Abstract
Starting with the work of Klein et al. in the early 1950s, there has been a concerted effort to apply the process of freeze-drying for the preservation of platelets in order to provide hemorrhagic patients with a stable infusible hemostatic agent to stop bleeding. The original attempts did not preserve platelet structural integrity and proved to be of little clinical benefit. However, it was known that fixation by various cross-linking agents rendered platelets able to withstand structurally intact the stresses of lyophilization but with (assumed) complete loss of functionality. Read and coworkers showed that fixed and freeze-dried platelets could respond to ristocetin-induced agglutination, and thus devised a widely accepted assay for von Willebrands factor that demonstrated that reconstituted platelets participated well in this in vitro model of an important interaction in primary hemostasis. This review chronicles the efforts of the authors to refine the fixation process so that the freeze-dried and reconstituted platelets retain fundamental hemostatic properties necessary to stop bleeding. The resultant product has demonstrated correction or reduction of the bleeding times in animal models with platelet deficits including the thrombocytopenic rabbit model of Blajchman and coworkers, a canine cardiopulmonary bypass model of open-heart surgery at East Carolina University (ECU), and a porcine trauma model at The University of North Carolina at Chapel Hill (UNC-CH) involving exsanguination and complete blood exchange with a hemoglobin-based oxygen carrier (HBOC). In addition, it has been shown that the fixation process kills viruses and bacteria spiked into the platelet suspension, indicating that the final material may indeed be the first truly sterile cellular transfusion product. The initial goal for clinical benefit is to prevent exsanguination and hypovolemic shock in combat casualties of armed services personnel, for whom platelet transfusions are most often unavailable. Commercial interests are being brought to bear by Entegrion Inc. (formerly known as Hemocellular Therapeutics Corporation) to transfer this technology to a scaleable manufacturing platform for the production of StasixTM, a pharmaceutical preparation of fixed and freeze-dried platelets for intravenous or topical use in the arrest of active hemorrhage in a wide variety of patients with a platelet-related bleeding diathesis. It has taken fifty + years from the first attempt at making a clinically useful freeze-dried platelet preparation to get to the rapidly-approaching clinical trials of StasixTM; stabilization of the platelets has been the key to realizing this advance.
INTRODUCTION
In 1935, freeze-drying (vacuum lyophilization of a previously frozen material to remove water by sublimation) was applied by Flosdorf [Citation[1]] and others to preservation of biological materials. This same technology became a real lifesaver when applied to the preservation of blood plasma for use as a resuscitation fluid on the battlefields of World War II. The freeze-drying of protein solutions without wholesale denaturation is far from a simple process. However, successful dehydration and rehydration of cells is much more complicated, so it was not too surprising that the first attempts to freeze-dry the cellular elements of blood resulted in loss of their structural integrity and function. In 1955, Klein and coworkers [Citation[2]] reported on their first experiments in testing the procoagulant properties of a freeze-dried preparation made from an extracted platelet suspension. The material was later infused into pediatric leukemia patients with thrombocytopenia in an effort to restore hemostasis [Citation[3], Citation[4]]. The results were controversial. Klein claimed a therapeutic benefit was obtained, stating that the clinical results were “suggestive” of a positive hemostatic effect. Others then tried to reproduce this work with infusion of a similar freeze-dried platelet preparation in a model of irradiated thrombocytopenic rats [Citation[5]] or dogs [Citation[6]], but no reduction in bleeding was observed. Further development of this material was discontinued.
Platelets are responsible for adhering to sites of vascular injury to stop hemorrhage in the primary step of hemostasis. The platelet surface membrane must be intact to accomplish this task efficiently. The lack of convincing efficacy of the Klein preparation was most likely due to structural disintegration of the platelets in their freeze-dried state. However, platelet structure can be well-preserved in a dry vacuum, like in an electron microscope, by appropriate use of cross-linking fixation agents [Citation[7]]. In 1975, Allain and coworkers at UNC-CH demonstrated that platelets cross-linked with 3.6% paraformaldehyde for 48 hours at 4°C retained the ability to agglutinate in the presence of vonWillebrand Factor (vWF) and ristocetin [Citation[8]]. These platelets appeared to be structurally intact under the microscope, but most likely were bereft of metabolic activity and incapable of the release reaction. Far from just being a laboratory curiosity, the fixed platelets became an important reagent in the diagnostic assay for quantitation of vWF activity in clinical testing. Preservation of ristocetin response and binding of vWF indicated an intact GPIb receptor, and suggested that some primary elements of hemostasis might be well supported by fixed platelets.
These preparations of fixed platelets were stable in terms of ristocetin response for several weeks refrigerated. Read and Brinkhous went further and adapted a benchtop freeze-drying protocol to extend the storage of the fixed platelets [Citation[9]]. The dried platelets remained intact and ristocetin response was now preserved for a year or more in the reconstituted product, making this an even more useful diagnostic reagent for the clinical lab. However, the question of their utility as an in vivo hemostatic agent remained untested until 1989 when Read and Bode began to evaluate these preparations further. It was apparent that the platelets as currently processed where incapable of other expressions of platelet responsiveness [unpublished data], and thus refinements of the fixation procedure where investigated to improve native function. The following sections of this review describe the changes in process and their effects as this project progressed to developments with commercial potential.
FIXATION PROTOCOL
After much trial and correction experimentation to target the “lightest” fixation conditions that still maintain cell integrity through the stresses of dehydration/rehydration, we settled on exposure of washed platelets at 1 × 10Citation9/mL to 1.8 g/dL paraformaldehyde at pH = 6.8–7.0 for 45–60 minutes at room temperature, followed by further washing and final bulking in 5g/dL bovine serum albumin. Yields depended somewhat on age of the input platelets and the method of washing platelets out of their native plasma milieu (centrifugation or gel filtration); typically we recovered 50–70% through the wet process steps. Recovery of intact platelets after freeze-drying and reconstitution was 90–100%. One indication of under-fixation was poor recoveries of platelet count and size after rehydration. The main indications of over-fixation were irreversible clumping of platelets during washing after the paraformaldehyde exposure and complete abrogation of metabolic activity in the reconstituted product.
The correctly processed preparation was capable of spreading on contact with glass surfaces albeit slower than fresh platelets [Citation[10]], expressed procoagulant activity that increased upon stimulation with thrombin and/or collagen [Citation[11]], adhered to physiologic thrombogenic surfaces under high shear through GPIb [Citation[12]], generated thromboxanes through inhibitable mechanisms [Citation[12]], and demonstrated intracellular phosphorylation reactions [Citation[13]] or activation neoantigen expression [Citation[12]] upon stimulation. These preparations have not been shown to aggregate macroscopically with agonists other than ristocetin, although the number of singlet platelets decreases with addition of ADP or collagen [data not published], and there is no clot retraction response as measured in classical assays. The real issue, though, was whether infusion of the reconstituted platelets could correct the bleeding time in animal models of platelet dysfunction.
ANIMAL MODELS
The thrombocytopenic rabbit model of Morris Blajchman (McMaster University) has gained worldwide acceptance for testing hemostatic efficacy of platelet preparations, and has the unique feature of supporting xenographic infusion of human platelet materials. Since the mid–1970s, data have been produced in this model for platelets stored under a variety of conditions, or for platelet derivatives or substitutes [Citation[14]]. Our initial studies in 1994 with fixed, freeze-dried platelets in this model produced very encouraging data that showed correction of the bleeding time nearly to the extent as seen with fresh human platelets [Citation[15]]. In this model we also saw that overfixed platelets did not stay in circulation very long and often caused extraordinary stress on the recipient subject [unpublished data]. For platelets fixed 45–60 minutes, the best results were obtained in this model with pools of preparations to reduce variability, suggesting that pooling of materials would be appropriate when dealing with pharmaceutical preparations at larger scale. Concurrently, Marjorie Read demonstrated in her laboratory that these preparations corrected the toenail bleeding times in rats made thrombocytopenic by anti-platelet antiserum [Citation[10]]. The positive results of these two models strongly advanced the notion that the fixed freeze-dried platelets should be developed further as a novel hemostatic agent for arrest of active bleeding in hemorrhagic thrombocytopenic patients, such as those treated in the historical trials conducted by Klein et al. [Citation[3]].
Soon after these initial successes in thrombocytopenic models, the fixed freeze-dried platelets were tested in a surgical setting. The model was one of open-heart surgery in dogs; this was primarily a medical practice regimen at ECU for training of cardiothoracic surgery residents in cardiopulmonary bypass (CPB) procedures. CPB is known to produce a platelet function deficit in human surgical patients [Citation[16]] and did so in these animal subjects as well. Our introduction of fixed freeze-dried canine platelets into this surgery model to correct the abnormal bleeding time caused by CPB was without known precedent but appeared to be a reasonable test of hemostatic efficacy in a clinical setting that is likely to benefit from the availability of a novel hemostatic agent. We infused the lyophilized platelets under a variety of surgical and support conditions in normal canine subjects and found that a large single bolus given just prior to weaning from the CPB pump gave an immediate correction of the bleeding time test that was sustained in continued testing for at least a 3–hour post-op period [Citation[17]]. This persistence of effect was remarkable in that it was not strictly dependant on circulating platelet count. Moreover, the subjects were splenectomized prior to CPB so it is unlikely that endogenous platelets played a role in the sustained hemostasis recovery we observed. In a very limited number of CPB procedures, lyophilized human platelets were introduced in place of lyophilized canine platelets and also produced a correction of the bleeding time, but the effect did not persist for more than 1–2 hours in the post-op phase. Comparison of effectiveness of the lyophilized platelets to fresh canine or human platelets or other preparations has not yet been undertaken.
Other in vivo tests of fixed freeze-dried platelets continued, especially to assess the effects of major changes in platelet washing procedure, or variation of input platelet sources, or for other information on circulation dynamics. Further testing in the Blajchman thrombocytopenic rabbit model demonstrated an effect on the bleeding time even after the infused lyophilized platelets had been in circulation for three hours and the count was as low as 40,000/µL [Citation[18]]. Read and colleagues used infusions of fixed freeze-dried porcine platelets to staunch the exsanguinating bleeding of an injured pig in the UNC von Willebrands animal colony [unpublished]. Tracer studies were performed in normal canine subjects with lyophilized canine platelets labeled with a fluorescent membrane dye to reveal aggregation at sites of intravascular injury but not in the systemic circulation [Citation[10]]. Multiple infusions of lyophilized canine platelets were initiated longterm in two normal canine subjects to see if new antibodies arose; none were observed [Citation[19]].
FURTHER DEVELOPMENT
For a period of time, commercial development of the fixed freeze-dried platelets was pursued by Centeon Corporation (formerly Armour Pharmaceuticals) under a license agreement with ECU and UNC. Their expertise in handling blood components in a GMP environment turned attention to issues of product safety and scale-up. Experiments conducted by Centeon demonstrated that the paraformaldehyde fixation procedure was completely microbicidal for at least eight test viruses spiked at high concentrations into washed platelet suspensions [Citation[17]]. Read and Becker at UNC also found a strong bacteriostatic effect of the fixation with strains relevant to transfusion concerns [Citation[18]]. These findings together were particularly striking because they demonstrated the possibility of creating for the first time a cellular transfusion product that was truly sterile. Centeon's timeline was set for initiating human clinical trials in the year 2000 with their pharmaceutical preparations, but commercial development was halted when their manufacturing facility came under a consent decree due to new safety concerns by the FDA for plasma-derived products in general. The technology license returned to ECU and UNC and further academic development was undertaken.
Fischer then began work with a new animal model in pigs undergoing controlled exsanguination and blood exchange using a hemoglobin-based oxygen carrier (HBOC) in collaboration with Emergency Medicine at UNC. This model offered a unique clinical scenario in which native hemostasis was compromised by hemodilution and hypovolemic shock. When fixed, freeze-dried porcine platelets were introduced in these subjects as the hematocrit neared zero, the bleeding time was corrected and continuing hemorrhage from prior bleeding time tests was arrested [Citation[20]]. The positive results in this model suggested that pharmaceutical preparations of lyophilized platelets would be of use in combination with new HBOC solutions in the resuscitation of trauma patients.
Thus the fixed, freeze-dried platelets showed hemostatic efficacy in animal models representing a broad spectrum of clinical hemorrhage scenarios: (a) excessive bleeding due to thrombocytopenia; (b) abnormal hemostasis caused by CPB in open-heart surgery; (c) excessive bleeding in vonWillebrands disease; and (d) restoration of normal hemostasis in extreme hemodilution resuscitation. It seemed appropriate to pursue further commercial development to bring this promising product to fruition. For this reason, a start-up company named Hemocellular Therapeutics Corporation was incorporated in 2002 as a joint venture by ECU and UNC so that advancement of the fixed freeze-dried platelets to clinical trials could continue.
REMAINING ISSUES
In order to bring any new hemostatic agent into FDA-approved clinical use, safety issues must be addressed in addition to the demonstration of efficacy. Some safety-related information can be inferred already from the foregoing academic research on the fixed, freeze-dried platelets, but Hemocellular developed a plan to specifically assess clinical effects. Preliminary and unpublished findings from their efforts include evidence that HLA antigens are ablated to a large extent by the fixation process. Also, there is no evidence as yet in serum samples from diverse human subgroups for any pre-existing antibodies to novel antigens induced by fixation on the freeze-dried platelets, and there is no detectable formaldehyde remaining in the final product after GMP manufacture. Registration toxicity and thrombogenicity studies have yet to be completed, but no toxic or thrombogenic event has been observed in the > 200 subjects of the several animal models receiving infusions of the correct formulation. If the impending IND submission is approved by the FDA, the first human exposure should be in late 2007.
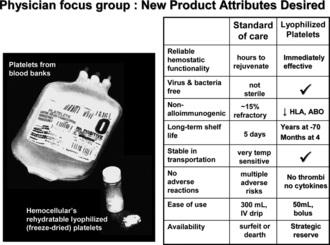
The advantages presented by a freeze-dried cellular hemostatic agent over the current blood bank therapeutic of stored platelet concentrates will be numerous and impressive. Platelet concentrates are not always available for bleeding patients, especially in scenarios involving armed service personnel in field or mass casualty events in civilian trauma, and stored blood bank platelets may not be fully effective in stopping active hemorrhage [Citation[21]]. Blood bank components at present are not guaranteed to be sterile, and are known to have unfavorable immunomodulatory effects [Citation[22]]. Alloimmunization of multiply transfused patients can lead to refractoriness against any beneficial effects of platelet concentrates in preventing spontaneous bleeding. A material that is sterile, immediately effective, non-immunogenic, safe and easy to use in emergent clinical situations to treat the hemorrhagic patient will have a very significant impact on transfusion medicine (). These are the attributes we hope to firmly establish in the fixed, freeze-dried platelets as clinical trials proceed, 50 years since the first recorded clinical use of a lyophilized platelet preparation.
Figure 1 Summary of interests from a survey of physicians for important desired attributes in a new cellular hemostatic agent; comparison of those attributes in standard of care blood bank stored platelet concentrates versus preclinical impressions of the lyophilized platelets. The comments used to describe blood bank concentrates should be regarded as opinions rooted in experiences and/or published statements of respondents in the survey.
The authors would like to recognize the collective contribution of the many scientific collaborators and advisors that have been involved in this project, and specifically thank Dr. Marjorie Read (UNC-CH) for leading this effort in its earlier stages. This work has been most generously supported by The US Office of Naval Research since 1989. The authors also acknowledge the support and continued development efforts of Entegrion Inc. and its investors.
REFERENCES
- Flosdorf, E.W., Mudd, S. (1935). Procedure and apparatus for preservation in “Lyophile” form of serum and other biological substances. J. Immunol. 29: 389–425.
- Klein, E., Farber, S., Freman, G. (1955). Effects of dried fraction from platelets on Ca clotting time of normal plasmas and plasma in thrombocytopenia and hemophilia. Fed Proc. 14: 410–1324.
- Klein, E., Farber, S., Djerassi, I., Toch, R., Freeman, G., Arnold, P. (1956). The preparation and clinical administration of lyophilized platelet material to children with acute leukemia and aplastic anemia. J. Pediatrics 49: 517–522.
- Klein, E., Farber, S., Djorassi, I., Arnold, P. (1956). Hemostasis following the administration of lyophilized platelet material in secondary thrombocytopenia. Sixth Intl. Congr. Hematology 47.
- Fliedner, T.M., Sorensen, D.K., Bond, V.P., Cronkite, E.P., Jackson, D.P., Adamik, E. (1958). Comparing effectiveness of fresh and lyophilized platelets in controlling irradiation hemorrhage in the rat. Proc. Soc. Exp. Biol. 99: 731–733.
- Jackson, D.P., Sorensen, D.K., Cronkite, E.P., Bond, V.P., Fliedner, T.M. (1959). Effectiveness of transfusions of fresh and lyophilized platelets in controlling bleeding due to thrombocytopenia. J. Clin. Invest. 38: 1689–1697.
- Murphy, M.J., Jr. (1972). The shape of blood platelets: An application of lyophilisation and scanning electron microscopy. Thrombos. Diath. Haemorrh. 28: 237–243.
- Allain, J.P., Cooper, H.A., Wagner, R.H., Brinkhous, K.M. (1975). Platelets fixed with paraformaldehyde: A new reagent for assay of von Willebrand Factor and platelet aggregating factor. J. Lab. Clin. Med. 85: 328–335.
- Brinkhous, K.M., Read, M.S. (1978). Preservation of platelet receptors for platelet aggregating factor/von Willebrand Factor by air drying, freezing or lyophilization: New stable platelet preparations for von Willebrand Factor assays. Thromb. Res. 13: 591–597.
- Read, M.S., Reddick, R.L., Bode, A.P., Bellinger, D.A., Nichols, T.C., Taylor, K., Smith, S.V., McMahon, D.K., Griggs, T.R., Brinkhous, K.M. (1995). Preservation of hemostatic and structural properties of rehydrated lyophilized platelets: Potential for long-term storage of dried platelets for transfusion. Proc. Natl. Acad. Sci. 92: 397–401.
- Read, M.S., Sanders, W., Fischer, T.H., Merricks, E.P., Nichols, T.C., Bode, A.P., Russel, K.E., Bellinger, D.A., Reddick, R.L., Khandelwal, G. (2002). Thrombus formation with rehydrated, lyophilized platelets. Hematology 7: 359–369.
- Bode, A.P., Read, M.S., Reddick, R.L. (1999). Activation and adherence of lyophilized human platelets on canine vessel strips in the Baumgartner perfusion chamber. J. Lab. Clin. Med. 133: 200–211.
- Fischer, T.H., Merricks, E.P., Russell, K.E., Raymer, R.A., White, G.C., Bode, A.P., Nichols, T.C., Read, M.S. (2000). Intracellular function in rehydrated, lyophilized platelets. British Journal of Haematology 111: 167–174.
- Blajchman, M.A., Lee, D.H. (1997). The thrombocytopenic rabbit bleeding time model to evaluate the in vivo hemostatic efficacy of platelets and platelet substitutes. Transf. Med. Rev. 11: 95–105.
- Bode, A.P., Blajchman, M.A., Bardossy, L., Read, M.S. (1994). Hemostatic properties of human lyophilized platelets in a thrombocytopenic rabbit model and a simulated bleeding time device. Blood 84(10): Suppl 1, #1840, 464a.
- Woodman, R.C., Harker, L.A. (1990). Bleeding complications associated with cardiopulmonary bypass. Blood 76(9): 1680–1697.
- Bode, A.P., Read, M.S., Summaria, L., Lust, R.M. (1997). Lyophilized platelets: Nearing clinical trials. Platelets 8: 436–437.
- Bode, A.P., Read, M.S. (2000). Lyophilized platelets for transfusion medicine, in Platelet Therapy: Current Status and Future Challenges, Elsevier Science: Amsterdam, pp. 131–167.
- Fischer, T.H., Merricks, E.P., Raymer, R.A., Nichols, T.C., Bode, A.P., Read, M.S. (2000). Multiple infusions of rehydrated, lyophilized platelets does not induce antiplatelet antibodies or thrombocytopenia in a canine model. Blood 96(Suppl): 106b.
- Fischer, T.H., Merricks, E.P., Nichols, T.C., Raymer, R.R., Hayes, P.M., Pearce, L.B., Bode, A.P., Xu, S-Z. (2001). The co-infusion of rehydrated, lyophilized platelets with HBOC-201 for hemostasis in dilutional thrombocytopenia. Blood 98(Suppl): #2250.
- Khuri, S., Healey, N., MacGregor, H., Barnard, M., Szymanski, I., Birjiniuk, V., Michelson, A., Gagono, D., Valeri, C. (1999). Comparison of the effects of transfusions of cryopreserved and liquid-preserved platelets on hemostasis and blood loss after cardiopulmonary bypass. J. Thorac Cardiovasc. Surg. 117: 172–184.
- Spiess, B.D. (2001). Blood transfusion: The silent epidemic. Annals of Thoracic Surgery 72(5): S1832–7.
