ABSTRACT
Nano-functionalized products such as UV protective paints additives, antimicrobial food packaging, and fuel additives offering reduced CO2 emissions have the potential to secure a significant Irish market share in the near future. This scoping study gives a first estimation of nanomaterial surface water concentrations and population ingestional exposure through drinking water resulting from these products. As nanomaterial behavior in wastewater treatment plants (WWTPs) and water treatment plants (WTPs) is currently unclear, bridging data relating to potentially relevant materials (pharmaceuticals and metal removal efficiencies in WWTPs; pathogen removal efficiencies in WTPs) are employed in this study. Mean nanomaterial removal efficiencies of 59.8% and 70.2% were predicted for Irish WWTPs, between 96.95% and 0% for Irish WTPs. Predicted nano-scale TiO2 concentrations in surface waters (resulting from exterior paints) were 2 orders of magnitude greater than that of Ag (resulting from food packaging) and CeO2 (resulting from fuel additives), respectively. Predicted surface and drinking water concentrations were unlikely to pose any ecotoxicological or human health risk, although nano-scale TiO2 and Ag may warrant monitoring as part of standard surface water monitoring schemes. Future research should be directed toward characterizing the behavior of different categories of nanomaterials within WWTP processes.
INTRODUCTION
Nanomaterials are currently found in many commercial and industrial products and processes, with many more under development (CitationO’Brien and Cummins 2008). Studies into the release potential of nanomaterial from nano-functionalized products and processes have indicated that release and transport in different environmental media is possible. The form in which a nanomaterial is present in a product will have a significant influence on its potential for release to different environments. Nanomaterials contained in a liquid suspension or present as free nanoparticles will have a much greater potential for significant release than those embedded on a surface or suspended in a solid construction (CitationFoss Hansen et al. 2009). A study into the release of TiO2 nanoparticles from paint coated onto an exterior façade indicated that a significant fraction of particles may be released to soil and aquatic environments (CitationKaegi et al. 2008a). Release of nanoparticles from nano-composites during usage has been detected for TiO2 and SiO2 nanoparticles (CitationReijnders 2009) and similar release of silver nanoparticles from anti-microbial coatings and composites to aquatic environments is expected (CitationSiegrist 2009; CitationBouwmeester et al. 2009). A number of studies incorporating the use of nano-scale CeO2 fuel additives into diesel fuels indicated release of a small fraction of these additives in exhaust fumes (HEI 2001). Many of these releases are a small fraction of the total nanomaterial content of the product or process in question, although with the market for nanomaterials constantly growing these small release fractions may result in significant exposure quantities.
Nanomaterials possess unique properties as a result of their scale and surface functionalization. These nano-specific characteristics may result in unique exposure routes related to inhalation, ingestion, conjunctival and dermal infiltration, and potential human and environmental toxicity (CitationHandy and Shaw 2007; CitationHandy et al. 2008). A potentially significant exposure route for the general population is ingestion of nanomaterials through foods or drinking water. Upon ingestion, adsorption of these particles depends on a number of characteristics, such as particle size, surface charge, ligand attachments, and surfactant coating (CitationHoet et al. 2004; CitationSzentkuti 1997; CitationHillery et al. 2004). There is no information on chronic or acute oral exposure to nanoparticles currently available; with little available on the acute toxic effects of metal oxide nanoparticles (excepting TiO2) through any exposure route (CitationHu et al. 2009).
A new EU legislative framework for chemical substances (REACH) was implemented in 2007, phased in over a number of years in order to protect human health and the environment and to maintain and enhance the competitiveness of the EU chemical industry. Under the tenants of REACH this burden of proof in establishing the safety of a substance is passed from the regulator to manufacturers, importers, and producers. The manufacturing, importation, occupational, and environmental exposure regulations and limits for potentially hazardous materials have so far largely been determined from toxicity and fate studies employing high doses of aggregated particles (CitationWarheit et al. 2008). Reassessing the potential nano-specific toxicity of all currently used materials would be an extremely complicated process. The resulting high cost of regulation could discourage development and investment in nanotechnology (CitationBowman and van Calster 2007). The lack of guidance and regulation as regards nano-specific risk has led some companies to tackle this uncertainty, and perhaps pre-empt future regulation, by performing their own nano-specific risk assessments (CitationPark et al. 2008; Environmental Defence and DuPont 2007).
In Ireland, the majority of drinking water (83%) originates from surface water (i.e., rivers and lakes), with the remainder originating from groundwater (11%) and springs (6%). Current Irish regulations describe water quality standards for 14 dangerous substances in surface waters (EPA 2006). The substances concerned include pesticides, solvents, metals, and other substances of high priority internationally (EU/OSPAR), with potential adverse impacts on waters by virtue of toxicity, persistence, and bioaccumulation. The regulation regarding these substances gives an indication of how potentially harmful substances with toxic, persistent, or bioaccumulative characteristics are monitored in the Irish system. The limits put in place to monitor these substances may be used as bridging limits until definitive fate and exposure studies have been performed for nanomaterials.
Determination of bioavailability in different environment media and representative organisms, for both the material itself and for any potential transformations, and distinguishing between different physicochemical forms of nanomaterials will be major factors in predicting environmental impact and eventual regulation. Environmental monitoring efforts must be selective and focused on areas of likely accumulation. Sites in which nanomaterials might accumulate, such as sediments at the outfall and downstream from manufacturing facilities and wastewater treatment facilities, are good examples of sites where semi-permeable membrane devices might be deployed to determine whether nanomaterials can be concentrated and detected more readily than random sampling as part of current surface water monitoring schemes (Royal Commission 2008). The monitored levels at these high exposure sites may be extrapolated, based on population and industrialization, to other potential exposure sites.
Full characterization of fate in wastewater treatment plants (WWTPs) and water treatment plants (WTPs) (for drinking water) is essential in assessing exposure to potential environmental pollutants. Sampling and analysing of influent and effluent streams for WWTPs and WTPs of high predicted nanomaterial load (i.e., industrial, urban or agricultural loads), as well as intermediate stages such as pre-treatment filtration, coagulation/flocculation/sedimentation, secondary filtration, and disinfection is required. This shall allow the development of baseline and maximum removal rates for nanomaterials, or categories of nanomaterials, at all stages in the treatment process.
In the absence of nano-specific data relating to fate and exposure in treatment processes, as well as ecotoxicity and human health effects, scoping models must employ bridging data from other potentially relevant areas to allow a first estimate of potential exposure. Where hazard or fate information is not available in literature for a specific material or application, this gap may be covered for risk assessment purposes by “bridging” to another, better-characterized material. Scoping models provide a first estimate and/or ranking of a number of exposure endpoints and human health hazards from limited material or product/ process data. Models must be developed that make the best use of available data, as well as provide a framework into which new research may be incorporated. Scoping models may be employed in making early stage research and regulatory prioritizations.
EXPOSURE MODELLING: NANO-SCALE TiO2, Ag, AND CeO2
Potential Nano-Pollutant Surface Water and Human Exposure Model
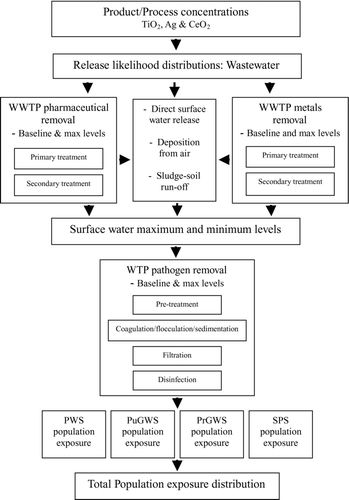
The model presented here employs data relating to wastewater and water treatment schemes in Ireland and removal efficiencies for representative pollutants commonly found in water supplies in Ireland to calculate potential surface water and human exposure to commercially available nanomaterials (nanoscale TiO2, Ag (metal and ionic forms) and CeO2). The model schematic may be seen in .
Product/Process Nano-Concentrations
Total concentrations of nano-scale TiO2, Ag, and CeO2 in commercially relevant products and processes (5% total Irish market penetration) were calculated as detailed in O’Brien and Cummins (in press). The range of nanomaterial quantities contained within a particular product or process were represented by probability distributions. The nano-quantities/distributions relating to TiO2, Ag (metal and oxide forms), and CeO2 containing products and processes may be seen in .
Table 1 Product/process categorization and wastewater release fractions.
Product/Process Release Likelihood
Release quantities of nanomaterial from environmentally relevant products and processes to wastewater treatment (Qx_ww) were calculated according to their categorization and form as detailed in O’Brien and Cummins (in press), the elements of which are represented by EquationEq. (1). These products and processes, categorizations, and fraction release to wastewater treatment plants (WWTPs) may be seen in . Release from paints and coatings (RT1) is a combination of direct release to surface water (2.5%) and 25% of runoff from degradation/abrasion (12.19%) going to wastewater treatment. Release from plastics and packaging (RA1) assumes a direct release for nanomaterial from plastic/packaging due to abrasion and water contact to wastewater treatment of 5%. In the case of Ag used as an anti-microbial coating, this 5% incorporates release of ionic silver and metal particles. Direct release of fuel additives to WWTPs (RC1) due to spillage and maintenance of filters was estimated at 0.5%. The market size and release values are subject to a standard deviation of 20% to represent variation and uncertainty.
where: x = TiO2, Ag or CeO2, a = T1, A1 or C1, b = T2, A2 or C2
Potential Nano-Pollutant Release from Wastewater Treatment Plants
Nanomaterial release to surface water was calculated using wastewater treatment levels employed in Ireland and removal efficiencies for the different treatment stages as defined in literature for pharmaceuticals and metals.
Wastewater arisings in Ireland undergo different levels of treatment in WWTPs before release into surface waters. As of 2005, 11% of wastewaters (for population equivalents more than 500) did not receive any form of treatment, 5% of wastewaters received preliminary treatment (removal or disintegration of gross solids in sewage and the removal of grit) only, 2% received primary treatment (physical and/or chemical process involving settlement of suspended solids) only, 70% of wastewaters received secondary treatment (biological treatment with a secondary settlement) only and 12% received nutrient reduction in addition to secondary treatment (EPA 2007a).
Pharmaceuticals in wastewaters have been indicated as an important focus for reducing micro-pollutant emissions into the aqueous environment (CitationJoss et al. 2005). The removal of pharmaceuticals in WWTPs may be attributed to biodegradation and adsorption onto solid surfaces. Compounds with low adsorption coefficients such as anti-inflammatories and antibiotics tend to remain in the aqueous phase, which favors their mobility through WWTPs and the receiving environment, while other compounds are adsorbed to the sludge, such as musks and estrogens (CitationCarballa et al. 2004). Pharmaceuticals in raw sewage have been detected through a number of international studies as being in the low mg m−3 range (CitationCarballa et al. 2004). CitationLacey et al. (2008) studied pharmaceutical concentration influent to three WWTPs in the greater Dublin area, with population equivalents of 60,000, 90,000, and 1.7 million. Twenty-four-hour composite samples resulted in detection of a number of pharmaceuticals, including salicylic acid and ibuprofen with maximum concentrations of 9.172 and 3.204 mg m−3, respectively. Removal of anti-inflammatories in wastewater treatment processes was selected as being potentially representative of nanomaterial removal. While nanomaterials are not expected to undergo degradation in the same way as pharmaceutical compounds, nanoparticles with surface coatings inhibiting aggregation or adsorption, or nanoparticles that may have associated with surfactants in the raw wastewater inlet may remain in the aqueous phase and so pharmaceutical compounds with low adsorption coefficients may offer a “worst-case” model for nanoparticle movement in WWTPs. The quantities of anti-inflammatories, such as ibuprofen, as detected in Irish wastewater influents by CitationLacey et al. (2008) are of a similar order to those predicted by nanomaterial exposure models (CitationO’Brien and Cummins, in press; Mueller and Nowack 2008; CitationBoxall et al. 2007). CitationCarballa et al. (2004) measured concentrations of ibuprofen and naproxen at the inlet of the WWTP, the inlet of the biological reactor, and the final effluent. These measurements are employed in the model to develop removal efficiencies for the primary treatment and biological reactor stages. The final removal efficiencies for metals and anti-inflammatories at different stages in wastewater treatment may be seen in . Minimum and maximum removal efficiencies for the anti-inflammatories over three sampling periods were applied as the 5% and 95% quantiles of normal distributions to represent variation in primary (Ppharm) and secondary (Spharm) removal efficiencies. The concentration of these pharmaceuticals detected by CitationCarballa et al. (2004) was in some cases higher after their passage through the primary sedimentation tank. The authors assumed this to be due to the analytical deviation caused by the different characteristics of the waters at the different sampling periods. WWTP removal rates for ibuprofen and naxopren have previously been reported as 75–90% and 66–82%, respectively (CitationLindqvist et al. 2005). The distribution employed in this assessment is at the lower end or below these reported removal rates and so removal is not over-estimated in this pharmaceutical model for nanomaterial removal.
Table 2 Pharmaceutical and metal removal efficiencies.
Metals are removed within wastewater treatment processes at varying rates depending on material and operating characteristics. Mechanisms of metal removal in activated sludge systems such as physical entrapment of insoluble particles into the floc, active cellular uptake, binding to extra cellular polymers, and volatilization (CitationStephenson and Lester 1987), are also seen as potential routes for nanoparticle removal. Metals that remain in the soluble phase during treatment are poorly removed and a similar result is expected for nanoparticles (CitationBoxall et al. 2007). Within primary treatment, various studies have reported average removal efficiencies of between 24 and 56% for metals of differing solubility (CitationBoxall et al. 2007). Within secondary treatment, the removal rates for these metals varied between 28 and 68%. These maximum and minimum removal rates were applied as the 5% and 95% quantiles of normal distributions to represent variation in primary (Pmetal) and secondary (Smetal) removal efficiencies.
Total nanomaterial release to surface water from WWTPs, employing pharmaceutical removal efficiencies, (Qsurface_water_pharm_x) was calculated as follows:
where: x = TiO2, Ag, or CeO2
Total nanomaterial release to surface water from WWTPs, employing metal removal efficiencies, (Qsurface_water_metal_x) was calculated as follows:
where: x = TiO2, Ag, or CeO2
Direct releases to surface waters from products/processes (Qsurface_water_direct), air deposition (Qsurface_water_air), and soil applied wastewater sludge runoff (Qsurface_water_soil) were calculated as detailed in O’Brien and Cummins (in press) and included in the total material released to surface waters.
Aquatic characteristics such as pH, calcium ion concentration, and the presence of natural colloids have been quoted as being important in predicting aquatic fate and behavior of nanomaterials. The aggregation and sedimentation of nanomaterials is likely to be dominated by natural aquatic colloids behavior, as the concentration of natural colloids in freshwaters are likely to be 2–3 orders greater than previously suggested engineered nanomaterial releases (CitationKlaine et al. 2008; CitationBoxall et al. 2007). The aquatic aggregation of engineered nanoparticles and absorption to organic matter may resemble that of metals, where the resultant sedimentation of agglomerates is an important process in the self-purification of water bodies (CitationKlaine et al. 2008). In this assessment, nanomaterials are assumed to persist in a mobile state where resulting surface water concentrations (Csurface_water_pharm_x; Csurface_water_metal_x) were calculated assuming a closed water system with initial zero presence of the specific nanomaterial, homogeneous dispersion of released nanomaterial from all sources over 1 year throughout all surface waters, mixing to a depth of 1.5 m (Dwater) and no degradation. The fraction of treated wastewater released to freshwaters in Ireland (Ffresh) is 91% (EPA 2007a). Approximately 3% of Ireland is covered by water, which equates to approximate surface area (Awater) of 2,100,000,000 m2.
where: x = TiO2, Ag, or CeO2
Potential Nano-Pollutant Release from Water Treatment Plants
Nanomaterial release to drinking water was calculated using surface water concentrations, water treatment levels employed in Ireland, and pathogen removal efficiencies at different treatment stages derived from literature. Supplies of drinking water in Ireland may be classified into four schemes (EPA 2007b). Public water supplies (PWS) are local authority operated schemes and supply water to 81.8% (Fpws) of the population. “Public” group water schemes (PuGWS) are supplied off larger public water supplies but distributed by group schemes and supply 3.2% (Fpugws) of the population. These water supplies may be assumed to undergo pre-treatment, coagulation/flocculation/sedimentation, filtration, and disinfection treatment stages. “Private” group water scheme (PrGWS) owners (usually representatives of the local community) source and distribute their own water, supplying 6% (Fprgws) of the population. A 2003 survey into “private” group water schemes in Ireland found that 6.7% of supplies underwent filtration, 48% chlorination, 12% UV treatment, and 45.3% underwent no treatment (CitationWims and Deane 2003). Small private supplies (SPS) comprise of industrial water supplies and boreholes serving single houses, serving 9% (Fsps) of the population. These supplies are generally not required to adhere to any specific regulation and so many would undergo the minimum level of treatment (i.e., disinfection or even no treatment).
Pathogens such as E. coli, salmonella, and campylobacter are regularly monitored for their presence in drinking water in Ireland. Their presence or absence is often taken as an indicator of water treatment plant efficiency. Typical measured concentrations of E. coli in surface waters range from 10,000 and 1,000,000 per liter, campylobacter 20 to 2500 per liter and salmonella 3 to 1000 per liter (WHO 2006). These bacteria are in the nano-range, although most nanomaterials are likely to be of higher densities, which will affect their removal efficiency in treatment processes. WHO guidelines for water treatment give baseline and maximum removal efficiencies of bacteria for different stages in the water treatment process. These baseline and maximum removal efficiencies were taken as the 5% and 95% quantiles, respectively, of normal distributions to represent variation in the treatment efficiency at different mechanical removal stages (except disinfection as this stage is not relevant to the potential removal of nanomaterials) ().
Table 3 Bacteria removal in WTPs.
where: x = pws, pugws, prgws or sps, y = TiO2, Ag, or CeO2
Predicted removal efficiencies (REx) for the different water supplies were calculated as follows (EquationEq. 10):
where: x = pws, pugws, prgws or sps, y = TiO2, Ag, or CeO2
Population Exposure through Drinking Water
Human exposure to nanomaterials through drinking water was calculated assuming an ingestion of 2 liters of water per person per day (WHO 1993) (0.002 m2) (Wdaily). A “worst-case” absorption and accumulation (Aaccum) of 10% of ingested nanomaterial dose over 1 year was assumed. This was based on an oral exposure study in rats where size dependent uptake of polystyrene particles by the GI tract was noted (CitationFlorence et al. 1995). 6.6% of the 50 nm particles administered in this study, 5.8% of the 100 nm particles, 1.8% of the 500 nm particles, 0.8% of the 1 μm particles, and 0% of the 3 μm particles were cumulatively taken up into the liver, spleen, blood, bone marrow, and kidney. Another study involving oral administration of carbon fullerenes to rats indicated a 10% absorption by the GI tract (CitationYamago et al. 1995). Annual exposure for the total Irish population (EquationEq. 11) was estimated using the predicted drinking water concentrations for each treatment scheme (EquationEq. 9) and the fraction of the population served by each of these schemes (Fpws; Fpugws; Fprgws; Fsps).
where: x = TiO2, Ag, or CeO2
RESULTS
The model was created in Microsoft Excel 2000 with the add-on packages @Risk (version 4.05, Palisade Corporation, New York, USA) and Matrix (version 2.3, Foxes Team, Italy). The model ran for 10,000 iterations to allow a fully representative selection of input values and distributions and convergence of results.
WWTP Nanomaterial Removal Rates
The overall mean nanomaterial removal efficiency predicted when employing pharmaceutical removal efficiencies for different treatment stages was 59.8% (90% CI: 50.6–68.7). The overall mean nanomaterial removal efficiency predicted when employing metals removal efficiencies for different treatment stages was 70.2% (90% CI: 56.9–82.2).
WTP Nanomaterial Removal Rates
The overall mean nanomaterial removal efficiency predicted when employing bacteria removal efficiencies for PWS and PuGWS was 96.95% (1–Dpws_x/Csurface_water_pharm_x) (90% CI: 91.66–99.79). The overall mean nanomaterial removal efficiency predicted for PrGWS was 4.93% (1–Dprgws_x/Csurface_water_pharm_x) (90% CI: 0–64.01). Removal efficiency for SPS was 0%, as no relevant treatment stages were assumed in the model.
Surface and Drinking Water Concentrations
TiO2
Predicted mean annual quantities of nano-scale TiO2, resulting from its usage in exterior paints, released to surface waters from WWTPs employing pharmaceutical (EquationEq. 2) and metal (EquationEq. 3) removal efficiencies were 1400 kg a−1 (90% CI: 2319–2719) and 1036 kg a−1 (90% CI: 165–2186), respectively. Predicted mean surface water concentrations of TiO2 for WWTPs employing pharmaceutical (EquationEq. 7) or metal removal efficiencies (EquationEq. 8), when other surface water releases are included (direct release, sludge leaching, and air deposition) were 1448 μg m−3 (90% CI: 240–2700) and 1333 μg m−3 (90% CI: 221–2495), respectively.
Drinking water concentrations were calculated based on the drinking water schemes available in Ireland and bacteria removal levels in WTPs. As the nanomaterial surface water concentrations resulting from pharmaceutical and metal removal rates used in estimating removal from WWTPs were relatively similar, as a pessimistic assumption the highest surface water concentrations were used in calculating drinking water concentrations. Predicted mean TiO2 drinking water concentrations (EquationEq. 9) for the four drinking water schemes (PWS, PuGWS, PrGWS, and SPS) were 44.1 μg m−3 (90% CI: 1.8–146), 44.1 μg m−3 (90% CI: 1.8–146), 1379.1 μg m−3 (90% CI: 193–2681), and 1448.2 μg m−3 (90% CI: 240–2700), respectively.
Ag
Predicted mean annual quantities of nano-scale Ag particles and silver ions (Ag+), resulting from its usage in food packaging, released to surface waters from WWTPs employing pharmaceutical (EquationEq. 2) and metal (EquationEq. 3) removal efficiencies were 92.5 kg a−1 (90% CI: 10.4–185) and 68.3 kg a−1 (90% CI: 7.6–149), respectively. Predicted mean surface water concentrations of nano-scale Ag for WWTPs employing pharmaceutical (EquationEq. 7) or metal removal efficiencies (EquationEq. 8), when other surface water releases are included (direct release, sludge leaching, and air deposition) were 29.5 μg m−3 (90% CI: 3.3–58.9) and 21.8 μg m−3 (90% CI: 2.4–47.4), respectively.
Predicted mean Ag drinking water concentrations (EquationEq. 9) for the four drinking water schemes (PWS, PuGWS, PrGWS, and SPS) were 0.9 μg m−3 (90% CI: 0.027–3.1), 0.9 μg m−3 (90% CI: 0.027–3.1), 28 μg m−3 (90% CI: 2.6–58.6), and 29.5 μg m−3 (90% CI: 3.3–58.9), respectively.
CeO2
Predicted mean annual quantities of nano-scale CeO2, resulting from its usage as a fuel additive, released to surface waters from WWTPs employing pharmaceutical (EquationEq. 2) and metal (EquationEq. 3) removal efficiencies were 22.6 kg a−1 (90% CI: 5.8–42.1) and 16.8 kg a−1 (90% CI: 4–33.9), respectively. Predicted mean surface water concentrations of nano-scale CeO2 for WWTPs employing pharmaceutical (EquationEq. 7) or metal removal efficiencies (EquationEq. 8), when other surface water releases are included (direct release, sludge leaching, and air deposition) were 24.3 μg m−3 (90% CI: 5.1–54.2) and 22.4 μg m−3 (90% CI: 4.4–51.4), respectively.
Predicted mean CeO2 drinking water concentrations (EquationEq. 9) for the four drinking water schemes (PWS, PuGWS, PrGWS, and SPS) were 0.74 μg m−3 (90% CI: 0.031–2.5), 0.74 μg m−3 (90% CI: 0.031–2.5), 23.1 μg m−3 (90% CI: 4–53.4), and 24.3 μg m−3 (90% CI: 5.1–54.3), respectively.
The predicted surface water concentrations of nano-scale TiO2, resulting from its usage in exterior paints, were 2 orders of magnitude greater than that of Ag and CeO2, as a result of their usage in food packaging and diesel fuel additives, respectively.
Annual Ingestion of Nanomaterial through Drinking Water
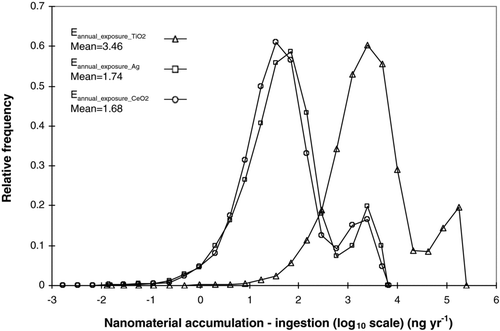
Predicted annual ingestion through drinking water (EquationEq. 10), assuming a 10% adsorption and accumulation rate, of the three nanomaterials investigated may be seen in .
It may be seen that the potential quantities of nano-scale TiO2, ingested through drinking water is ∼2 orders greater than that for ingestion of nano-scale Ag and nano-scale CeO2. These nanomaterial ingestion quantities are as a result of their usage in the products explored in this model.
Regulatory Levels of Concern
The predicted surface water and drinking water nanomaterial concentrations (resulting from the products and scenarios considered) were compared to potential regulatory limits that may be of concern to nanomaterials. Initial release quantities, WWTP and WTP removal efficiencies were then varied to compare to these regulatory values ().
Table 4 Regulatory levels of concern—Varying model inputs.
DISCUSSION
WWTP Nanomaterial Removal Rates
The mean nanomaterial removal rates from WWTPs when employing pharmaceutical and metal removal rates for each treatment stage, attributing relevant mass fractions to each treatment level (no treatment, primary treatment only, secondary treatment), were ∼60% and 70%, respectively. Other exposure studies have assumed higher removal rates for nanomaterials, that is, 97% (CitationMueller and Nowack 2008) and 94% (CitationBlaser et al. 2008), based on individual materials or particulate removal. The treatment stages are not broken down in these studies however, and total wastewater is not distributed to the correct treatment level (i.e., no treatment, pre-treatment, and primary treatment, secondary treatment), by total mass fraction. A study into the influence of surfactants on the removal of nano-scale CeO2 in model wastewater activated sludge treatment stage found that up to 6% of the nanoparticles mass was not retained by the treatment (CitationLimbach et al. 2008). The scaling up of laboratory scale models to real plants is a difficult process, which is increased in this case as the behavior of nanoparticles in aquatic environments has yet to be fully characterized. Estimation of nano-scale Ag removal from WWTP activated sludge treatment stages predicted a high level of removal efficiency, ∼99.9%, (CitationBenn and Westerhoff 2008), although variation of operating parameters and efficiencies in WWTPs must be taken into consideration.
Removal rates in WWTPs when employing anti-inflammatory removal rates (ibuprofen and naproxen) may under-estimate removal as these compounds remain in the aqueous phase due to their low absorption coefficients, increasing their mobility in WWTPs. Removal of nanomaterials as compared to that of metals may be increased due the relative surface area of nanomaterials, although the WWTP operating characteristics such as pH, retention time, sludge age, and so on and the influent wastewater characteristics such as presence of surfactants, and so on may have a significant influence on potential removal rates. Theoretical and laboratory scale nanomaterial removal rates are greater than those reported for pharmaceuticals and metals, although the measured removal rates for these pollutants take into account real world WWTPs where efficiency may not be 100%, which will have an impact on the potential for nanomaterial removal. While the WWTP removal rates predicted in this model may underestimate potential nanomaterial removal, until nano-specific studies into fate of a range of nanomaterials in the different stages of wastewater treatment, such as those performed by CitationLimbach et al. (2008), under different conditions of pH, duration, and so on have been undertaken, the distributions used to describe potential removal offer a realistic worst-case scenario of potential nanomaterial exposure.
WTP Nanomaterial Removal Rates
The predicted nanomaterial drinking water concentrations to which the general population shall be exposed vary as many different supply schemes are in use in Ireland. The majority of the population (85%) are supplied by PWS and PrGWS in which drinking water undergoes a number of treatment stages before release for public consumption. Employing pathogen removal efficiencies for each stage (WHO 2006), results in a mean removal efficiency (REpws; REpugws) for nanomaterials of ∼97% for PWS and PuGWS (EquationEq. 10). A fraction of PrGWS undergo filtration, which may be a relevant treatment stage for nanomaterial removal, while SPS are likely to undergo only disinfection, resulting in mean removal efficiencies (REprgws; REsps) for these schemes of ∼5% and 0%, respectively. As the high surface area to mass ratio of nanomaterials promotes adsorption to organic material, and the density of nanomaterials is likely to be higher than that of bacteria resulting in higher settling velocities, nanomaterials may have even higher removal efficiency than that predicted during these treatment stages. The exposure concentrations predicted in this model do not, however, consider failing WTPs where removal efficiencies may be considerably lower, although as treatment facilities undergo rigorous, regular testing in Ireland it is unlikely that a water treatment plant would under-perform for a significant period of time (EPA 2007b).
Surface and Drinking Water Nanomaterial Concentrations
It is important to view potential nano-exposure scenarios in the context of total environmental/human exposure to these materials. The predicted WWTP releases and surface water concentrations are as a result of one particular product that is likely to be of environmental relevance in the Irish market.
TiO2
TiO2 is an inorganic compound and as such would be expected to persist and not biodegrade upon release to surface waters. The predicted quantities of nano-scale TiO2 released to surface water as a result of its usage in exterior paints (90% CI: 240–2700 μg m−3) represents a 25% to 300% increase on median TiO2 levels as detected in European stream waters (FOREGS 2006). The predicted surface water concentrations of nano-scale TiO2 (resulting from its usage in exterior paints) are a number of orders lower than those producing lethal and sub-lethal effects in ecotoxicity tests (i.e., Daphnia magna—10 g m−3 (CitationLovern and Klaper 2006); Rainbow trout—1 g m−3 (CitationFederici et al. 2007); Algae growth inhibition—40 g m−3 (CitationHund-Rinke and Simon 2006)). The predicted exposure levels are not of initial concern, although binding of nano-scale TiO2 particles to other substances such as arsenic may influence potential uptake of secondary pollutants (CitationSun et al. 2008). Micron-scale TiO2 has also been shown to produce persistent reactive oxygen species such as hydrogen peroxide (H2O2) in aqueous environments (CitationHarbour and Hair 1985). These secondary concerns warrant a more in depth study of environmental fate and exposure to nano-scale TiO2.
The overall population exposure to the nanomaterials investigated assumed a “worst-case” scenario of absorption and accumulation of 10% of ingested material in the body over 1 year. The log distributions for predicted nanomaterial accumulation in the body through drinking water may be seen in . The raw statistical data (not shown) indicated a mean TiO2 accumulation level through exposure over 1 year of 18.1 μg (95% quantile 135.6 μg). Studies have shown that nanoparticles may be absorbed through the gut based on characteristics such as particle size, surface charge, ligand attachments, and surfactant coating (Hoet et al.2004; Szentkuti 1997; Florence et al. 1995). Biodistribution experiments have indicated that nano-scale TiO2 could be transported to other tissues and organs after uptake by gastrointestinal tract, such as the liver, spleen, and kidneys (CitationWang et al. 2007). The quantities of nano-scale TiO2 employed in ingestion studies eliciting no response (i.e., rat—5 g/kg (CitationWang et al. 2007)) are a number of orders greater than that those drinking water quantities predicted by the model (1.8–2700 μg m−3) and so exposure through ingestion of nanomaterials is extremely unlikely to result in a nano-specific toxicological response.
Ag (metal and oxide forms)
Some formulations of silver nanoparticles may dissolve or degrade in slightly acidic conditions and at temperatures not much above room temperature, with the presence of chloride or dissolved organic materials potentially accelerating the rate of dissolution (CitationLuoma 2008). This suggests that nano-scale Ag is oxidized into a dissolved ionic form when subjected to prolonged exposure to water (CitationBenn and Westerhoff 2008). Particle surface charge and water salinity will encourage aggregation and reaction with dissolved natural organic matter and ultimate deposition into sediments. The predicted quantities of nano-scale Ag particle and Ag+ released to surface water (resulting from its usage in food packaging) (90% CI: 3.3–58.9 μg m−3) represents a 1 to 20% increase on average Ag levels as suggested for European stream waters (FOREGS 2006). There are many uncertainties in transferring the findings of chronic and acute invertebrate and fish laboratory studies involving silver and nano-scale silver to natural environments, due to potential aggregation behavior and bioavailability. Sulfides present in freshwaters may offer greater than five orders of magnitude protection against chronic toxicity, due to reduced bioaccumulation of silver ions (CitationHogstrand and Wood 1998). Nano-scale Ag exposure studies into the development of Zebrafish embryos indicated that exposure of up to 120 hours to 19 μg m−3 resulted in deformed and dead Zebrafish (CitationLee et al. 2007), although the particles employed in this experiment were formulated to avoid aggregation and so may not be representative of natural ligand containing freshwaters. Considering the limitations of laboratory toxicity testing, the quantities potentially released to surface waters as a result of nano-scale Ag usage in food packaging and as a fraction of total products (CitationBlaser et al. 2008) are unlikely to be of eco-toxicological concern when compared to silver toxicity (i.e., invertebrates 0.2–6.3 mg m−3 (CitationLuoma 2008); Fish—0.09–0.65 mg m−3 (CitationLuoma 2008)), although they are of sufficient levels to warrant regular monitoring.
Accumulation of nano-scale Ag through drinking water (resulting from food packaging) over 1 year was predicted by the raw statistical data (not shown) to result in a mean level of 0.37 μg for the general population (95% quantile 2.73 μg). Nano-scale Ag ingestion studies in rats with concentrations up to 1000 mg/kg indicated no significant changes in body weight relative to dose, though significant dose-dependent changes were found in the alkaline phosphatase and cholesterol values (CitationKim et al. 2008). The authors concluded that exposure to more than 300 mg of silver nanoparticles may result in slight liver damage. A study incorporating nano-scale Ag exposure concentrations of up to 25 ppm resulted in increases in populations of lactic acid bacteria in the gut of a quail, although no other major disruption of gut micro-flora and no cell damage were observed (CitationSawosz et al. 2007). The relative nano-scale Ag accumulation levels predicted by the exposure model () are not of toxicological concern, however, as they are below any current Ag toxicological limits and there are currently colloidal silver products on the market for oral consumption resulting in greater daily ingestion of nano-scale Ag (POEN 2009).
CeO2
Cerium would be expected to persist upon release to the environment due to its ion-exchange positions or associated with carbonates, organic matter, and iron and manganese oxides/hydroxides (NTP 2006). The predicted quantities of nano-scale CeO2 released to surface waters as a result of its use as a fuel additive (90% CI: 5.1–54.2 μg m−3) would represent a 10% to 100% increase on median stream water concentrations as reported for Europe (FOREGS 2006). The relationship between surface area and the bactericidal properties of nanoparticles has been investigated in published studies, although interactions with the cell membrane have also been implicated (CitationHandy et al. 2008). The predicted surface waters concentrations of nano-scale CeO2 as a result of its usage as a fuel additive (90% CI: 5.1–54.2 μg m−3) are relatively low compared to those quantities employed in bactericidal tests (i.e., E. coli—1 mg m−2 (CitationThill et al. 2006)) and unlikely to be of ecotoxicological concern.
A mean level of 0.3 μg (95% quantile 2.1 μg) was predicted for CeO2 accumulation through drinking water over 1 year was predicted by the raw statistical data (not shown). A study into the possible health risk arising from nano-scale CeO2 concluded that because cerium compounds are poorly absorbed through the digestive system, toxicity of cerium delivered orally is not likely to be a major concern (HEI 2001). A number of LD50s were identified in this study based on ingestion studies on mice and rats with cerium nitrates and cerium oxides, ranging from 622 to >5000 mg kg−1. A lowest observable adverse effect level (LOAEL) of 1000 mg kg−1 was identified for Cerium chloride, resulting in gastritis and enteritis, spleen hypertrophy, and hyperplasia upon ingestion by mice (HEI 2001). The toxicity limits and effect levels predicted for CeO2 are a number of orders greater than the annual accumulation levels predicted by the model () and so nano-scale CeO2 exposure through drinking water, resulting from 5% diesel fuel additive market penetration, is extremely unlikely to result in a nano-specific toxicological response.
While the population exposure to nanomaterials from specific products and processes were seen to pose an extremely low exposure risk for ingestion through drinking water, should the market penetrations or number of products incorporating these materials increase, the issue of WWTP and WTP removal efficiency may become an important issue. One potential solution to improve nanomaterial sequestering in water treatment facilities is that of ultra filtration, especially in regards to areas of relatively increased nanomaterial exposure (i.e., industrial and/or urban areas). In characterizing nanoparticles removal from raw water (2.7 × 1011 particles L−1; 4.2 nL L−1 volume; 31 nm average particle size), CitationKaegi et al. (2008b) found that ultra-filtration methods (cutoff approximately 10 nm) resulted in a 5–6 fold lower particle number density (1.3 and 7.0 particles L−1, respectively) and a 6–7 fold volume reduction (0.2 and 1.3 nL L−1, respectively) when compared to conventional drinking water treatments, with similar average particle sizes resulting from each technology (13 and 15 nm, respectively).
Basis of Potential Environmental Regulation and Monitoring
Due to the vast range of materials, characteristics, and functionalities covered by the terms “nanomaterial” and “nanoparticle,” their regulation may ultimately not be nano-specific but determined by their usage, material, environmental behavior/impact, or human health impact.
Classification of nanomaterials into categories based on physicochemical characteristics for testing and risk assessment purposes has been proposed by a number of authors (CitationWarheit et al. 2008; CitationTsuji et al. 2006; CitationMaynard and Aitken 2007; CitationTervonen et al. 2008). These categorizations may be based on particle shape, size distribution, material chemistry, surface charge, aggregation state, surface area, and surface coatings. However, attempting to classify into limited categories for regulatory purposes will be a difficult process as even within a single chemical there may be diversity in almost every other physical characteristic, altering potential chemical reactivity and toxicity (CitationHandy et al. 2008).
Regulating by usage implies that TiO2 nanoparticles, for example, with similar characteristics but used in different products and processes due to their anti-microbial and UV filtration properties may be regulated in different ways, with potentially different controls and restrictions. UV filters, as used in sunscreens, cosmetics, and other personal care products, have come under increased interest as an emerging environmental pollutant due to their presence in surface waters, their endocrine and developmental toxicity and estrogenicity (la Farre et al. 2008). TiO2 is currently used at the nano-scale as a UV filter in personal care products such as sunscreen and in exterior paint products and so may be classified as such. A recent ruling by the EU on the regulation of cosmetics containing nanomaterials, applicable from 2012, indicates a move toward regulation according to specific usage (EU Business 2009). A legislative definition of nanomaterials is incorporated into this regulation. This definition covers biopersistent and insoluble nanomaterials, signifying the incorporation of environmental behavior and impact into nano-regulatory issues.
A number of pesticides are currently monitored in Irish waters under the Water Quality (Dangerous Substances) Regulations (EPA 2006). The anti-microbial characteristics of Ag and TiO2, as employed in surface coatings, packaging and water treatment may result in their classification as a pesticide and monitoring and restriction as such. In 2006 the U.S. Environmental Protection Agency (USEPA) announced plans to regulate as a pesticide certain types of washing machines incorporating embedded Ag nanoparticles as an anti-microbial agent under the Federal Insecticide, Fungicide, and Rodenticide Act (FIFRA) (CitationHoyt and Mason 2008). Current pesticide limits in Irish surface waters under the Water Quality (Dangerous Substances) Regulations range from 1 to 10 mg m−3. The lower of these pesticide limits is within the range predicted for the release of TiO2 to surface waters (90% CI: 240–2700 μg m−3), solely from the use of nano-scale TiO2 in exterior paints, and within two orders of the range predicted for the release of nano-scale Ag to surface waters (90% CI: 3.3–58.9 μg m−3), solely from its use in food packaging. A WWTP removal efficiency of 100% for TiO2 would still result in the predicted mean nanomaterial concentrations in surface waters (resultant from the individual products and exposure scenarios investigated) reaching the lower “dangerous substance” limit (1 mg m−3) () whereas at the exposure scenarios investigated Ag and CeO2 would not reach this level, even at zero removal. For the predicted mean nanomaterial concentrations in surface waters to reach the lower “dangerous substance” limit (1 mg m−3) at the WWTP removal efficiencies predicted in this assessment (∼60%), initial release quantities of TiO2, Ag, and CeO2 would have to increase above those exposure scenarios assessed in this study by factors of 0.7, 35, and 42, respectively.
Irish drinking waters are monitored for a number of microbiological, chemical, and indicator values as defined by EU regulation (EU 2000). If nano-scale UV filters, anti-microbial coatings, and fuel additives come into widespread usage in Ireland, these materials may be present in surface waters in quantities greater than those predicted in this study for single product usage and so may be required to be monitored in drinking waters. Lower parametric values for individual pesticides and heavy metals (e.g., mercury), are 0.1 mg m−3 and 1 mg m−3, respectively. Applying similar values to nanomaterials (TiO2: 1.8–2700 μg m−3) indicates that drinking water concentrations may be in range of these parametric values, especially PrGWS and SPS supplies. When other nanomaterial sources are considered these values may increase, and may be subject to consideration under total nanomaterial concentrations, as for pesticides, although more research is needed into potential toxicity (e.g., carcinogenicity), before any parametric values or limits may be applied to drinking water. For the predicted mean nanomaterial concentrations in PWS and PrGWS drinking water supplies (resultant from the individual products assessed and predicted surface water concentrations) to reach the lower parametric value for individual pesticides (0.1 mg m−3) the WTP removal efficiency for TiO2 would have to be in the order of 93% (), whereas at the exposure scenarios investigated Ag and CeO2 would not reach this level, even at zero removal. For the predicted mean nanomaterial concentrations in PWS and PrGWS drinking water supplies to reach the lower parametric value for individual pesticides (0.1 mg m−3) at the WTP removal efficiencies predicted in this assessment (∼97%), surface water concentrations of TiO2, Ag, and CeO2 would have to increase above those concentrations predicted in this study by factors of 2.3, 111, and 135, respectively.
Nano-Relevant Exposure Metrics
There is uncertainty over the most appropriate metric by which to assess the risk posed to human health by nanoparticles. Many commentators and studies have suggested surface area as a more appropriate metric than the mass metric currently employed (CitationTsuji et al. 2006; CitationTran et al. 2000; CitationStoeger et al. 2006; CitationNel et al. 2006; CitationOberdoerster et al. 2005; CitationGrassian et al. 2007); although studies have also shown that this may not be universally true (CitationWarheit et al. 2006; CitationWang et al. 2007; CitationSoto et al. 2007). Biologically based, mechanistic models to predict lung burdens of material have been adapted and extended to account for the potentially enhanced reactivity, clearance mechanisms, and potential translocation of nanomaterials by investigating these burdens under surface area and number concentration mechanisms (CitationKuempel et al. 2006).
While the effect of surface area and number concentration has been considered and examined in inhalation/instillation studies, the effect of these exposure metrics has yet to be fully explored in ingestion studies. If common TiO2, Ag, and CeO2 nanoparticle characteristics are applied to the predicted drinking water mass concentrations, assuming no aggregation, spherical particles and homogeneous density, corresponding surface area and number concentrations may be predicted. Assuming a commercial nano-scale TiO2 mean particle size of 21 nm, surface area of 50 m2 g−1 and a density of 3.8 g cm−3 (Aerosil 2009), a drinking water mass concentration of 2.7 mg m−3 corresponds to a surface area concentration of 0.14 m2 m−3 and a number concentration of ∼1.47E+14 m−3. Assuming a commercial nano-scale Ag mean particle size of 10 nm, surface area of 10 m2 g−1 and a density of 10.5 g cm−3 (American Elements 2009), a drinking water mass concentration of 0.059 mg m−3 corresponds to a surface area concentration of 0.0006 m2 m−3 and a number concentration of ∼1.07E+13 m−3. Assuming a commercial nano-scale CeO2 mean particle size of 9 nm, surface area of 94.7 m2 g−1 (CitationPark et al. 2008) and a density of 7.65 g cm−3, a drinking water mass concentration of 0.054 mg m−3 corresponds to a surface area concentration of 0.005 m2 m−3 and a number concentration of ∼1.86E+13 m−3. The predicted mass concentration (95th quantile) of nano-scale TiO2 in drinking water was 50 times greater than that for nano-scale Ag and CeO2. On a surface area exposure basis, the predicted concentration of TiO2 was 230 and 30 times that of nano-scale Ag and CeO2, respectively. On a number concentration exposure basis, the predicted concentration of TiO2 was 14 and 8 times that of nano-scale Ag and CeO2, respectively. These calculations do not take into account the probable aggregation of nanoparticles in aquatic environments and absorption to organic matter, which will reduce surface area and number concentration exposures, but does give an idea of the varying exposure metrics that may be applied to nanomaterials. The use of these different exposure metrics in tandem may give a more complete description of potential nanomaterial exposure than a mass metric alone.
CONCLUSIONS
This study has incorporated current knowledge in pollutant removal from WWTPs and WTPs to estimate nanomaterial surface water concentrations and population ingestion exposure through drinking water. The nanomaterials selected were those with environmental application and of relevance to the Irish market. At the conservative market penetrations (5%) considered for a number of nano-functionalized products in the Irish market, surface water and drinking water concentrations were predicted to be unlikely to pose any ecotoxicological or human health risk, though levels of nano-scale TiO2 and Ag in surface waters may warrant monitoring as part of standard surface water monitoring schemes. The impact of environmental transformation products and secondary pollutants that may associate with nanomaterials must be considered once more data on the aquatic behaviors and mechanisms of nanomaterials becomes available. The potential application and management of nanomaterials in current pollutant monitoring and regulation schemes in Ireland was been investigated. The use nanomaterial surface area and number concentration measurements as potentially more relevant exposure metrics than mass based metrics has been explored for use in exposure studies.
The data employed in predicting the release of nanomaterials from specific products and processes to different exposure pathways and the removal efficiencies assumed for WWTPs and WTPs may not be nano-specific, but until more complete studies are undertaken, this bridging data may provide a first estimate of environmental and population exposure in Ireland. Initial nanomaterial release from a specific product or process and WWTP removal efficiency are crucial points in the exposure chain and future research should be directed toward characterizing the behavior of different categories of nanomaterials during these processes.
ACKNOWLEDGMENT
This work was funded by the Environmental Protection Agency, Ireland, through the Environmental RTDI Programme 2000–2006.
Notes
aEPA lower “dangerous substances” limit (pesticides).
bEU lower parametric value limit (pesticides)
REFERENCES
- Aerosil . 2009 . “ Aeroxide TiO2 P25 Product information ” . Available at www.aerosil.com
- American Elements . 2009 . “ Silver nanoparticles S-MITE product information ” . Available at www.americanelements.com/agnp.html
- Benn , T M and Westerhoff , P . 2008 . Nanoparticle silver released into water from commercially available sock fabrics . Environ Sci Technol , doi:10.1021/es7032718
- Blaser , S A , Scheringer , M A MacLeod , M . 2008 . Estimation of cumulative aquatic exposure and risk due to silver: Contribution of nano-functionalized plastics and textiles . Sci Total Environ , 390 : 396 – 409 .
- Bouwmeester , H , Dekkers , S Noordam , M Y . 2009 . Review of health safety aspects of nanotechnologies in food production . Regul Toxicol Pharmacol , doi:10.1016/j.yrtph.2008.10.008
- Bowman , D M and van Calster , G. 2007 . Does REACH go too far? . Nat Nanotechnol , 2 : 525 – 6 .
- Boxall , A BA , Chaudhry , Q Sinclair , C . 2007 . Current and Future Predicted Environmental Exposure to Engineered Nanoparticles: Report to the Department for the Environment, Food and Rural Affairs York, , UK : Central Science Laboratory .
- Carballa , M , Omil , F Lema , J M . 2004 . Behavior of pharmaceuticals, cosmetics and hormones in a sewage treatment plant . Water Res , 38 : 2918 – 26 .
- Environmental Defence and DuPont . 2007 . Nano Risk Framework Available at http://www.nanoriskframework.com
- EPA (Environmental Protection Agency) . 2006 . Dangerous Substances Regulations: National Implementation Report Dublin, , Ireland
- EPA . 2007a . Dublin, , Ireland : Urban waste Water Discharges in Ireland .
- EPA . 2007b . The Provision and Quality of Drinking Water in Ireland: A Report for the Years 2006–2007 Dublin, , Ireland
- EU (European Union) . 2000 . European Communities (Drinking Water) Regulations The Minister for the Environment & Local Government. Available at: http://www.irishstatutebook.ia/home.html SI No. 439 of 2000
- Business , E U . 2009 . “ Nanomaterials in Cosmetics: BEUC Cautiously Welcomes New Regulation ” . Available at http://www.eubusiness.com/Consumer/beuc-press.09-03-25/?searchterm=cosmetics
- Federici , G , Shaw , B J and Handy , R D . 2007 . Toxicity of titanium dioxide nanoparticles to rainbow trout, (Oncorhynchus mykiss): Gill injury, oxidative stress, and other physiological effects . Aquat Toxicol , 84 : 415 – 30 .
- Florence , A T , Hillery , A M Hussain , N . 1995 . Nanoparticles as carriers for oral peptide absorption: Studies on particle uptake and fate . J Contr Release , 36 : 39 – 46 .
- FOREGS . 2006 . Geochemical Atlas of Europe Available at http://www.gsf.fi/publ/foregsatlas/index.php
- Foss Hansen , S , Baun , A Michelson , E S . 2009 . “ Nanomaterials in consumer products: Categorisation and exposure assessment ” . Edited by: Linkov , I and Steevens , J . 359 – 67 . Dordrecht, , The Netherlands : Springer . Nanomaterials: Risks and Benefits
- Grassian , V H , O’Shaughnessy , P T Adamcakova-Dodd , A . 2007 . Inhalation exposure study of titanium dioxide nanoparticles with a primary particle size of 2 to 5 nm . Environ Health Perspect , 115 : 397 – 402 .
- Handy , R D and Shaw , B J . 2007 . Toxic effects of nanoparticles and nanomaterials: Implications for public health, risk assessment and the public perception of nanotechnology . Health Risk Soc , 9 : 125 – 44 .
- Handy , R D , von der Kammer , F Lead , J R . 2008 . The ecotoxicology and chemistry of manufactured nanoparticles . Ecotoxicol , 17 : 287 – 314 .
- Harbour , J R and Hair , M L . 1985 . Transient radicals in heterogeneous systems: Detection by spin trapping . Adv Colloid Interface Sci , 24 : 103 – 41 .
- HEI (Health Effects Institute) . 2001 . “ Evaluation of human Health Risk from Cerium Added to Diesel Fuel ” . Available at http://pubs.healtheffects.org/view.php?id=172
- Hillery , A M , Jani , P U and Florence , A T . 2004 . Comparative, quantitative study of lymphoid and non-lymphoid uptake of 60 nm polystyrene particles . J Drug Target , 2 : 151 – 6 .
- Hoet , P HM , Bruske-Hohfeld , I and Salata , O V . 2004 . Nanoparticles—Known and unknown health risks . J Nanobiotech 2 , doi:10.1186/1477-3155-2-12
- Hogstrand , C and Wood , C M . 1998 . Toward a better understanding of the bioavailability, physiology, and toxicity of silver in fish: Implications of water quality criteria . Environ Toxicol Chem , 17 : 547 – 61 .
- Hoyt , W V and Mason , E . 2008 . Nanotechnology: Emerging health issues . J Chem Health Safety , 15 : 10 – 5 .
- Hu , X , Cook , S Wang , P . 2009 . In vitro evaluation of cytotoxicity of engineered metal oxide nanoparticles . Sci Total Environ , doi:10.1016/j.scitotenv.2009.01.033
- Hund-Rinke , K and Simon , M . 2006 . Ecotoxic effect of photocatalytic active nanoparticles TiO2 on algae and daphnids . Environ Sci Pollut Res , 13 : 225 – 32 .
- Joss , A , Keller , E Alder , A C . 2005 . Removal of pharmaceuticals and fragrances in biological wastewater treatment . Water Res , 39 : 3139 – 52 .
- Kaegi , R , Ulrich , A Sinnet , B . 2008a . Synthetic TiO2 nanoparticle emission from exterior facades into the aquatic environment . Environ Pollut , 156 : 233 – 9 .
- Kaegi , R , Wagner , T Hetzer , B . 2008b . Size, number and chemical composition of nanosized particles in drinking water determined by analytical microscopy and LIBD . Water Res , 42 : 2778 – 86 .
- Kim , Y S , Kim , J S Cho , H S . 2008 . Twenty-eight-day oral toxicity, genotoxicity, and gender-related tissue distribution of silver nanoparticles in Sprague-Dawley rats . Inhalation Toxicol , 20 : 575 – 83 .
- Klaine , S J , Alvarez , P JJ Bately , G E . 2008 . Nanomaterials in the environment: Behavior, fate, bioavailability and effects . Environ Toxico Chem , 27 : 1825 – 51 .
- Kuempel , E D , Tran , C L Castranova , V . 2006 . Lung dosimetry and risk assessment of nanoparticles: Evaluating and extending current models in rats and humans . Inhalation Toxicol , 18 : 717 – 24 .
- Lacey , C , McMahon , G Bones , J . 2008 . An LC–MS method for the determination of pharmaceutical compounds in wastewater treatment plant influent and effluent samples . Talanta , 75 : 1089 – 97 .
- la Farre , M , Perez , S Kantiani , L . 2008 . Fate and toxicity of emerging pollutants, their metabolites and transformation products in the aquatic environment . Trends Anal Chem , 27 : 991 – 1007 .
- Lee , K J , Nallathamby , P D Browning , L M . 2007 . In vivo imaging of transport and biocompatibility of single silver nanoparticles in early development of zebrafish embryos . ACS Nano , 1 : 133 – 43 .
- Limbach , L K , Bereiter , R Mueller , E . 2008 . Removal of oxide nanoparticles in a model wastewater treatment plant: Influence of agglomeration and surfactants on clearing efficiency . Environ Sci Technol , 42 : 5828 – 33 .
- Lindqvist , N , Tuhkanen , T and Kronberg , L . 2005 . Occurrence of acidic pharmaceuticals in raw and treated sewages and in receiving waters . Water Res , 39 : 2219 – 28 .
- Lovern , S B and Klaper , R D . 2006 . Daphnia magna mortality when exposed to titanium nanoparticles and fullerene (C60) nanoparticles . Environ Toxicol Chem , 25 : 1132 – 7 .
- Luoma , SN. 2008 . Silver Nanotechnologies and the Environment: Old Problems or New Challenges? Project on Emerging Nanotechnologies Washington, , DC, USA : Woodrow Wilson Centre .
- Maynard , A D and Aitken , R J . 2007 . Assessing exposure to airborne nanomaterials: Current abilities and future requirements . Nanotoxicology , 1 : 26 – 41 .
- Mueller , N C and Nowack , B . 2008 . Exposure modeling of engineered nanoparticles in the environment . Environ Sci Technol , 42 : 4447 – 53 .
- Nel , A , Xia , T Maedler , L . 2006 . “ Toxic potential of materials at the nanolevel Science 311 ” . doi:10.1126/science.1114397
- NTP (National Toxicity Program) . 2006 . Chemical Information Profile for Ceric Oxide: Supporting Nomination for Toxicological Evaluation by the National Toxicology Program. Integrated Laboratory Systems Available at http://ntp.niehs.nih.gov/files/Ceric_oxide2.pdf
- Oberdoerster , G , Maynard , A Donaldson , K . 2005 . Principles for characterizing the potential human health effects from exposure to nanomaterials: elements of a screening strategy . Part Fibre Toxicol 2 , doi:10.1186/1743-8977-2-8
- O’Brien , N and Cummins , E . 2008 . Recent developments in nanotechnology and risk assessment strategies for addressing public and environmental health concerns . Hum Ecol Risk Assess , 14 : 568 – 92 .
- O’Brien , N and Cummins , E . A risk assessment framework for assessing metallic nanomaterials of environmental concern: Aquatic exposure and behavior . Risk Anal , (in press)
- Park , B , Donaldson , K Duffin , R . 2008 . Hazard and risk assessment of a nanoparticulate cerium oxide-based diesel fuel additive—A case study . Inhalation Toxicol , 20 : 547 – 66 .
- POEN (Project on Emerging Nanotechnologies) . 2009 . Consumer Product Database , Washington, , DC, USA : Woodrow Wilson Centre . Available at http://www.nanotechproject.org/44/consumer-nanotechnology
- Reijnders , L. 2009 . The release of TiO2 and SiO2 nanoparticles from nanocomposites . Polym Degrad Stab , doi:10.1016/j.polymdegradstab.2009.02.005
- Royal Commission . 2008 . Novel Materials in the Environment: The Case of Nanotechnology, 27th Report TSO : Royal Commission on Environmental Pollution .
- Sawosz , E , Binek , M Grodzik , M . 2007 . Influence of hydrocolloidal silver nanoparticles on gastrointestinal microflora and morphology of enterocytes of quails . Arch Anim Nutr , 61 : 444 – 51 .
- Siegrist , M , Stampfli , N Kastenholz , H . 2009 . Perceived risks and perceived benefits of different nanotechnology foods and nanotechnology food packaging . Appetite , 51 : 283 – 90 .
- Soto , K , Garza , L E and Murr , L E . 2007 . Cytotoxic effects of aggregated nanomaterials . Acta Biomater , 3 : 351 – 8 .
- Stephenson , T and Lester , J N . 1987 . Heavy metal behaviour during the activated sludge process I: Extent of soluble and insoluble metal removal . Sci Total Environ , 63 : 199 – 214 .
- Stoeger , T , Reinhard , C and Takenaka , S . 2006 . Instillation of six different ultrafine carbon particles indicates a surface area threshold dose for acute lung inflammation in mice . Environ Health Perspect , 114 : 328 – 33 .
- Sun , H , Zhang , X Zhang , Z . 2008 . Influence of titanium dioxide nanoparticles on speciation and bioavailability of arsenite . Environ Pollut , doi:10.1016/j.envpol.2008.08.022
- Szentkuti , L. 1997 . Light microscopial observations on luminally administered dyes, dextrans, nanospheres and microspheres in the pre-epithelial mucus gel layer of the rat distal colon . J Contr Release , 46 : 233 – 42 .
- Tervonen , T , Linkov , I Figueira , J R . 2008 . Risk-based classification system of nanomaterials . J Nanopart Res , doi:10.1007/s11051-008-9546-1
- Thill , A , Zeyons , O Spalla , O . 2006 . Cytotoxicity of CeO2 nanoparticles for Escherichia coli. Physico-chemical insight of the cytotoxicity mechanism . Environ Sci Technol , 40 : 6151 – 6 .
- Tran , C L , Buchanan , D Cullen , R T . 2000 . Inhalation of poorly soluble particles II: Influence of particle surface area on inflammation and clearance . Inhalation Toxicol , 12 : 1113 – 26 .
- Tsuji , J S , Maynard , A D Howard , P C . 2006 . Research strategies for safety evaluation of nanomaterials, Part IV: Risk assessment of nanoparticles . Toxicol Sci , 89 : 42 – 50 .
- Wang , J , Zhou , G Chen , C . 2007 . Acute toxicity and biodistribution of different sized titanium dioxide particles in mice after oral administration . Toxicol Lett , 168 : 176 – 85 .
- Warheit , D B , Webb , T R Sayes , C M . 2006 . Pulmonary instillation studies with nanoscale TiO2 rods and dots in rats: toxicity is not dependent upon particle size and surface area . Toxicol Sci , 91 : 227 – 36 .
- Warheit , D B , Sayes , C M Reed , K L . 2008 . Health effects related to nanoparticle exposures: Environmental, health and safety considerations for assessing hazards and risks . Pharmacol Ther , 120 : 35 – 42 .
- WHO (World Health Organisation) . 1993 . Guidelines for Drinking Water Quality: Volume 1. Recommendations: 2nd edit Geneva, , Switzerland
- WHO . 2006 . Guidelines for Drinking-Water Quality Available at: http://www.who.int/water_sanitation_health/dwq/guidelines/en/
- Wims , P and Deane , B . 2003 . The management and activities of group water schemes in the Republic of Ireland . Tearmann , 3 : 87 – 96 .
- Yamago , S , Tokuyama , H and Nakamura , E . 1995 . In vivo biological behaviour of a water-miscible fullerene: 14C labelling, absorption, distribution, excretion and acute toxicity . Chem Biol , 2 : 385 – 9 .