Abstract
The PEGylation that adds an extension arm on protein amino groups with the conservation of their positive charge masks the A and D antigens of erythrocytes efficiently. In the present study, the efficiency of masking the antigens of RBC by PEGylation protocols that do not conserve the charge with and without adding extension arms is compared. The conjugation of PEG-5000 to RBCs through the addition of extension arms masked the D antigen more efficiently than the other protocol. A combination of PEG-5 K and PEG-20 K is needed to mask the A antigen, irrespective of the PEGylation approach. The oxygen affinity of the PEGylated RBCs increased by the extension arm facilitated PEGylation. The protocol involving the conjugation of PEG-chains without adding extension arm did not alter the oxygen affinity of RBCs. A combination of PEGylation protocols is an alternate strategy to generate universal red blood cells with good levels of oxygen affinity.
INTRODUCTION
Masking of blood group antigens from their antibodies by conjugating bulky PEG chains to erythrocyte membrane proteins is an approach that is being investigated in recent years to generate universal red blood cells for transfusion [Citation[1-8]]. Red blood cells of group O Rh(D)− are considered as universal erythrocytes. Accordingly, in order to generate universal red blood cells it is essential to mask the A, B and D antigens of RBCs. In addition to masking the blood group antigens of RBCs, it is essential to ensure that the PEGylation protocols chosen are such that they endow the RBCs enough coverage of the antigens while maintaining a satisfactory level of functional/biochemical properties to be useful for transfusion applications.
We have developed “Extension Arm Facilitated PEGylation” to increase the accessibility of the functional groups at the surface of proteins to macromolecular PEG reagents [Citation[9]]. In this protocol, an extension arm carrying a thiol group at the distal end as the targeted sites for conjugating the PEG-chains of desired molecular size using maleimide PEG is engineered on the amino groups of proteins by reaction with 2-iminothiolane (, Scheme 1). We have employed this thiolation mediated PEGylation protocol to mask the blood group antigens of RBC [Citation[10]]. This protocol has been optimized to mask the A, B, D and CE antigens of RBC. The masking of these antigens appears to be much more efficient than that seen with the PEGylation using cyanuric chloride PEG. This approach has been reported to be the most efficient of all the PEG-reagents tried earlier in the PEGylation studies to mask the antigens [Citation[3], Citation[4], Citation[8]].
Figure 1 Schematic representation of Protocols used for PEGylation of RBC. Scheme 1: Iminothiolane-thiolation mediated PEGylation. Iminothiolane reacts with amino groups generating thiol groups in situ. These thiols are modified by maleimide-PEG (Mal-Phe-PEG). Scheme 2: DTSSP-thiolation mediated PEGylation. DTSSP is a cross-linking reagent and uses acylation chemistry to react with amino groups. On treatment with reducing agents, the S–S bond generates free thiols that can be modified by Mal-Phe-PEG. Scheme 3: Acylation chemistry mediated PEGylation. Succinimidyl propionate-PEG (SPA-PEG) acylates amino groups of proteins. In schemes 1 and 2 an extension arm is added on protein amino groups for PEGylation and in scheme 3 PEGylation of proteins is carried out without adding extension arm.
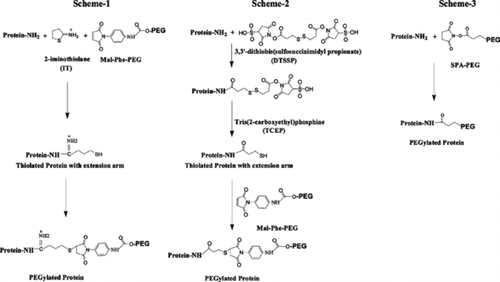
In the thiolation mediated PEGylation protocol described above, the positively charged ε-amino groups of proteins are converted to substituted amidines (amidination) and hence, the net positive charge of the protein is conserved. In the present study, we have compared the efficiency of masking the RBC antigens and the influence of the PEGylation of the RBCs on their oxygen affinity by two other PEGylation protocols wherein the positive charge of the protein amino group is neutralized. Like the iminothiolane based thiolation mediated PEGylation, the first protocol also involves maleimide chemistry based PEGylation mediated through the addition of an extension arm on the amino groups that carry a thiol group at its distal end. However, the extension arm is added using acylation chemistry and hence thiolation reaction results in the neutralization of the charge of the amino groups derivatized. In this new modified protocol of the thiolation mediated PEGylation, the thiolation reagent IT has been replaced by 3,3′-dithiobis(sulfosuccinimidyl propionate) (DTSSP). DTSSP links to protein amino group through an isopeptide linkage to add a γ-mercapto propionyl moiety as an extension arm on the ε-amino groups of lysine residues (, Scheme 2). The formation of isopeptide bond neutralizes the positive charge on the lysine residues. The thiol groups at the distal end of the extension arm are derivatized by Mal-Phe-PEG reagent just as in the earlier iminothiolane based thiolation mediated PEGylation protocol. The second protocol is also based on acylation chemistry, but without the introduction of the extension arm and results in the neutralization of the positive charge of the protein amino groups. Thus, in this case, the PEG chains are conjugated directly on the protein amino groups without involving an extension arm. In this protocol, succinimidyl propionate-PEG (SPA-PEG) was used to conjugate PEG-chains to RBC (, Scheme 3). The oxygen affinity of these PEGylated RBCs was determined to evaluate the primary function of these RBCs. A comparison of the blood group antigen masking efficiency of these protocols and the influence of PEGylation on the oxygen affinity of the RBCs clearly establish the beneficial effects of the extension arms engineered on to the membrane proteins.
MATERIALS AND METHODS
All Mal-Phe-PEG reagents were synthesized as described earlier [Citation[11]]. SPA-PEG reagents were purchased from Nektar Therapeutics (San Carlos, CA). DTSSP and IT were purchased from Pierce (Rockford, IL). Blood typing monoclonal, IgG type antisera were purchased from ImmucorGamma (Norcross, GA). Rabbit anti-mouse IgG-FITC was obtained from Sigma Chemical CO. (St. Louis, MO). Blood samples from healthy volunteers were collected in heparinized tubes. The red blood cells were washed at least three times with the incubation buffer (50 mM sodium phosphate, 105 mM sodium chloride, pH 7.4) by centrifugation at 3000 rpm.
Thiolation Mediated PEGylation of RBCs Using DTSSP and Mal-Phe-PEG
A Rh(D) + RBCs (at 5% hematocrit in the incubation buffer) were incubated with 5 mM DTSSP at room temperature for 1 h. After washing off the excess reagent the cells were re-incubated with 20 mM Tris(2-carboxyethyl)phosphine (TCEP) for 30 min in the same buffer at room temperature. The cells were washed to remove excess TCEP, incubated with 15 mM Mal-Phe-PEG-5000 for 2 h at room temperature and washed again to remove excess reagents. A second step DTSSP mediated PEGylation of the RBCs was also carried out in the same way as the 1st step PEGylation, except that 10 mM Mal-Phe-PEG-20000 was used in place of Mal-Phe-PEG-5K.
Thiolation Mediated PEGylation of RBCs Using IT and Mal-Phe-PEG
A Rh(D) + RBCs (5% hematocrit) were incubated with 20 mM IT and 15 mM Mal-Phe-PEG-5000 at room temperature for 2 h in incubation buffer. After incubation the cells were washed for three times with the same buffer. In the second step modification, the PEGylated cells were incubated with 10 mM Mal-Phe-PEG-20000 in the presence of 20 mM IT.
PEGylation of RBCs with SPA-PEG
A Rh(D) + RBCs (5%) were reacted with 10 mM SPA-PEG-5000 for 2 h at room temperature in the incubation buffer and washed with the same buffer. The second step PEGylation involved 10 mM SPA-PEG-20000.
Blood Typing
RBCs were tested for blockage of the blood group antigens by PEGylation by the standard blood typing test using antibodies against the respective antigens. Agglutination was evaluated at the macroscopic level using an agglutination viewer (Beckton Dickinson, San Jose, CA). Samples that gave negative results for agglutination at the macroscopic level were examined under light microscope (Nikon Eclipse 50i, Morrell Instrument CO. Inc., Melville, NY) for further confirmation. Images were collected at 10 × 0.30 lens.
Oxygen Affinity Studies
The oxygen affinity of the PEGylated RBCs was determined in the incubation buffer using Hem-O-Scan, at 37°C.
RESULTS
Masking of the D Antigen of RBCs from the Respective Antibodies by Different PEG-5000 Chains
Group A Rh(D) + RBCs PEGylated with Mal-Phe-PEG-5000 after linking γ-mercapto propionyl moieties on to the ε-amino groups of Lys residues of membrane proteins via DTSSP mediated thiolation did not agglutinate in the presence of anti-D, indicating that Mal-Phe-PEG-5000 chains could mask the D antigen sufficient enough to prevent agglutination with the respective antibodies at the microscopic level (, B). These results are comparable to the ones that were obtained using IT as the thiolation agent to introduce the extension arm (, A). RBCs PEGylated with SPA-PEG-5000 agglutinated with anti-D (, C), however, to a lower level than the control unPEGylated cells. Thus, direct conjugation of PEG-chains to the RBC membrane proteins using SPA-PEG-5000 chains did not mask the D antigen as well as that achieved by the conjugation of Mal-Phe-PEG-5000 chains with an intermediate step of engineering extension arms using either IT or DTSSP. It may be noted that the chemistry used for linking the γ-mercapto propionyl moiety to the protein amino groups is the same as the chemistry used to conjugate the PEG-chains to RBCs using SPA-PEG-5000. Apparently, the increased accessibility of the protein sites to the PEG reagent has facilitated a better masking of the D-antigen. However, none of these PEGylation protocols using PEG-5000 chains could block the agglutination of RBCs with anti-A ().
Table 1 Functional and serological properties of PEGylated RBCs
Figure 2 Microscopic evaluation of agglutination of PEGylated RBCs in the presence of anti-D. RBCs are PEGylated by (A) imnothiolane-thiolation mediated PEGylation using Mal-Phe-PEG-5000, (B) DTSSP-based-thiolation mediated PEGylation using Mal-Phe-PEG-5000, and (C) Acylation chemistry mediated PEGylation using SPA-PEG-5000.

Masking of the A Antigen of RBCs Using a Combination of Short and Long PEG-chains
Our previous studies with GroupA Rh(D) + RBCs using IT thiolation mediated PEGylation has shown that effective masking of the A antigens can be achieved using a combination of PEG-5000 and PEG-20000 chains [Citation[10]]. Hence, this stategy was also evaluated with the DTSSP thiolation mediated PEGylation. Group A Rh(D) + RBCs PEGylated with Mal-Phe-PEG-5000 were subjected to a second thiolation step with DTSSP and then PEGylated with Mal-Phe-PEG-20000. These cells did not agglutinate either in the presence of anti-A or of anti-D (). No clumps were detected in these samples in the presence of anti-A or anti-D even under microscope (, C and D, respectively), indicating that optimal coverage of the A and D antigens has been achieved by the extension arm facilitated PEGylation. Again, these results are similar to those where IT was used to engineer the extension arm in the thiolation mediated PEGylation protocol.
Figure 3 Microscopic evaluation of agglutination of PEGylated RBCs in the presence of anti-A (A, C & E) and anti-D (B, D and F). RBCs are PEGylated by (A & B) imnothiolane-thiolation mediated PEGylation using Mal-Phe-PEG-5000 in step-1 and Mal-Phe-PEG-20000 in step-2, (C & D) DTSSP-thiolation mediated PEGylation using Mal-Phe-PEG-5000 in step-1 and Mal-Phe-PEG-20000 in step-2 (E & F) acylation chemistry mediated PEGylation using SPA-PEG-5000 in step-1 and SPA-PEG-20000 in step-2.
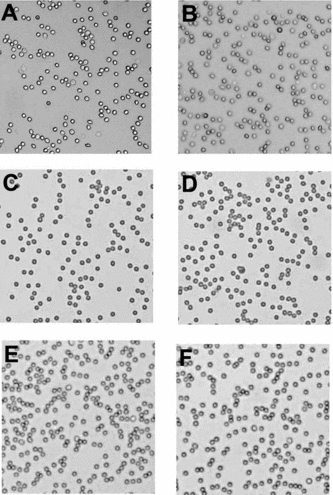
Group A Rh(D) + RBCs PEGylated with SPA-PEG-5000 (without the introduction of extension arm) were also subjected to a second step PEGylation with SPA-PEG-20000. These PEGylated cells showed no signs of agglutination with anti-A or anti-D at macroscopic level (). No clumps were detected in these PEGylated cells under microscope in the presence of anti-A and also anti-D (, E and F, respectively). Thus, although SPA-PEG-5000 could not mask the D antigen efficiently, the second step modification with SPA-PEG-20000 masked the D antigen efficiently. This second step PEGylation also masked the A antigen efficiently. All the three samples of PEGylated RBCs generated by the two-step PEGylation are indistinguishable from one another in terms of masking the D and A antigens as reflected by the agglutination assay.
Influence of PEGylation on the Oxygen Affinity of RBCs
The oxygen affinity of the PEGylated cells has been measured to determine the influence of PEGylation on this property of RBCs. IT based thiolation mediated PEGylation increased the oxygen affinity of RBC (). This increase in the oxygen affinity was very pronounced with the first step of PEGylation that conjugates PEG-5000 to RBC. The second step of PEGylation that conjugates PEG-20000 did not influence the oxygen affinity further.
The influence of DTSSP thiolation mediated PEGylation on the oxygen affinity of RBCs is less pronounced with the first step of PEGylation (conjugation of Mal-Phe-PEG-5000) relative to IT based thiolation mediated PEGylation. These PEGylated RBCs exhibited slightly increased oxygen affinity as compared to the control unPEGylated RBCs (). The second step of this PEGylation reaction (conjugation of PEG-20000 chains) exhibited a much more pronounced influence on the oxygen affinity. RBCs PEGylated with SPA-PEG reagents in both the steps exhibited an oxygen affinity comparable to that of the unmodified RBCs (). Thus, although the PEGylated RBCs generated by the three different chemistries are indistinguishable from one another in terms of masking the D and A antigens, their functional properties are very distinct.
Combination of Two Different PEGylation Protocols to Generate Functional Universal Red Blood Cells
DTSSP based thiolation mediated PEGylation of RBCs with Mal-Phe-PEG-5000 masked the D antigen very effectively, whereas direct conjugation with SPA-PEG-5000 without adding extension arm was less efficient in that respect. However, DTSSP based thiolation mediated PEGylation resulted in a slight increase in the oxygen affinity of RBC, whereas PEGylation with SPA-PEG-5000 did not alter the oxygen affinity of RBC. Furthermore, the PEGylated RBCs maintained normal oxygen affinity even after two steps of PEGylation using SPA-PEG reagents. Accordingly, it was of interest to determine whether the two PEGylation steps of these two different PEGylation protocols (one using the extension arm and the other without using extension arm) are interchangeable. Therefore, a combination of these two protocols; DTSSP based thiolation mediated PEGylation using Mal-Phe-PEG-5000 in the first step and acylation mediated PEGylation with SPA-PEG-20000 in the second step and vice versa has been carried out. One can expect to generate PEGylated RBCs with optimal coverage of antigens with little influence on the oxygen affinity by such combination approaches. Indeed, the PEGylated RBCs generated by modifying first with Mal-Phe-PEG-5000 using DTSSP based thiolation mediated PEGylation and then with SPA-PEG-20000 exhibited an oxygen affinity comparable to that of the RBCs PEGylated by DTSSP based thiolation mediated PEGylation using Mal-Phe-PEG-5000 alone (). However, the strategy of PEGylation protocols employed in the reverse direction; decorating the RBC surface with SPA-PEG-5000 in the first step and with Mal-Phe-PEG-2000 by DTSSP based thiolation mediated PEGylation in the second step increased the oxygen affinity of the RBCs to the level that is comparable to that of RBCs PEGylated by DTSSP based thiolation mediated PEGylation in both the steps (). The masking of the A and D antigens by these combinatorial PEGylation protocols was sufficient enough to inhibit the agglutination of the cells with the respective antibodies at the microscopic level ().
DISCUSSION
Several PEG reagents have been used by various investigators for masking the blood group antigens on RBC from the cognate antibodies with the hope of generating universal red blood cells [Citation[1-8]]. These PEGylation reactions are generally targeted to the amino groups of membrane proteins. It is the general observation that small molecular size PEG reagents, such as PEG-5000, could not mask the Rh system antigens, efficiently [Citation[3], Citation[4]]. The Rh system antigens are multi-pass-transmembrane proteins and the antigenic epitopes reside very close to the cell membrane [Citation[12]]. Therefore, accessibility of the protein amino groups to bulky PEG reagents to efficiently mask the Rh system antigens may be difficult. The fact that the “extension arm facilitated PEGylation” gives a better masking of the Rh antigens suggests that the accessibility of the amino groups of proteins can be enhanced by adding an extension arm on the ε-amino groups. When iminothiolane is used to engineer an extension arm (charge-conservative thiolation), the new thiol group at the distal end of the extension arm is placed approximately at 8.0 Å away from the amino group to which δ-mercapto butirimidyl chain (extension arm) is added. This extension arm provided new sites for PEGylation with maleimide PEG that endow enough protection to the RBC from anti-D in a single step PEGylation using Mal-Phe-PEG-5000 reagent.
The new charge-nonconservative thiolation mediated PEGylation platform that is developed here uses acylation chemistry to link extension arm to the amino groups. γ-mercapto propionyl side chains engineered onto the amino groups in the DTSSP based thiolation chemistry places the new thiol group at about 6.5 Å away from the amino group as the target site for the thiolation mediated PEGylation with the loss of the positive charge at the site of modification. Thus this thiol group is 1.5 Å closer to the amino group than that engineered by iminothiolane. In spite of this shorter chain length of the extension arm, the overall efficiency of this approach to mask the amino groups appears to be comparable to that seen with the use of iminothiolane. This suggests that engineering of the extension arm rather than the chemistry of engineering as the primary molecular aspect that has facilitated the efficient masking of the D-antigen.
The same acylation chemistry is used for the conjugation of the SPA-PEG-5000 chains to RBCs in the PEGylation protocol that does not involve addition of extension arm. This protocol could not mask the D antigen as efficiently as the DTSSP-thiolation mediated PEGylation. Therefore, the more efficient coverage of the D antigen by the DTSSP-thiolation mediated PEGylation as compared to that by SPA-PEG-5000 seems to be due to the introduction of the extension arm (γ-mercapto propionyl chain) on lysine residues.
ABO system antigens are carbohydrate based and are located on the membrane proteins and lipids [Citation[13], Citation[14]]. These antigens are extended more into the solvent phase than the Rh system antigens. Therefore, longer PEG chains are needed to mask these antigens. However, PEGylation of RBCs with PEG-20000 alone induces self aggregation of RBCs [Citation[10]]. This aggregation was prevented by prior PEGylation of RBCs by PEG-5000 before modifying with PEG-20000 reagents. The two-step PEGylation masked the A antigen efficiently without any aggregation. In addition, the second step PEGylation by SPA-PEG-20000 in the protocol without using extension arm also facilitated the masking of the D antigen that was not masked by first step of PEGylation using SPA-PEG 5000.
Group O Rh(D) − RBCs are considered as universal RBCs for emergency conditions. Conversion of Group O Rh(D) + RBCs into RBCs with serological behavior comparable to that of Group O Rh(D) − cells needs masking of the D antigen alone. Based on the results presented here, this can be accomplished by single step PEGylation using the DTSSP-thiolation mediated platform. Since this protocol does not influence the oxygen affinity of RBCs, significantly this protocol could be used to achieve the optimal masking of the D antigen. However, preparation of universal RBCs from Group A Rh(D) + or Group B Rh(D) + RBCs needs covering of the A and D or the B and D antigens, respectively. This needs two-step PEGylation. The DTSSP mediated PEGylation using Mal-Phe-PEG-5000 followed by PEGylation with SPA-PEG-20000 can be used for effective masking of the A, B and D antigens, with only a moderate influence on the oxygen affinity. Alternatively, since PEGylation with SPA-PEG-5000 in the first step and with SPA-PEG-20000 in the second step masked both the A and D antigens efficiently, acylation chemistry PEGylation alone may be used in both the steps in order to preserve the oxygen affinity of the RBCs.
Abbreviations: DTSSP, 3,3′-dithiobis(sulfosuccinimidyl propionate); IT, 2-iminothiolane; Mal-Phe-PEG; maleimide-phenyl-PEG; RBC, red blood cell; SPA-PEG; succinimidyl propionate-PEG; TCEP; tris(2-carboxyethyl)phosphine.
This study was supported by a grant-in aid from the American Heart Association Heritage Affiliate (9951021T), National Institutes of Health grants (HL58247 and HL71064) and Army grant (PR023085).
REFERENCES
- Jeong, S.T., Byun, S.M. (1996). Decreased agglutinability of methoxy-polyethylene glycol attached red blood cells: Significance as a blood subsstitute. Art Cells Blood Subs. and Immob. Biotech. 24: 503–511.
- Scott, M.D., Murad, K.L., Koumpouras, F., Talbot, M., Eaton, J.W. (1997). Chemical camouflage of antigenic determinations: Stealth erythrocytes. Proc. Natl. Acad. Sci. USA 94: 7566–7571.
- Fisher, T.C., Armstrong, J.K. (2001). Red blood cells covalently bound with two different polyethylene glycol derivatives. US Patent 6312685.
- Bradley, A.J., Murad, K.L., Regan, K.L., Scott, M.D. (2002). Biophysical consequences of linker chemistry and polymer size on stealth erythrocytes: Size does matter. Biochim. Biophys. Acta. 1561: 147–158.
- Sabolovic, D., Sestier, C., Perrotin, P., Guillet, R., Tefit, M., Boynard, M. (2000). Covalent binding of polyethylene glycol to the surface of red blood cells as detected and followed up by cell electrophoresis and rheological methods. Electrophoresis 21: 301–306.
- Blackall, D.P., Armstrong, J.K., Meiselman, H.J., Fisher, T.C. (2001). Polyethylene glycol-coated red blood cells fail to bind glycophorin A-specific antibodies and are impervious to invasion by the Plasmodium falciparum malaria parasite. Blood 97: 551–556.
- Bradley, A.J., Test, S.T., Murad, K.L., Mitsuyoshi, J., Scott, M.D. (2001). Interactions of IgM ABO antibodies and complement with methoxy-PEG-modified human RBCs. Transfusion 41: 1225–1233.
- Mathur, S., Clark, B., Castro, G., Zhou, X.M., Bowers, S., Stassinopoulos, A. (2003). Efficient full unit Pegylation of human red blood cells (RBC) using linear methoxy-capped polyethylene glycol (mPEG). Blood 102: 556A.
- Acharya, A.S., Manjula, B.N., Smith, P.K. (1996). Hemoglogin crosslinkers. US Patent 5585484.
- Nacharaju, P., Boctor, F.N., Manjula, B.N., Acharya, S.A. (2004). Surface decoration of red blood cells with maleimidophenyl-polyethylene glycol facilitated by thiolation with iminothiolane: An approach to mask A, B, and D antigens to generate universal red blood cells. Transfusion 45: 374–383.
- Manjula, B.N., Tsai, A., Upadhya, R., Perumalsamy, K., Smith, P.K., Malavalli, A., Vandegriff, K., Winslow, R.M., Intaglietta, M., Prabhakaran, M., Friedman, J.M., Acharya, A.S. (2003). Site-specific PEGylation of hemoglobin at Cys-93(beta): Correlation between the colligative properties of the PEGylated protein and the length of the conjugated PEG chain. Bioconjug. Chem. 14: 464–472.
- Avent, N.D., Reid, M.E. (2003). The Rh blood group system: A review. Blood 95: 375–387.
- King, M.-J. (1994). Blood group antigens on human erythrocytes-distribution, structure and possible functions. Biochim. Biophys. Acta. 1197: 15–44.
- Reid, M.E., Mohandas, N. (2004). Red blood cell blood group antigens: Structure and function. Seminars Hematol. 41: 93–117.
