoretical predictions of this model with actual clinical data, several results taken from the literature have also been plotted (). Data were only plotted from published work in which an average degree of blood sparing was reported or could be calculated. In the case of data derived from studies sponsored by Baxter Healthcare Corporation, an average value for HBOC dose could also be calculated, whereas only ranges could be determined for data derived from clinical trials sponsored by Biopure Corporation. In the latter case, data points were placed in the center of the range of possible HBOC doses.
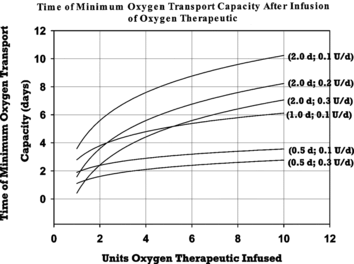
The time after HBOC treatment at which total circulating hemoglobin content reaches a minimum was found to vary from less than one day to over ten days within the range of parameter variation studied in this exercise (). This nadir occurs later as the HBOC circulating half-life becomes more prolonged. Increasing the dose of HBOC also increases the time interval between treatment and the total hemoglobin minimum. Increases in the rate of enhanced erythropoesis have the opposite effect, resulting in a minimum in circulating hemoglobin content that occurs more rapidly after treatment.
Figure 3 Variation in time after HBOC treatment at which total blood hemoglobin concentration attains a minimum value. Minimum points are plotted as a function of HBOC dose for HBOC half-lives and rates of erythropoesis enhancement noted at the right of each line. The notation for HBOC half-life and erythropoesis enhancement is as described in the caption of Figure 2.
DISCUSSION
The assumptions used in deriving the equations used in this paper were taken to be the simplest set of suppositions that could reasonably be expected to predict key aspects of the degree of blood sparing that would be observed in selected surgical patient populations suffering blood loss that is controlled and then treated by the infusion of an HBOC solution. While mathematical convenience drives the tacit assumption of a homogeneous patient population that is never realized in actual clinical practice, it is believed that the model is predictive of the average behavior observed in relatively well defined populations of patients.
With regard to more specific assumptions, the modeling of HBOC vascular pharmacokinetics as an exponential decay is consistent with the empirical observations made for some, but not all, hemoglobin solutions infused into animals and humans [Citation[8-14]]. For the purposes of this exercise the HBOC half-life is taken to be the half-life of functional hemoglobin in the circulation. Oxidation of hemoglobin to the inactive met form is observed after infusion into mammals [Citation[13], Citation[15]], but this process does not appear to have a profound impact on the assumption of exponential decay kinetics, but rather results in an effective decrease in the functional half-life relative to the circulating half-life of plasma hemoglobin. The range of HBOC half-lives chosen for evaluation in this exercise encompasses the range of values reported in the literature for the current generation of HBOC solutions in humans and large animals [Citation[10], Citation[13-16]].
Perhaps more problematic is the assumption of a constant rate of excess red cell production for up to ten days after HBOC infusion. Clearly, this assumption becomes false at some point since patients do not generate excess red blood cells indefinitely. On the other hand, it is known that most patients suffering blood loss do replenish lost red blood cells as a result of an increase in erythropoesis, which can be enhanced by several-fold above homeostatic baseline values [Citation[17]]. In this modeling exercise the author chose to utilize the simplest approximation that may hold for a time after HBOC infusion. No long–term blood sparing will be observed in the absence of some period of enhanced erythropoesis since the total circulating hemoglobin content will return to the pre-treatment value as the HBOC solution is eliminated from the circulation. The same is also true after blood transfusion, albeit with a more prolonged period of hemoglobin elevation. The time period during which erythropoesis must remain enhanced above replacement values to support the predicted level of blood sparing will vary depending on the circulating half-life of the HBOC dose and the degree of assumed erythropoesis enhancement. For example, for HBOC doses less than four blood equivalent units, circulating half-lives of 24 hours or less, and erythropoesis enhancement of 0.2 units/day or greater, the rate of assumed erythropoesis enhancement need only be maintained for 4–5 days at this level in order for the model predictions to hold.
Another assumption underlying this analysis is that the total oxygen carrying capacity present in circulation is of paramount importance with respect to the need for blood transfusion. Changes in vascular volume are not explicitly considered. It is recognized that maintenance of vascular volume at appropriate levels is critical for tissue perfusion and that fluid perfusion may be used to compensate for low to moderate blood loss in many patients. On the other hand, each patient has a critical point at which oxygen delivery becomes limiting to metabolism even in the face of adequate vascular volume [Citation[18]]. At this point the oxygen carrying capacity of the blood must be augmented to maintain health, and indeed good clinical practice is directed to avoiding putting patients in critical oxygen debt. While the factors that determine the critical oxygen delivery threshold are complex and vary from patient to patient, it is assumed that the clinical transfusion decisions ultimately relate to the maintenance of at least a minimal level of overall oxygen transport capacity in the circulation that is measurable, at least by clinical indices that are used to determine transfusion decisions. It should be noted that because HBOC solutions have vasoactive properties, volume expansion properties, and oxygen delivery properties very different from those of red blood cells, some of the indices that have been historically used to determine the need for blood transfusion may not be applicable after HBOC infusion [Citation[5], Citation[16], Citation[19-21]]. In the mathematical model described in this publication, it is assumed that, regardless of the criteria used to determine the initial patient need for blood transfusion, these criteria ultimately relate to a fundamental need to increase the circulating oxygen transport capacity. Furthermore, it is assumed that patients should be maintained at a total oxygen transport capacity above the level that triggered the initial transfusion. Insofar as an HBOC infusion, coupled with enhanced erythropoesis, prevent the total oxygen carrying capacity from decreasing to the pretreatment levels, the need for blood transfusion is reduced. In light of the fact that the non–oxidized (i.e., non–met) hemoglobin contained in current generation HBOC solutions can bind and release the same amount of oxygen on a gram for gram basis as the hemoglobin contained in human red blood cells, a gram for gram equivalence between HBOC solution hemoglobin and red cell hemoglobin is assumed. Although it has been claimed that some HBOC solutions are more efficacious in oxygen delivery than blood in certain situations [Citation[22]], it has yet to be demonstrated that such claims are generally applicable to typical human surgical patients. If such demonstrations are forthcoming, the model can accommodate this fact by a straightforward alteration in the unit equivalency assumption. Furthermore, since no assumptions are made with respect to the chemical or physical nature of the oxygen transporting medium, this model could also be used to predict the degree of blood sparing to be expected from the use of perfluorocarbon emulsions if an appropriate equivalency factor with respect to oxygen carrying capacity between blood and perfluorocarbon emulsions can be identified.
With respect to the predictions of the model, it was noted above that the degree of blood sparing increases as the HBOC dose is increased, although the incremental improvement in blood sparing diminishes as the HBOC dose is increased. Blood sparing also increases as half-life and rate of erythropoesis enhancement are increased. Examination of these predictions suggests that an average reduction in required blood transfusion of one to two units is achievable with current technology at HBOC doses of one to four units. This implies that the use of HBOC solutions to treat patient populations that currently receive an average of less than three units of blood should result in a substantial number of those patients completely avoiding the need for blood transfusion. Patients who lose a large volume of blood will probably ultimately need to receive a blood transfusion unless repeat HBOC dosing over a prolonged period of time is feasible.
These predictions are consistent with the results obtained from some of the clinical trials performed with HBOCs. In two small clinical studies performed in cardiac surgery patients and vascular, orthopedic and abdominal surgery patients using diaspirin crosslinked hemoglobin (DCLHb) from Baxter Healthcare Corporation, blood sparing and the percentage of patients completely avoiding blood transfusion were assessed after treatment with an average of one blood unit equivalent of HBOC solution [Citation[5], Citation[6]]. In both trials, patients were randomized to receive either HBOC or blood transfusion after surgery when a need for blood transfusion was established. HBOC transfusions could be given over a 24-hour period following randomization, but for the purposes of comparison to the model it was assumed that the total dosage was given as a single bolus infusion. No overall blood sparing was reported for HBOC treated patients in the cardiac surgery study despite the fact that 19% of these patients completely avoided blood transfusion during their course of treatment. One would expect there to be a correlation between these two parameters. This discrepancy may be a result of post treatment bleeding episodes in a few treated patients unrelated to HBOC infusion and/or an adverse effect of HBOC treatment that influenced blood transfusion. However, with respect to the latter possibility, Lamy et al. note that DCLHb treated and control patients received the same amount of non red cell blood products calculated on both a daily and cumulative basis [Citation[5]]. These authors also note that varying transfusion practices among the participating clinical sites might have contributed to the lack of overall reduction in blood transfusion in the treated patient group. In particular, the desire among some clinicians to maintain the total blood hemoglobin concentration ≥ 10 g/dl despite recommendations and recent clinical results that suggest that maintenance of patient hemoglobin values at this level may be unnecessary, and even detrimental, could have contributed to the lack of blood sparing [Citation[23-25]]. These authors go on to note that adherence to a 10 g/dl target could be particularly problematic after treatment with DCLHb solutions since the volume expansion properties of this formulation result in a smaller increase in blood hemoglobin concentration on a gram for gram basis than treatment with red cell containing preparations.
In the clinical trial with vascular, orthopedic and abdominal surgery patients, an average reduction in blood usage of 1.0 unit was observed, with 24% of the DCLHb treated patients completely avoiding blood transfusion by Day 7 after surgery [Citation[6]]. In a companion paper derived from the same set of patients, a 10–hour harmonic mean half-life of DCLHb in plasma was reported [Citation[15]]. The degree of blood sparing observed in this study is higher than that predicted by the model for an HBOC with a 10–hour half-life and an enhancement of erythropoesis of up to three-fold. In fact, the model would require an unreasonably high enhancement of erythropoesis in excess of ten-fold to explain the observed degree of blood sparing, suggesting that factors other than those encompassed within the model may be influencing the clinical outcome. One such factor may be the ability of patients to engage other mechanisms of adaptation to anemia due to surgical blood loss over the several day time period that they are partially supported by the presence of the HBOC. The difference in blood sparing observed in this study compared to the cardiac trial may be due to differences in the response of these two patient populations to surgical blood loss and/or HBOC treatment, or some other aspect of the trial protocol or implementation.
Data with respect to blood sparing in human patients treated with the modified bovine hemoglobin solution HBOC-201 have also been reported [Citation[3], Citation[4]]. As was the case with the studies with DCLHb, patients were treated with HBOC-201 after surgery and randomization following the determination that a transfusion was indicated. However, the reported data do not allow for a calculation of the average total HBOC dose. Therefore, for comparison to model predictions, data points were plotted in the center of the range of possible dosing values (). This comparison also assumes that the model predictions are valid for these patient populations even though dosing occurred over a three to six day time period. In a double-blinded study performed with cardiac surgery patients, Levy et al. reported an average reduction in blood transfusion of 0.47 units (from 2.19 to 1.72 units) when one to two blood unit equivalents of HBOC-201 hemoglobin were infused during the 72–hour period following the initial transfusion decision. Total blood transfusion avoidance was achieved in 34% of the treated patients. The plasma half-life of HBOC-201 was reported to be 24 hours. The observed degree of blood sparing in this patient population is consistent with that predicted by the model for patients exhibiting a doubling of erythropoesis during the several day period post treatment. In 19 orthopedic surgery patients treated with one to five blood unit equivalents of HBOC-201, Jahr reported an average blood transfusion reduction of 1.8 units (3.1±0.4 to 1.3±0.3 units) when compared to the control group of 16 patients treated at the same site. Treatment occurred over a six–day time period and the observed difference was statistically significant. Total avoidance of blood transfusion was observed in 47% of the treated patients. This report was addressed to a small subset of a much larger multicenter clinical trial and it is not known whether the published results are typical of the study as a whole. The reported degree of blood sparing is somewhat higher than that predicted by the model, but the repetitive dosing of HBOC-201 over a six–day time interval may have effectively mimicked the pharmacokinetics of a product with a half-life significantly greater than 24 hours. In addition, as noted above for surgical patients treated with DCLHb, adaptation to anemia during the treatment period may have reduced the clinical need for blood transfusion. The larger degree of blood sparing observed in studies with HBOC-201 compared to DCLHb would be expected based on the higher doses utilized in studies with the former, as well as a longer reported circulating half-life for HBOC-201. It is interesting to note that higher blood sparing was again observed in orthopedic patients compared to cardiac surgery patients with HBOC-201, although much higher doses of HBOC-201 were permitted in the orthopedic surgery study.
Another aspect of the use of HBOC solutions of some clinical importance is the time after treatment at which the total hemoglobin concentration reaches a minimum. The results of the model calculations imply that this nadir varies with dose, HBOC half-life, and degree of erythropoesis enhancement, ranging from one to ten days after treatment over the parameter values assessed in this study. This may prove to be an important clinical monitoring consideration when high doses of relatively long-lived HBOC solutions are used to treat patients.
In summary, the mathematical model presented in this publication suggests that the use of current generation HBOC solutions should indeed result in an overall reduction in blood usage when used to treat surgical patients post-operatively. Greater or lesser degrees of blood sparing may be observed clinically insofar as the assumptions of the model are valid. Greater degrees of blood sparing may be observed than predicted if patients can engage other mechanisms to adapt to anemia and/or if the use of HBOC solutions enhances erythropoesis over and above the usual surgical response. Greater blood sparing may also be observed if for some reason the blood equivalency ratio assumed in this study is too conservative. It also suspected that the repeated dosing of patients with HBOC solutions over a multiple day time period may result in more blood sparing than predicted by the results presented in the preceding, although this more complex scenario has not been modeled in this study. On the other hand, less blood sparing may be observed if the use of HBOC solutions has adverse effects that contravene the beneficial effects of their oxygen transport properties such as inducing a greater susceptibility to bleeding or compromising the perfusion of critical tissues. Perhaps the most significant conclusion from this study is the prediction that some degree of long–term blood sparing should be expected from the appropriate use of HBOC solutions in the treatment of blood loss despite the relatively short intravascular persistence of these formulations relative to red blood cells. In light of these characteristics, and the fact the ultimate level of blood loss in specific surgical patients is often unpredictable, the results of this modeling exercise, as well as the clinical data obtained with HBOC solutions, imply that the best use of HBOC solutions would be as a first line treatment for hemorrhage, thus preserving red blood cell preparations for the treatment of high blood loss patients. Such usage would also take advantage of the fact that HBOC solutions do not require patient blood typing or crossmatching before administration.
REFERENCES
- Fratantoni, J.C. (1994). Points to consider on efficacy evaluation of hemoglobin- and perflurocarbon-based oxygen carriers. Transfusion 34: 712–713.
- Stehling, L.C., Doherty, D.C., Faust, R.J. (1996). Practice guidelines for blood component therapy: A report by the American Society of Anesthesiologists Task Force on Blood Component Therapy. Anesthesiology 84: 732–747.
- Levy, J.H., Goodnough, L.T., Grelich, P.E., Parr, G.V.S., Stewart, R.W., Gratz, I., Wahr, J., Williams, J., Comunale, M.E., Doblar, D., Silvay, G., Cohen, M., Jahr, J.S., Vlahakes, G.J. (2002). Polymerized bovine hemoglobin solution as a replacement for allogeneic red blood cell tranfusion after cardiac surgery: Results of a randomized, double-blinded trial. J. Thorac. Cardiovasc. Surg. 124: 35–42.
- Jahr, J.S. (2002). A Novel Blood Substitute: Use of HBOC-201 (Hemopure®) to Decrease Need for RBCs: Results of Pivotal Trial in Orthopedic Surgery Patients, paper presentation to Society of Critical Care Medicine meeting, San Diego, CA.
- Lamy, M.L., Daily, E.K., Brichant, J.-F., Larbuisson, R.P., Demeyere, R.H., Vandermeersch, E.A., Lehot, J.-J., Parsloe, M.R., Berridge, J.C., Sinclair, C.J., Baron, J.-F., Przybelski, R.J. (2000). Randomized trial of diaspirin cross-linked hemoglobin solution as an alternative to blood transfusion after cardiac surgery. The DCLHb Cardiac Surgery Trial Collaborative Group. Anesthesiology 92: 646–656.
- Schubert, A., Przybelski, R.J., Eidt, J.F., Lasky, L.C., Marks, K.E., Karafa, M., Novick, A.C., O'Hara, J.F. Jr., Saunders, M.E., Blue, J.W., Tetzlaff, J.E., Mascha, E. (2003). Diaspirin-crosslinked hemoglobin reduces blood transfusion in noncardiac surgery: A multicenter, randomized, controlled, double-blinded trial. Anesth. Analg. 97(2): 323–332.
- Lee, R., Neya, K., Svizzero, T.A., Vlahakes, G.J. (1995). Limitations of the efficacy of hemoglobin-basaed oxygen-carrying solutions. J. Appl. Physiol. 79(1): 236–242.
- DeVenuto, F., Friedman, H.I., Neville, J.R., Peck, C.C. (1979). Appraisal of hemoglobin as a blood substitute. Surg. Gynecol. Obstet. 149: 417–436.
- Bleeker, W.K., van der Plas, J., Feitsma, R.I.J., Agterberg, J., Rigter, G., de Vries-van Rossen, A., Pauwels, E.K.J., Bakker, J.C. (1989). In vivo distribution and elimination of hemoglobin modified by intramolecular cross-linking with 2-nor-formylpyridoxal 5′-phosphate. J. Lab. Clin. Med. 113: 151–161.
- Lenz, G., Junger, H., Schneider, M., Kothe, N., Lissner, R., Prince, A. (1991). Elimination of pyridoxylated polyhemoglobin after partial exchange transfusion in chimpanzees. Biomat. Art Cells Immob. Biotech. 19(4): 699–708.
- Boswell, G., Rodkey, W.G. (1983). Pharmacokinetics of hemoglobin infusion. Progr. Clin. Biol. Res. 122: 139–147.
- Migita, R., Gonzales, A., Gonzales, M.L., Vandegriff, K.D., Winslow, R.M. (1997). Blood volume and cardiac index in rate after exchange transfusion with hemoglobin-based oxygen carriers. J. Appl. Physiol. 82(5): 1995–2002.
- Wicks, D., Wong, L.T., Sandhu, R., Stewart, R.K., Biro, G.P. (2003). The intravascular persistance and methemoglobin formation of Hemolink (hemoglobin raffimer) in dogs. Art. Cells Blood Substit. Biotechnol. 31: 1–17.
- Pearce, L.B., Rentko, V.T., Moon-Massat, P.F., Gawryl, M.S. (2003). Comparative pharmacokinetics of a hemoglobin-based oxygen carrier. Academic Emergency Medicine 10(5): 557–558.
- O'Hara, J.F., Colburn, W.A., Tetzlaff, J.E., Novick, A.C., Angermeier, K.W., Schubert, A. (2001). Hemoglobin and methemoglobin concentrations after large-dose infusions of diasprin cross-linked hemoglobin. Anesth. Analg. 92: 44–48.
- Hughes, G.S., Antal, E.J., Locker, P.K., Francom, S.F., Adams, W.J., Jacobs, E.E. (1996). Physiology and pharmacokinetics of a novel hemoglobin-based oxygen carrier in humans. Crit. Care Med. 24: 756–764.
- Goodnough, L.T., Skikne, B., Brugnara, C. (2000). Erythropoietin, iron, and erythropoiesis. Blood 96(3): 823–833.
- Van der Linden, P. (1998). Anemic hypoxia, in Tissue Oxygenation in Acute Medicine, W.J. Sibbald, K. Messmer, M.P. Fink, Eds., Springer: New York, pp. 116–127.
- Posner, L.P., Moon, P.F., Bliss, S.P., Gleed, R.D., Erb, H.N. (2003). Colloid osmotic pressure after hemorrhage and replenishment with Oxyglobin solution, hetastarch, or whole blood in pregnant sheep. Vet. Anaesth. Analg. 30: 30–36.
- Fischer, S.R., Burnet, M., Traber, D.L., Prough, D.S., Kramer, G.C. (1999). Plasma volume expansion with solutions of hemoglobin, albumin, and Ringer lactate in sheep. Am. J. Physiol. 276 ( Heart Circ. Physiol., 45): H2194–H2203.
- Vane, L.A., Funston, J.S., Kirschner, D.H., Harpter, D., Deyo, D.J., Traber, D.L., Traber, L.L., Kramer, G.C. (2002). Comparison of tranfusion with DCLHb or pRBCs for treatment of intraoperative anemia in sheep. J. Appl. Physiol. 92: 343–353.
- Standl, T., Horn, P., Wilhelm, S., Greim, C., Freitag, M., Freitag, U., Sputtek, A., Jacobs, E., am Esch, J.S. (1996). Bovine haemoglobin is more potent than autologous red blood cells in restoring muscular tissue oxygenation after profound isovolaemic haemodilution in dogs. Can. J. Anaesth. 43(7): 714–723.
- Wedgewood, J.J., Thomas, J.G. (1996). Peri-operative haemoglobin: An overview of current opinion regarding the acceptable level of haemoglobin in the peri-operative period. Eur. J. Anaesthesiol. 13: 316–324.
- Goodnough, L.T., Despotis, G.J., Hogue, C.W., Fergueson, T.B. (1995). On the need for improved transfusion indicators in cardiac surgery. Ann. Thorac. Surg. 60: 473–480.
- Herbert, P.C., Wells, G., Blajchman, M.A., Marshall, J., Martin, C., Pagliarello, G., Tweeddale, M., Schweitzer, I., Yetsir, E. (1999). A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators, Canandian Critical Care Trials Group. New Engl. J. Med. 340: 409–417.
