ABSTRACT
Background: Impairments in arm function are a common problem in stroke survivors and have a large impact on health-related quality of life (HRQoL). Little is known about the longitudinal relationship between recovery of upper limb strength and changes in HRQoL.
Objectives: This study aimed to determine to what extent changes in HRQoL are related to changes in upper limb strength after discharge from inpatient rehabilitation.
Methods: 250 patients from an RCT were assessed at discharge from inpatient rehabilitation (baseline) and at 12 weeks post-discharge (follow-up). The Stroke Impact Scale was used to measure HRQoL, and the Motricity Index Arm was used to measure upper limb strength. Hierarchical regression analysis was performed to determine the predictive value of upper limb strength on HRQoL, relative to demographic and clinical characteristics. Regression analysis was used to determine the relation between upper limb strength improvement and HRQoL improvement.
Results: Upper limb strength at baseline was a major predictor of HRQoL at follow-up, after accounting for demographic and clinical characteristics (p < .05). Improvement in HRQoL was positively related to improvement in upper limb strength (F(1, 240) = 18.351, p <.0005).
Conclusions: These findings highlight the importance of upper limb strength in HRQoL, as HRQoL is associated with improvement in upper limb strength recovery. Better monitoring of recovery and treatment of upper limb strength during the outpatient rehabilitation period and beyond, i.e. outside the typical time-window of recovery in the first 3 months post-stroke, might contribute to higher quality of life for stroke survivors.
Introduction
Stroke is a major health problem across the world causing complex disability.Citation1 The impact of this common and serious condition on an individual’s life is considerable: physical, psychological, and social consequences can be experienced.Citation2 Citation3–Citation4 Upper limb paresis, a muscle weakness in the affected limb to one side of the body, is one of the most frequently occurring conditions (up to 85% of stroke survivors).Citation5,Citation6 Improvement in upper extremity motor function occurs mainly in the first few months after stroke.Citation7 At 6 months post-stroke, estimates pointed out that some dexterity in the paretic arm is found in 38% of the stroke patients who show no dexterity in the first week post-stroke.Citation7 Citation8–Citation9
Arm function plays a critical role in the performance of daily life activities. Most everyday activities require the use of both hands, and because of this, performance of bimanual activities receives considerable attention in the rehabilitation setting.Citation10 Improved arm and hand function positively contribute to societal participation and (health-related) quality of life.Citation1,Citation10,Citation11 Health-related quality of life (HRQoL) can be defined as an individual’s (or group’s) perceived physical and mental health over time.Citation12 There is a growing body of literature that recognizes that different factors influence HRQoL after stroke.Citation1,Citation4,Citation13,Citation14 A cross-sectional study has shown that the extent of upper limb improvement positively influences a patient’s perception of what activities can be performed, which in turn enhances HRQoL.Citation1 Incomplete motor recovery of the upper and lower extremities has been found to be the strongest predictor of a lower HRQoL in an observational study.Citation4
Whilst some research has been carried out on the association between arm function and HRQoLCitation1,Citation4,Citation13,Citation14, there has been no longitudinal investigation of improvement in HRQoL in relation to improvement in arm recovery. Obtaining insights into this relationship will be useful for understanding problems faced by patients and for planning and optimization of rehabilitation treatment after stroke.
The first aim of the present study was to identify the relation between upper limb strength and HRQoL at discharge from inpatient rehabilitation and at follow-up (12 weeks later). Second, we aimed to determine whether upper limb strength at discharge from inpatient rehabilitation predicts HRQoL at follow-up, when corrected for patient and stroke characteristics. Third, we aimed to determine whether a change in upper limb strength is related to a change in HRQoL over time. We hypothesized that an improvement in upper limb strength is positively related to an improvement in HRQoL.
Methods
Data for this study were collected during the FIT-stroke trial.Citation15 The FIT-Stroke trial is a stratified, multicentre single-blinded randomized controlled trial to investigate the effectiveness of task-oriented circuit class training. Stroke patients were recruited in nine outpatient rehabilitation centers in the Netherlands, between June 2008 and December 2010. All included patients completed an inpatient rehabilitation period (average of 3 months) and were included at the start of their outpatient rehabilitation period. The inpatient rehabilitation period consisted of a multidisciplinary approach to reach complex (physical and cognitive) rehabilitation goals. The patients were treated by nurses, physical therapists, occupational therapists, speech therapists, social workers, and psychologists. Outcomes from assessments at baseline (start outpatient rehabilitation period) and 12 weeks after randomization were used in this study. Full details of the FIT-Stroke trial have been reported elsewhere.Citation15
The FIT-Stroke protocol has been approved by the Medical Ethics Committee of the University Medical Center Utrecht and all the participating rehabilitation centers. The trial is registered in the Dutch Trial Register (NTR1534). The content of this manuscript conforms to STROBE guidelines.Citation16
Participants
For inclusion, patients were eligible if they 1) had a verified stroke according to the WHO definition; 2) were discharged from a rehabilitation center; 3) needed to continue physiotherapy during outpatient care to improve walking competency or physical condition, or both; and 4) were able to give informed consent and motivated to participate in a 12-week intensive program of physiotherapy. Patients affected by cognitive deficits (Mini Mental Status Examination, <24 points), who were not able to communicate (Utrechts Communicatie Onderzoek (UCO), <4 points) or lived more than 30 km from the rehabilitation center were excluded. All participants provided written informed consent. The study size was based on a power analysis.Citation15
Outcome measures
Health-related quality of life: stroke impact scale (SIS), version 3
The SIS 3.0 is a multidimensional, self-reported stroke-specific 59-item instrument that assesses HRQoL in eight domains related to activities and participation (including strength, memory, emotion, communication, activities of daily living (ADL), mobility, hand function, and participation). The SIS has been shown to have good psychometric properties in a group of stroke survivors.Citation17 All 59 items are rated using a 5-point Likert scale and scored from “unable to complete the item” to “no difficulty experienced completing the item.” Each domain of the SIS has a range of 0–100 with higher scores indicating better quality of life. An extra question in the recovery domain assesses how much the patient feels that he/she has recovered from his/her stroke.Citation17
Independent variables
Motricity index (MI), arm
The upper extremity subscale of the Motricity Index (MI arm) was used to assess muscle strength of the paretic arm. The MI arm gives a rapid and reliable measure of the upper limb strength and uses a 6-point ordinal scale, with higher scores indicating better upper limb strength. The MI has been shown to provide good validity and reliability as a tool in stroke research.Citation18 Pinch grip, elbow flexion, and shoulder abduction were assessed. On each dimension, scores ranged from 0 (no activity) to 33 (maximal muscle force).Citation18
Motricity index (MI), leg
The lower extremity subscale of the Motricity Index (MI leg) was used to assess muscle strength and voluntary movement of the paretic leg. Ankle dorsiflexion, knee extension, and hip flexion were assessed. The scoring system is similar to the MI arm described above. The MI has been shown to provide good validity and reliability as a tool in stroke research.Citation18
Hospital anxiety and depression scale (HADS)
The HADS is a self-reported measure for assessing anxiety and depression in patients, but it does not provide a specific diagnosis.Citation19 It is a 14-item scale with 7 items for anxiety and 7 items for depression. The items use a four-point rating scale and patients can score between 0 and 3 points per item. The HADS has been proven to be a reliable, valid, and practical psychological screening tool, with lower scores indicating lower risk of anxiety and/or depression disorders.Citation19
Fatigue severity scale (FSS)
The FSS is a self-administered questionnaire with nine questions that examine the perceived severity of fatigue symptoms in different situations in the past week.Citation20 The patient indicates to what extent fatigue determines functioning. Scores for each item range from 1 (strong disagreement) to 7 (strong agreement). The FSS is a valid and reliable scale to measure fatigue in stroke.Citation20
Demographic and clinical variables
Age, gender, stroke type, side of hemiplegia and dominant side affected were assessed as potential predicting variables of HRQoL.
Data analysis
We statistically tested for differences in change scores on HRQoL, arm- and leg function between the circuit class training group and the usual care group 12 weeks after randomization to determine if both groups could be included in the analysis. SIS total score was the dependent variable in the regression analysis. Age, gender, clinical variables (stroke type, side of hemiplegia and dominant side affected) and clinical scales (MI arm and leg, HADS, FSS) at baseline and at follow-up were considered as independent variables. We tested whether the assumptions for a linear regression analysis were met. Change-from-baseline scores of the SIS and MI-arm were computed to describe the data.
To determine the relationship between HRQoL and upper limb strength, cross-sectionally at baseline and follow-up, univariate regression analysis was performed. Variables demonstrating p-values <0.20 were included in the hierarchical regression analysis.
To determine how much variance in HRQoL at follow-up was explained by upper limb strength and other demographic and clinical variables at baseline, hierarchical regression analysis was performed. HRQoL at baseline post-stroke was entered as first block into the analysis (to control for the effect of the dependent variable) and upper limb strength as second block. Clinical variables that could be included in the hierarchical regression analysis were combined as the third block and demographic and stroke characteristics were added in the fourth block. Potential predictor variables were examined for collinearity by inspection of the correlation coefficients (no multicollinearity when coefficients <0.7) and Tolerance/VIF values (Tolerance needed to be >0.1 and VIF values <10).
Univariate regression analysis was also performed with change-from-baseline scores for HRQoL, as dependent variable, and change-from-baseline scores in upper limb strength. Effect sizes were classified as .02, .15 and .35 as small, medium, and large effect sizes.Citation21 Significance levels were set at p = .05. Statistical analyzes were performed with SPSS, version 25.0.
Results
In total, 250 participants were included in this study (flowchart; Supplementary Figure 1). Of the 250 included patients, one patient in the circuit training group and seven in the usual care group were excluded from the analysis. Reasons were withdrawal from participation (n = 3), death from cancer (n = 2), and recurrent stroke (n = 2), while one patient missed the 12-week assessment visit because of change of address.Citation15 The change scores on HRQoL, arm- and leg function from circuit class training group and control group did not differ significantly from each other 12 weeks after randomization. lists the clinical and demographic characteristics of the patients. The baseline assessment was done 102 days (SD 64) post-stroke and the follow up assessment 12 weeks after baseline. The average age in the population was 57 years (SD 10), 65% (n = 162) was male, the majority (n = 203) had an ischemic stroke, and almost half of the patients (n = 116) had a right hemisphere lesion. From this table, we can see that there are significant improvements in mean scores over time for most clinical variables (p < .05), except the emotion subscale (SIS), FSS, and HADS.
Table 1. Baseline characteristics (n=250)
For HRQoL (SIS), the mean score at baseline was 70.30 (SD 11.54) and 3 months later the mean was 75.61 (SD 13.78). The highest score at baseline was 94. One patient reached the maximum score of 100 at the follow-up assessment. Change-from-baseline scores show that 57 patients (24%) had a negative change score (decline), and the remaining patients had a neutral (change score of 0; 3%) or positive change score (improvement; 73%). The lowest and highest change-from-baseline scores were −23 and +40, respectively.
Mean upper limb strength (MI-arm) was 60.37 (SD 26.41). Eleven patients (4%) had a baseline score of 0 in comparison to 21 patients (8%) with the maximum score of 99 at baseline. Six months post-stroke, mean MI-arm was 65.35 (SD 26.27), and only seven patients (3%) had a score of 0 while the number of patients reaching the maximum score increased to 40 (17%). Change-from-baseline scores showed that 39 patients (16%) had a negative change score, and the remaining patients had a neutral (no change) (28%) or positive change score (56%). The lowest and highest change-from-baseline scores were −23 and +52 points, respectively.
Upper limb strength and health-related quality of life cross-sectionally in time
Linear regression analysis showed that HRQoL was statistically significantly related to upper limb strength at baseline, F(1, 248) = 165.023, p < .0005 and upper limb strength accounted for 40% of the explained variability in HRQoL (). At follow-up HRQoL was also significantly related to upper limb strength, F(1, 240) = 225.191, p < .0005 and upper limb strength accounted for 48% of the explained variability in HRQoL (). also shows that age, sex, side of hemiplegia, MI-leg, HADS, and FSS in relation to HRQoL at baseline can be included as independent variables in the regression analysis (all p < .2). At follow-up, age and sex (baseline), MI-leg, HADS, and FSS in relation to HRQoL emerged as independent variables (all p < .2).
Table 2. Univariate regression analyzes SIS and potential predictors, baseline, and follow-up.
Upper limb strength, stroke- and clinical variables, and health-related quality of life over time
HRQoL at baseline (Model 1) was significantly related to HRQoL at follow-up (R2 = .644, F(1, 229) = 414.546, p < .0005) (). The addition of (improvement in) upper limb strength to the prediction of HRQoL at follow-up (Model 2) led to a statistically significant increase in R2 of .078, F(2, 227) = 31.983, p < .0005. The addition of MI-leg, FSS, HADS (Model 3) led to small, non-significant changes in R2 (.004) (). The addition of age, sex, and hemiplegia (full model) led to a small increase in R2 of .010, which was statistically significant (p < .05, for full details). Clinical variables, like leg function (MI-leg), did not show a significant correlation with HRQoL (p > .05). Sex was the only stroke-related characteristic with a significant relation with HRQoL (p < .05).
Table 3. Hierarchical regression analysis.
Changes in upper limb strength and changes in health-related quality of life over time
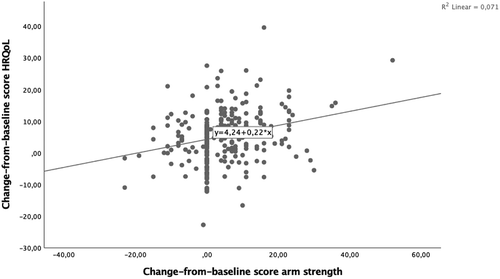
Change scores in upper limb strength between baseline and follow-up accounted for 7.1% of the variation in change scores in HRQoL between baseline and follow-up, a small to medium effect size ().Citation21 Change scores in upper limb strength between baseline and follow-up significantly predicted change scores in HRQoL between baseline and follow-up (F(1, 240) = 18.351, p < .0005).
Discussion
This is the first study that assesses the degree and relative impact of the relationship, both cross-sectionally and longitudinally, between upper limb strength and HRQoL. Upper limb strength was significantly related to HRQoL at baseline and follow-up and is an important significant predictor of HRQoL, even after correcting for clinical and stroke characteristics. Furthermore, an improvement in upper limb strength was positively related to an improvement of HRQoL, which suggests that recovery of upper limb strength is also important during the outpatient rehabilitation period and beyond.
Our finding that upper limb strength is independently related to HRQoL is in line with findings from other studies.Citation1,Citation4,Citation13,Citation14,Citation22,Citation23 In contrast to earlier studies, however, our study is able to demonstrate the relation between upper limb strength and HRQoL in the sub-acute outpatient rehabilitation phase. In addition, patients were enrolled at a specific moment during stroke rehabilitation, namely upon discharge from inpatient rehabilitation. We considered it useful to explore this sub-acute phase, since it is characterized by minimal rehabilitation support and is beyond the sensitive time-window of recovery.Citation24,Citation25 Most earlier studies assessed patients within a broad time frame after stroke (e.g. 2–68 months after stroke onset), and took place in the chronic phase.Citation4,Citation13,Citation14,Citation22,Citation23
Our study highlights the unique contribution of upper limb strength to HRQoL and its importance compared to other predictors. In previous studies arm function was included as one of the many potential predictors of HRQoL and can, therefore, have been easily overlooked, as these studies did not focus solely on arm function.Citation1,Citation4,Citation13,Citation14,Citation22 Some previous studies did show that specific domains of HRQoL, i.e. ADL and participation were related to arm functionCitation13,Citation14, and that autonomy in daily life activities was strictly related to recovery of the affected arm.Citation26 The current study also demonstrates that upper limb strength contributes more to HRQoL than other predictors, like leg function, which is consistent with the findings of a comparable studyCitation4, indicating that upper limb strength is the strongest predictor of HRQoL. It should be noted that this has only been demonstrated in mildly to moderately impaired stroke patients, because the inclusion criteria were limited to this group of patients.Citation4
In addition to the importance of upper limb strength, there are several factors that predict HRQoL in stroke patients. Our study demonstrated that HRQoL was also cross-sectionally predicted by age, sex, side of hemiplegia, anxiety and depression, leg function, and fatigue. In accordance with most studies, symptoms of anxiety and depression and post-stroke fatigue could affect a patient’s motivation to participate in rehabilitation programs and influence (the rate) of recovery.Citation1,Citation13,Citation27 Older age is associated with poorer HRQoL in stroke patients as in the general population. The association between (female) gender and poorer HRQoL might be explained by a higher prevalence of anxiety post-stroke.Citation1 Dominant side of hemiplegia has been repeatedly shown to be associated with HRQoL. Most patients are right-handed with a left hemisphere stroke. Since the left hemisphere controls speech and language function may affect HRQoL through an altered communication ability.Citation1,Citation28
In contrast to the current study, previous studies have not assessed the effect of changes in upper limb strength over time on HRQoL, as HRQoL was assessed at a single time-point.Citation1,Citation4,Citation23,Citation29 The current study shows that improvements in upper limb strength are positively related to improvements in HRQoL during outpatient rehabilitation. This suggests that there should also be a focus on upper limb strength recovery in the outpatient rehabilitation phase, in order to possibly expect improvement in HRQoL. The improvement in upper limb strength found during outpatient rehabilitation raises the possibility that there is potential to train outside the sensitive time-window (i.e. 0–3 months post-stroke).Citation30,Citation31 A recent study that investigated an intensive upper limb neurorehabilitation programme in chronic stroke patients, found large clinical improvements in measures of impairment and activity.Citation32 However, future studies are recommended to confirm the results and unanswered questions about potential for arm recovery beyond the sensitive time-window. Currently, patients receive little to no rehabilitation support when discharged to the community.Citation24,Citation25
The present study had some limitations. Firstly, included patients were indicated for inpatient rehabilitation when discharged from the hospital and had only mild to moderate stroke, which limits the generalizability of the trial. In addition, the included patients were specifically selected for inclusion in a task-oriented circuit class training because of primary problems in walking competency, what might result in a selection of patients with relatively mild upper limb impairment. Nonetheless, only a few patients reached a maximum score on the upper limb clinical measures at baseline. Secondly, we chose to limit this study to upper limb strength, while sensory function may play a prominent role in a patient’s perception of arm function.Citation33 Future studies should determine the role of sensory function in the relation between upper limb strength and HRQoL.
Our study provides evidence that upper limb strength is an important independent predictor of HRQoL in the sub-acute phase after stroke. Upper limb strength appears to be the most important predictor of HRQoL, besides other predictors as leg function and anxiety, and an improvement of upper limb strength contributes positively to HRQoL. Current arm function recovery therapies, e.g. constraint-induced movement therapy,Citation34 should be optimized and evaluated since improvement in upper limb strength positively influences HRQoL. Future studies on the current topic, and outside the sensitive time-window of recovery, are therefore recommended.
Additional information
Funding
References
- Morris JH, Van Wijck F, Joice S, Donaghy M. Predicting health related quality of life 6 months after stroke: the role of anxiety and upper limb dysfunction. Disabil Rehabil. 2013;35(4):291–299. doi:10.3109/09638288.2012.691942.
- Northcott S, Moss B, Harrison K, Hilari K. A systematic review of the impact of stroke on social support and social networks: associated factors and patterns of change. Clin Rehabil. 2016;30(8):811–831. doi:10.1177/0269215515602136.
- Northcott S, Hilari K. Why do people lose their friends after a stroke? Int J Lang Commun Disord. 2011;46(5):524–534. doi:10.1111/j.1460-6984.2011.00079.x.
- Franceschini M, La Porta F, Agosti M, Massucci M. Is health-related-quality of life of stroke patients influenced by neurological impairments at one year after stroke? Eur J Phys Rehabil Med. 2010: 46(3):389–399. R33102182 [pii]
- Langhorne P, Bernhardt J, Kwakkel G. Stroke rehabilitation. Lancet. 2011;377(9778):1693–1702. doi:10.1016/S0140-6736(11)60325-5.
- Broeks JG, Lankhorst GJ, Rumping K, Prevo AJ. The long-term outcome of arm function after stroke: results of a follow-up study. DisabilRehabil. 1999;21:357–364.
- Kwakkel G, Kollen BJ, Van der Grond JV, Prevo AJH. Probability of regaining dexterity in the flaccid upper limb: impact of severity of paresis and time since onset in acute stroke. Stroke. 2003;34(9):2181–2186. doi:10.1161/01.STR.0000087172.16305.CD.
- Persson H, Parziali M, Danielsson A, Sunnerhagen K. Arm function within 72 hours after first occasion of stroke and stroke outcome in an unselected population. Neurorehabil Neural Repair. 2012;26(6):732. doi:10.1177/1545968312449454.
- Nijland RHM, Van Wegen EEH, Harmeling-Van Der Wel BC, Kwakkel G. Presence of finger extension and shoulder abduction within 72 hours after stroke predicts functional recovery: early prediction of functional outcome after stroke: the EPOS cohort study. Stroke. 2010;41(4):745–750. doi:10.1161/STROKEAHA.109.572065.
- Ekstrand E, Rylander L, Lexell J, Brogårdh C. Perceived ability to perform daily hand activities after stroke and associated factors: a cross-sectional study. BMC Neurol. 2016;16(1):1–9. doi:10.1186/s12883-016-0733-x.
- Waddell KJ, Birkenmeier RL, Bland MD, Lang CE. An exploratory analysis of the self-reported goals of individuals with chronic upper-extremity paresis following stroke. Disabil Rehabil. 2016;38(9):853–857. doi:10.3109/09638288.2015.1062926.
- Harper A, Power M, Orley J, et al. Development of the World health organization WHOQOL-BREF quality of life assessment. Psychol Med. 1998. doi:10.1017/S0033291798006667.
- Chen C-M, Tsai -C-C, Chung C-Y, Chen C-L, Wu KP, Chen H-C. Potential predictors for health-related quality of life in stroke patients undergoing inpatient rehabilitation. Health Qual Life Outcomes. 2015;13(1):118. doi:10.1186/s12955-015-0314-5.
- Nichols-Larsen DS, Clark PC, Zeringue A, Greenspan A, Blanton S. Factors influencing stroke survivors’ quality of life during subacute recovery. Stroke. 2005;36(7):1480–1484. doi:10.1161/01.STR.0000170706.13595.4f.
- van de Port IGL, Wevers L, Roelse H, van Kats L, Lindeman E, Kwakkel G. Cost-effectiveness of a structured progressive task-oriented circuit class training programme to enhance walking competency after stroke: the protocol of the FIT-Stroke trial. BMC Neurol. 2009;9:43. doi:10.1186/1471-2377-9-43.
- von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008;61(4):344–349. doi:10.1016/j.jclinepi.2007.11.008.
- Vellone E, Savini S, Fida R, et al. Psychometric evaluation of the stroke impact scale 3.0. J Cardiovasc Nurs. 2015;30(3):229–241.doi:10.1097/JCN.0000000000000145.
- Collin C, Wade D. Assessing motor impairment after stroke: a pilot reliability study. J Neurol Neurosurg Psychiatry. 1990;53(7):576–579. doi:10.1136/jnnp.53.7.576.
- Spinhoven P, Ormel J, Sloekers PPA, Kempen GIJM, Speckens AEM, Van Hemert AM. A validation study of the Hospital anxiety and depression scale (HADS) in different groups of Dutch subjects. Psychol Med Copyr. 1997;27:363–370. doi:10.1017/S0033291796004382.
- Learmonth YC, Dlugonski D, Pilutti LA, Sandroff BM, Klaren R, Motl RW. Psychometric properties of the Fatigue Severity Scale and the Modified Fatigue Impact Scale. J Neurol Sci. 2013;331(1–2):102–107. doi:10.1016/j.jns.2013.05.023.
- Cohen J. Statistical power analysis for the behavioral sciences. Stat Power Anal Behav Sci. 1988;2nd:567. doi:10.1234/12345678.
- Lee J, Chang T, Yang S, Huang C, Hsieh F. Prediction of quality of life after stroke rehabilitation. 2016;6:369–375. doi:10.4172/Neuropsychiatry.1000163.
- Chang WH, Sohn MK, Lee J, et al. Predictors of functional level and quality of life at 6 months after a first-ever stroke: the KOSCO study. J Neurol. 2016;263(6):1166–1177.doi:10.1007/s00415-016-8119-y.
- Meyer M, Foley N, Pereira S, Salter K, Teasell R. Organized stroke rehabilitation in Canada: redefining our objectives. Top Stroke Rehabil. 2012;19(2):149–157. doi:10.1310/tsr1902-149.
- Duncan Millar J, van Wijck F, Pollock A, Ali M. Outcome measures in post-stroke arm rehabilitation trials: do existing measures capture outcomes that are important to stroke survivors, carers, and clinicians? Clin Rehabil. 2019;33(4):737–749. doi:10.1177/0269215518823248.
- Smania N, Paolucci S, Tinazzi M, et al. Active finger extension a simple movement predicting recovery of arm function in patients with acute stroke. Eur J Phys. 2009;45:349–354.
- Chen YK, Qu JF, Xiao WM, et al. Poststroke fatigue: risk factors and its effect on functional status and health-related quality of life. Int J Stroke. 2015;10(4):506–512.doi:10.1111/ijs.12409.
- Foerch C, Misselwitz B, Sitzer M, Berger K, Steinmetz H, Neumann-Haefelin T. Difference in recognition of right and left hemispheric stroke. Lancet. 2005;366(9483):392–393. doi:10.1016/S0140-6736(05)67024-9.
- Hartman-Maier A, Soroker N, Ring H, Katz N. Awareness of deficits in stroke rehabilitation. J Rehabil Med. 2002;34(4):158–164. http://www.embase.com/search/results?subaction=viewrecord&from=export&id=L34873750%5Cnhttp://dx.doi.org/10.1080/16501970213236%5Cnhttp://elvis.ubvu.vu.nl:9003/vulink?sid=EMBASE&issn=16501977&id=doi:10.1080%2F16501970213236&atitle=Awareness+of+deficits+in+.
- Zeiler SR, Krakauer JW. The interaction between training and plasticity in the poststroke brain. Curr Opin Neurol. 2013;26(6):609–616. doi:10.1097/WCO.0000000000000025.
- McDonnell MN, Koblar S, Ward NS, Rothwell JC, Hordacre B, Ridding MC. An investigation of cortical neuroplasticity following stroke in adults: is there evidence for a critical window for rehabilitation? BMC Neurol. 2015;15(1):109. doi:10.1186/s12883-015-0356-7.
- Ward NS, Brander F, Kelly K. Intensive upper limb neurorehabilitation in chronic stroke: outcomes from the queen square programme. J Neurol Neurosurg Psychiatry. 2019;90(5):498–506. doi:10.1136/jnnp-2018-319954.
- Meyer S, De Bruyn N, Krumlinde-Sundholm L, et al. Associations between sensorimotor impairments in the upper limb at 1 week and 6 months after stroke. J Neurol Phys Ther. 2016;40(3):186–195.doi:10.1097/NPT.0000000000000138.
- Corbetta D, Sirtori V, Castellini G, Moja L, Gatti R. Constraint-induced movement therapy for upper extremities in people with stroke (Review) SUMMARY OF FINDINGS FOR THE MAIN COMPARISON. Cochrane Database Syst Rev. 2015;10. doi:10.1002/14651858.CD004433.pub3.www.cochranelibrary.com.