Abstract
Objective: To examine the extent to which pain acceptance, pain catastrophising and alexithymia moderate associations between pain intensity and psychological pain medication dependence. Methods: Participants (106 hospital outpatients with chronic spinal pain) completed the Leeds Dependence Questionnaire (LDQ) to measure psychological dependence on pain medication, and the Chronic Pain Acceptance Questionnaire-8 (CPAQ-8), the Pain Catastrophising Scale (PCS) and the Toronto Alexithymia Scale-20 (TAS-20), plus the Depression, Anxiety and Stress Scale-21 (DASS-21). Results: Multiple linear regression showed that degree of psychological dependence (measured dimensionally across the range of LDQ scores) was associated with TAS subscale difficulty identifying feelings (DIF) (β = 0.249, p = <0.002) and PCS subscale rumination (β = 0.193, p = 0.030), independently of pain intensity and risk behaviors for medication misuse. The effect of pain intensity was moderated by rumination, with pain intensity more strongly associated with dependence when rumination was high (interaction β = 0.192, p = 0.004). Logistic regression showed that the effect of pain intensity on severe dependence (measured categorically as LDQ score ≥ 20) was moderated by alexithymia, so that severe dependence was independently associated with the combination of intense pain and high alexithymia (interaction odds ratio = 7.26, 95% CIs = 1.63–32.42, p = 0.009). Conclusions: Rumination and alexithymia moderated the associations between pain intensity and psychological pain medication dependence, consistent with emotion regulation theory. This raises the possibility that specifically targeting rumination about pain and symptoms of alexithymia could potentially improve the effectiveness of psychological interventions for chronic pain and help people to avoid or reduce their psychological dependence on pain medication.
Introduction
Between 55% and 80% of the population are affected by chronic back or spinal pain at some point in life and the annual prevalence is between 15% and 45% (Andersson, Citation1999). Chronic back or spinal pain is a major cause of disability and impaired mental health and is a significant healthcare burden in terms of consultations, referrals to secondary care and pain medications (Hong et al., Citation2013; Manchikanti et al., Citation2009; Stubbs et al., Citation2016).
Pain medications are compounds that relieve pain by altering perception of nociceptive stimuli without producing anesthesia or loss of consciousness, including opioids, non-steroidal anti-inflammatory drugs (NSAIDs) and a range of other natural and synthetic compounds. Pain medications contribute significantly to the costs of treatment for spinal pain (Manchikanti et al., Citation2009) and people with spinal injuries are at high risk of pain medication misuse (Clark et al., Citation2017; Heinemann et al., Citation1992; Krause et al., Citation2015).
Pain medication dependence is an important treatment outcome for people with pain because it may increase the chronicity of pain and may be a precursor of more harmful outcomes. Most previous research on pain medication dependence emphasized opioid medications and physical dependence (e.g., Larance et al., Citation2016; Walid et al., Citation2007) but it is also important to consider non-opioid medications and psychological dependence. Dependence on non-opioid pain medications such as NSAIDS is not common, but some cases met DSM-IV criteria for substance dependence and/or DSM-V criteria for substance use disorder (Etcheverrigaray et al., Citation2014; Godersky et al., Citation2017). One study of 400 hospital inpatients found that 7% met DSM-IV-TR criteria for dependence on non-opioid analgesics (Bonnet et al., Citation2019). A randomized controlled study comparing chronic pain patients prescribed NSAIDs, tramadol (a weak opioid) or hydrocodone (a strong opioid) found that rates of abuse or dependence over a 12-month follow-up period were equivalent for NSAIDs and tramadol, although both those were significantly lower than for hydrocodone (Adams et al., Citation2006).
The Leeds Dependence Questionnaire measures a dependence construct that is “viewed as a purely psychological phenomenon, explained by learning theory and departing from the psychobiological view in that tolerance and the resulting withdrawal symptoms are relegated to the realm of consequences of regular, excessive use” (Raistrick et al., Citation1994, p. 564). Psychological dependence develops in a process and can be measured dimensionally (on a continuum) and categorically (by applying a cut-point for severe dependence) (Raistrick et al., Citation1994, Citation2014). This means it could be targeted both by early-stage preventative interventions to stop mild or moderate psychological dependence developing further and by later-stage treatment interventions for severe psychological dependence.
To develop effective interventions, it is important to understand the psychological risk factors for dependence (Jamison & Edwards, Citation2013), including emotion regulation, defined as “the conscious and non-conscious strategies we use to increase, maintain or decrease one or more components of an emotional response” (Gross, Citation2001, p. 215). Emotion regulation is an approach that is increasingly applied to chronic pain because it can help to explain differences between individuals in how they respond to chronic pain (Aaron et al., Citation2020; Koechlin et al., Citation2018).
Acceptance has been identified as an aspect of emotion regulation (Ding et al., Citation2015) and acceptance and commitment therapy (ACT) can be viewed as involving emotion regulation strategies (Gaudiano, Citation2011). Pain acceptance involves being willing to engage in everyday activities despite experiencing pain and has been associated with reduced pain medication use and dependence (Elander et al., Citation2014; Kratz et al., Citation2018).
Pain catastrophising involves perceiving a painful situation as worse than it is, and alexithymia involves difficulties describing and identifying emotions, leading to confusion between physical pain and distressing emotions. Both catastrophising and alexithymia involve distressing emotional responses to pain and both have been investigated as emotion regulation strategies (Ghandehari et al., Citation2020; Safavi et al., Citation2016; Sheppes et al., Citation2015; Wong & Fielding, Citation2013). Pain catastrophising and alexithymia have also been associated with increased pain medication use, misuse and dependence among clinical samples of people taking opioid pain medications and general population samples of people taking opioid and non-opioid pain medications (Arteta et al., Citation2016; Elander et al., Citation2014; Oberleitner et al., Citation2019).
If pain acceptance, catastrophising and alexithymia affected the outcomes of chronic pain by acting as emotion-regulation factors, they would be expected to moderate the effects of pain intensity, with greater pain acceptance reducing and both catastrophising and alexithymia increasing the impact of pain intensity. Pain acceptance and alexithymia were shown to moderate the effects of pain intensity on psychological dependence on pain medication among people with chronic pain in the general population (Elander et al., Citation2014), but those moderation effects have not to our knowledge been examined among clinical samples of spinal pain patients.
The present study aimed to test the moderation of pain intensity by pain acceptance, catastrophising and alexithymia among a clinical sample of people with chronic spinal pain. To obtain conservative estimates of moderation effects, we examined the extent to which they accounted for unique variance in psychological dependence, over and above that accounted for by other known risk factors, including depression, anxiety, stress, pain medication use, and drug use behaviors associated with misuse of pain medications, including opioid and non-opioid medications.
Methods
Participants
The participants were 106 people with chronic spinal pain recruited at the spinal and physiotherapy outpatient departments of two UK hospitals. The inclusion criteria were being over 18 years old, having chronic spinal pain and using prescribed or over-the-counter pain medications. Chronic spinal pain was defined as pain over three months duration related to the spine and associated structures. The exclusion criteria were being unable to speak English, having an additional diagnosed pain condition (eg., cancer) or having a serious mental illness or cognitive impairment that could affect participants’ ability to make reasonably accurate self-reports about themselves and their medication use. This meant that the threshold for serious mental illness was high; participants with severe depression would not have been excluded. In fact, no one was excluded because of serious mental illness or because of addictions or substance use disorders. Two people were excluded because of cancer pain and two because of fibromyalgia, but no one was excluded because of concomitant arthritis pain in other locations.
Measures
Outcomes
The Leeds Dependence Questionnaire (LDQ) assesses psychological dependence and is designed to be sensitive through the range from mild to severe dependence, with scores of 20 or more indicating severe dependence (Raistrick et al., Citation1994). It has good internal consistency, test-retest reliability and convergent validity (Kelly et al., Citation2010; Raistrick et al., Citation1994) and has been used to assess pain medication dependence among people with chronic pain (Corbelli et al., Citation2012; McDonough et al., Citation2019). We used a version previously adapted to assess pain medication dependence by replacing the words “drink and drugs” in each item with “painkillers” (e.g., “do you find yourself thinking about when you will next be able to take painkillers?”) (Said et al., Citation2019).
Potential moderators
The Chronic Pain Acceptance Questionnaire-8 (CPAQ-8) assesses willingness to engage in everyday activities despite experiencing pain and gives subscale scores for Activity Engagement and Pain Willingness, each with good internal consistency and convergent validity (Fish et al., Citation2010).
The Pain Catastrophising Scale (PCS) assesses frequency of catastrophic thoughts about pain and gives subscale scores for Rumination, Magnification and Helplessness (Sullivan et al., Citation1995). It has good factor structure and each scale has good reliability and validity (Sullivan et al., Citation1995; Osman et al., Citation1997; Wheeler et al., Citation2019). Clinical levels of catastrophising are indicated by scores above 11 for Rumination, 5 for Magnification, 13 for Helplessness, and 30 for the PCS total score (Sullivan, Citation2009).
The Toronto Alexithymia Scale-20 (TAS-20) assesses difficulties identifying and describing emotions and gives a total score as well as subscale scores for Difficulty Describing Feelings (DDF), Difficulty Identifying Feelings (DIF) and Externally Oriented Thinking (EOT), each with good internal reliability (Bagby et al., Citation1994; Parker et al., Citation1993, Citation2003). A three-factor structure was confirmed with individuals with musculoskeletal disorders (Swift et al., Citation2006). TAS-20 Total scores of 61 or more indicate high alexithymia (Bagby et al., Citationn.d.; Taylor et al., Citation1992).
Potential confounds
The Depression, Anxiety and Stress Scale-21 (DASS-21) gives scores for depression, anxiety and stress, each with good internal reliability and concurrent validity and with a cleaner factor structure and smaller inter-factor correlations than the 42-item version (Antony et al., Citation1998; Lovibond & Lovibond, Citation1995a). The cut-points for at least moderate depression, anxiety and stress are 14 for Depression, 10 for Anxiety and 19 for Stress (Lovibond & Lovibond, Citation1995b).
Participants also reported how long they had experienced spinal pain, their pain intensity in the last 24 h (10-point rating scale), whether they were disabled by spinal pain, their use of prescribed and non-prescribed pain medication, and whether they had experienced at least sometimes a range of behavioral risk factors for opioid medication misuse. These included feeling addicted to pain medication, using pain medication for other problems, saving up unused pain medications, losing pain medications and needing to have them replaced, being taken off pain medication to reduce tolerance, obtaining pain medications from a different prescriber than usual, taking pain medications differently from how they were prescribed, taking more pain medication than prescribed, borrowing pain medication, being worried about use of pain medications, others being worried about use of pain medications, and having to make emergency clinic visits or phone calls because of pain.
These are behaviors that have been associated with medication misuse and dependence (Butler et al., Citation2007; Compton et al., Citation2008) although they do not in themselves constitute misuse or dependence. All the participants were asked about all these behaviors whether or not they were taking opioids and/or prescribed pain medications, and the wording of the questions was reviewed to ensure they were appropriate for people taking pain medication of any type, not just opioids. This information was used to assess the extent to which the moderation of pain intensity by emotion regulation factors accounted for variability in psychological dependence that was not accounted for by known risk factors for medication misuse.
Procedure
The study protocol was approved by the NHS North-West—Greater Manchester West Research Ethics Committee (Reference 19/NW/0031) and by the NHS Health Research Authority. The participants were a series of consecutive attenders at two outpatient clinics over a period of three months, at which point it was considered that most of those eligible had been invited to participate. Consenting participants completed paper copies or electronic questionnaires during routine hospital outpatient visits.
Analytic strategy
The data analysis was conducted using IBM SPSS v27. Bivariate associations between psychological dependence and other factors were tested using Pearson’s product-moment coefficients for associations with continuous measures (age, duration of spinal pain, pain intensity) and independent groups t-tests for associations with binary measures (gender, pain-related disability, use of each type of pain medication, and presence/absence of risk behaviors for medication misuse).
Multiple linear regression was then used to test whether pain acceptance, pain catastrophising and alexithymia moderated the effect of pain intensity on degree of psychological dependence (dimensional LDQ scores across the range). Multiple logistic regression was used to test the same moderation effects on the presence vs absence of severe psychological dependence (LDQ scores dichotomized using a cut-point of 20 or more).
To assess whether moderation of pain intensity by pain acceptance, pain catastrophising and alexithymia accounted for unique variance in psychological dependence, over and above that accounted for by the other known risk factors, predictor variables were added to the regression models in two blocks: 1) demographic and clinical factors, medication use and risk behaviors for medication misuse that were significantly associated with dependence in the bivariate analyses; 2) pain acceptance, pain catastrophising and alexithymia measures and terms for the interactions between those measures and pain intensity (to test moderation effects), plus depression, anxiety and stress. Terms for all the interactions between pain intensity and the alexithymia, pain catastrophising and pain acceptance measures were computed and tested. We used subscale rather than total scores wherever possible to be as specific as possible about emotion regulation processes that could be potential targets for therapeutic interventions.
In the linear regression analysis, the dependent variable was LDQ score across the range. Interaction terms were computed as products of pairs of standardized scores. Significant interactions were explored using the PROCESS v4.0 macro for SPSS (Hayes, Citation2022) and simple slopes analyses (Aiken et al., Citation1991). Independence of error terms was assessed using the Durbin-Watson statistic and multicollinearity using Tolerances and Variance Inflation Factors (VIFs). Durbin-Watson values should be close to 2, with values less than 1 and greater than 3 indicating potential non-independence (Field, Citation2009, p. 221). Multicollinearity is indicated by Tolerances below 0.2 and VIFs greater than 10.0 (Field, Citation2009, pp. 224, 242).
In the logistic regression analysis, the dependent variable was LDQ score dichotomized using a cut-point of 20 or more. Predictor variable scores were dichotomized using recognized cut-points wherever possible (for alexithymia, rumination, magnification, helplessness, depression, anxiety and stress) and median splits only where recognized cutoffs were not available (for pain acceptance subscales). Because there is a recognized cut-point for the TAS-20 total score but not the subscales, the dichotomized total score was used rather than subscale scores for alexithymia. Interaction terms were computed as the products of dichotomized scores so that 0 = below cut-point or median for one or both, 1 = above cut-point or median for both.
Results
Descriptive information
Of the 106 participants who completed the survey, 91 (85.8%) completed it in paper form and 15 (14.2%) completed it electronically. Ages ranged from 23 to 88 years, with a mean of 56.57 years (SD 14.72). There were 36 (34%) males and 70 (66%) females. There were 97 (91.5%) from White family backgrounds and nine (8.49%) from Asian (n = 3), African (n = 2), Caribbean (n = 3) and African-Caribbean (n = 1) backgrounds. There were 63 (59.4%) who were married or cohabiting and 91 (85.8%) who had children. There were 57 (53.8%) employed, in occupations including healthcare, education and administration, 15 (14.2%) unemployed and 34 (32.1%) retired.
Mean duration of spinal pain was 85.55 months (range four months to over 40 years). There were 84 (79.2%) who had spinal pain for at least a year. Pain intensity ratings ranged from 3 to 10, with a mean of 7.41 (SD 1.70). There were 96 (91%) who reported intense pain every day and 67 (63.2%) who were disabled by pain. The mean LDQ score was 11.52 (SD 7.35) and there were 15 participants (14.2%) with LDQ scores of at least 20 points.
Prescribed pain medications were used by 94 participants (88.7%), over-the-counter pain medications by 66 (62.3%), and both prescribed and over-the counter pain medications by 54 (50.9%). The prescribed medications included strong opioids (eg., buprenorphine, fentanyl, oxycodone and morphine), weak opioids and compound medications (eg., codeine, co-codamol, co-dydramol, dihydrocodeine, Tramadol and Zapain), anticonvulsant medications (eg., gabapentin, pregabalin), and NSAIDs (eg., diclofenac, ibuprofen, naproxen, meloxicam). There were 17 (16.0%) participants who reported taking strong opioids, 56 (52.8%) weak opioids, 44 (41.5%) NSAIDs, 41 (38.7%) anticonvulsant medication, 12 (11.3%) antidepressant medication and 3 (2.8%) antianxiety medication). The over-the counter medications were almost all NSAIDs and paracetamol (acetaminophen).
Descriptive statistics for study measures are given in . Applying DASS-21 cutoffs (Lovibond & Lovibond, Citation1995b), there were 36 (34.0%) with at least moderate depression, 46 (43.4%) with at least moderate anxiety, and 38 (35.9%) with at least moderate stress. Applying PCS cutoffs (Sullivan, Citation2009), there were 33 (31.1%) with clinically significant rumination, 50 (47.2%%) with clinically significant magnification and 41 (38.7%) with clinically significant helplessness. Applying the TAS-20 total score cutoff (Bagby et al., n.d.; Taylor et al., Citation1992), there were 27 (25.5%) with high alexithymia.
Table 1. Descriptive statistics for study measures (n = 106).
Bivariate analyses
LDQ score was significantly correlated with pain intensity (r = 0.53, p < 0.001), but not with age or pain duration. LDQ scores were higher among participants who were disabled by pain (14.49 [SD 6.79] compared with 6.41 [SD 5.20], t = 6.42, p < 0.001) but there were no significant differences in LDQ scores between males and females or between those who were married or cohabiting versus those who were not.
LDQ scores were higher among those taking versus not taking prescribed medication (12.09 [SD 7.35] compared with 7.08 [SD 5.95], t = 2.26, p = 0.026); those taking versus not taking strong opioids (15.06 [SD 7.77] compared with 10.84 [SD 7.12], t = 2.21, p = 0.030); and those taking versus not taking anticonvulsant medication (13.32 [SD 7.90] compared with 10.38 [SD 6.81], t = 2.03, p = 0.045), but there were no significant differences in LDQ scores between those taking versus not taking over-the-counter pain medication, weak opioids, any opioids, NSAIDs, antidepressants or antianxiety medications.
LDQ scores were higher among those who felt addicted to pain medication versus those who did not (19.50 [SD 8.36] compared with 11.04 [SD 7.05], t = 2.83, p = 0.006); those who used pain medication for other problems versus those who did not (13.40 [SD 7.02] compared with 10.13 [SD 7.34], t = 2.31, p = 0.023); those who had taken more pain medication than prescribed versus those who had not (16.45 [SD 5.48] compared with 9.48 [SD 7.08], t = 4.90, p < 0.001); those who had borrowed pain medication versus those who had not (18.17 [SD 6.80] compared with 11.12 [SD 7.22], t = 2.33, p = 0.022); those who had worried about their own use versus those who had not (16.62 [SD 7.30] compared with 10.81 [SD 7.11], t = 2.75, p = 0.007); those who felt others worried about their use versus those who did not (17.29 [SD 7.18] compared with 11.11 [SD 7.23], t = 2.19, p = 0.031); and those who had made an emergency phone call or clinic visit versus those who had not (18.30 [SD 7.70] compared with 10.81 [SD 6.99], t = 3.20, p = 0.002). There were no significant differences in mean LDQ scores between those who had versus those who had not saved up unused pain medication, lost pain medications and needed to have them replaced, been taken off pain medications, used other prescribers for pain medications, or taken pain medications differently from how they were prescribed.
Correlations among study measures are given in . There were no significant correlations with age or duration of pain. Because depression, anxiety and stress were all intercorrelated above 0.70, only anxiety was included in the linear regression analysis. Because rumination, magnification and helplessness were all intercorrelated above 0.70, only rumination, which had the highest correlation with LDQ score, was included in the regression analysis. Because difficulty describing feelings and difficulty identifying feelings were correlated above 0.70, only difficulty identifying feelings, which had the higher correlation with LDQ score, was included in the linear regression analysis, along with externally oriented thinking, the third TAS-20 subscale. This meant that the highest correlation among variables used as predictor variables in the analysis was 0.57 (between rumination and difficulty identifying feelings).
Table 2. Correlations among predictor variables in the linear regression analyses (n = 106).
Table 2 continued
Based on that bivariate analysis, the control variables included in block 1 of both the linear and logistic regression analyses were pain intensity, pain-related disability, taking prescribed medication, taking strong opioid medication, taking anticonvulsant medication, feeling addicted to pain medication, using pain medication for other problems, taking more pain medication than prescribed, borrowing pain medication, worrying about their own use of medication, feeling that others worried about their use of medication, and making emergency phone calls or clinic visits. The predictor variables added in block 2 of the linear regression were anxiety, rumination, difficulty identifying feelings, externally oriented thinking, activity engagement, pain willingness, pain intensity x rumination, pain intensity x difficulty identifying feelings, pain intensity x externally oriented thinking, pain intensity x activity engagement, and pain intensity x pain willingness. In the logistic regression, dichotomized versions of the same variables were used, except that the alexithymia total score was used instead of subscales, as described in the analytic strategy.
Multivariate regression analyses
In the linear regression analysis, the F ratio for the final model was 22.23 (df = 7, 98; p < 0.001), indicating a good fit, and the Durbin-Watson statistic was 1.90, indicating independence of errors. The lowest Tolerance was 0.51 and the highest Variance Inflation Factor (VIF) was 1.96, indicating no multicollinearity.
shows standardized regression coefficients for variables retained in each model. Variables that were not independently associated with psychological dependence were not retained and are not shown in . A full listing of Beta weights and p values for all predictor variables including those not retained in the final models of each block is given in Table S1, Supplementary Material.
Table 3. Summary of hierarchical multiple linear regression analysis to predict degree of pain medication dependence (n = 106).
In block 1, being disabled by pain, greater pain intensity, borrowing pain medication, and making an emergency phone call or clinic visit were all independently associated with psychological dependence and together accounted for 45.2% of the variance in psychological dependence on pain medication. In block 2, difficulty identifying feelings, rumination and the pain intensity x rumination interaction were also independently associated with psychological dependence and increased the proportion of variance in psychological dependence accounted for to 61.4%.
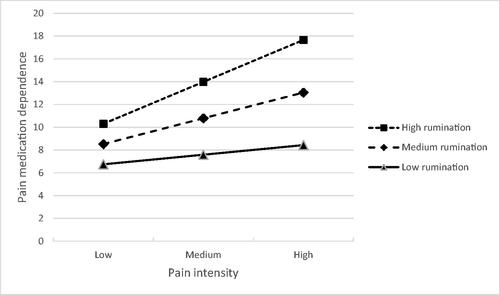
The pain intensity x rumination interaction was explored using an unadjusted simple slopes analysis (Aiken et al., Citation1991). This showed that the effect of pain intensity on psychological dependence was not significant for low levels of rumination (b = 0.4944, 95% CIs −0.3219 to 1.3108, t = 1.2014, p = 0.2324), but was significant for medium (b = 1.3294, 95% CIs 0.6436 to 2.0152, t = 3.8448, p = 0.0002) and high levels of rumination (b = 2.1643, 95% CIs 1.1882 to 3.1404, t = 4.3979, p < 0.0001). shows that rumination moderated the association between pain intensity and psychological dependence, which was stronger when rumination was higher.
Figure 1. Effects of pain intensity and rumination on degree of pain medication dependence (LDQ scores across the range).
Note: LDQ = Leeds Dependence Questionnaire
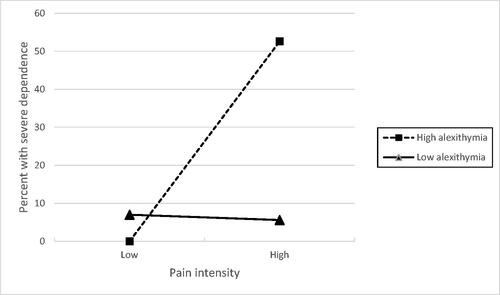
shows the results of the logistic regression analyses for variables that were retained in each model (variables that were not independently associated with severe psychological dependence were not retained and are not shown in ). In block 1, only pain intensity and borrowing pain medication were independently associated with severe psychological dependence, and the block 1 model correctly classified 84.9% of cases. In block 2, the pain intensity x high alexithymia interaction was also significantly associated with severe psychological dependence and this model correctly classified 87.7% of cases. Borrowing pain medication increased by 16-fold the odds of severe psychological dependence. Having high pain intensity and high alexithymia increased seven-fold the odds of severe psychological dependence.
Table 4. Summary of multiple logistic regression analysis to predict severe pain medication dependence (n = 106).
The unadjusted effect of the interaction between pain intensity and alexithymia is shown in . This shows that the proportion of participants with severe psychological dependence increased only when high pain intensity was combined with high alexithymia. Among participants with low alexithymia, 3/43 (7%) of those with low pain intensity and 2/36 (5.6%) of those with high pain intensity had severe psychological dependence. Among participants with high alexithymia, 0/8 (0%) of those with low pain intensity and 10/19 (52.6%) of those with high pain intensity had severe psychological dependence.
Discussion
Most participants had mild-to-moderate psychological dependence on pain medication, but a significant minority were severely dependent. The fact that pain intensity was significantly associated with both dimensional and severe psychological dependence reinforces how chronic pain makes people vulnerable to dependent use of pain medication. However, emotion regulation factors added to the explained variance and cases correctly classified in the two analyses, and the key factors were rumination and alexithymia.
Rumination was a significant factor in the linear regression, where it moderated the influence of pain intensity so that more intense pain was more strongly associated with increased psychological dependence when rumination was high. This suggests rumination may be an important aspect of the negative and distressing impact of pain, effectively amplifying pain intensity and linking pain with negative outcomes.
In an interview study, people with chronic spinal pain described and explained their use of pain medications predominantly in terms of their pain (Elander et al., Citation2021). The role of rumination in the present findings gives further insights into how people’s experiences of pain influence their use of medication. The four rumination items in the Pain Catastrophising Scale are: “I anxiously want the pain to go away”; “I can’t seem to keep it out of my mind”; “I keep thinking about how much it hurts”; and “I keep thinking about how badly I want the pain to stop.” The present findings suggest that people with thoughts like that are much more sensitive to pain and more vulnerable to psychological dependence on pain medication, probably because they are more strongly motivated to control or reduce their pain by taking medication.
Reducing catastrophic thinking about pain is already a key element of operant behavioral therapy, cognitive behavioral therapy, mindfulness-based stress reduction, and acceptance and commitment therapy for chronic pain (Sturgeon, Citation2014; Smeets et al., Citation2006). There is also evidence that interventions designed to target rumination can be effective (Watkins & Roberts, Citation2020), and rumination-focused cognitive-behavioral therapy has been shown to reduce depression, anxiety and pain intensity among people with chronic low back pain (Soleymani et al., Citation2020). Rumination about pain could therefore be specifically targeted by interventions for people with problematic pain medication use, to help them reduce this distressing way of thinking about their pain.
Alexithymia was a significant factor in both analyses. In the linear regression, greater Difficulty Identifying Feelings (DIF) was independently associated with degree of psychological dependence on pain medication. DIF involves difficulties with distinguishing emotions or feelings from bodily sensations like pain, and the fact that this subscale was associated with degree of dependence suggests that alexithymia may be associated with negative emotional feelings that are attributed to pain.
In the logistic regression, the interaction of high alexithymia and more intense pain was independently associated with severe psychological dependence on pain medication, showing how pain and alexithymia act together to increase the risk of severe dependence. The interaction effect is consistent with the hypothesis that alexithymia affects people’s experiences of symptoms (Lumley et al., Citation1996, Citation2008), for example by causing “hypersensitivity to aversive bodily sensations” (Kano & Fukudo, Citation2013, p. 5). Alexithymia reflects problems with emotion regulation, and difficulty managing negative emotions can increase impulsive tendencies and behaviors associated with pain medication misuse (Martel et al., Citation2014; Schreiber et al., Citation2012; Vest et al., Citation2016), so there is a plausible mechanism for the influence of alexithymia on dependent pain medication use.
In a previous study, alexithymia moderated the association between pain intensity and psychological dependence on pain medication among people with pain in the general population, whose levels of pain medication dependence were much lower than the present sample (Elander et al., Citation2014). The fact that alexithymia has now been shown to moderate the association between pain intensity and psychological dependence on pain medication both among the general population of people with pain and the present clinical sample of people with spinal pain suggests alexithymia is a robust correlate of psychological dependence.
The fact that difficulty identifying feelings (DIF) was the key element of alexithymia and the fact that high alexithymia was associated with severe dependence are both consistent with previous evidence about the impact of alexithymia on experiences of chronic back pain. A systematic review concluded that DIF was the TAS subscale most closely associated with chronic pain and that studies employing cut-points for high alexithymia produced more consistent associations with chronic pain than those using dimensional alexithymia scores (Kapadi et al., Citation2021).
Anhedonia, which refers to reduced capacity for positive emotional experiences and proneness toward negative emotions, has been associated with both chronic pain and opioid misuse (Garland et al., Citation2020). Alexithymia and anhedonia overlap, although the two constructs are distinct (Loas et al., Citation1997). In one study, the association between alexithymia and mental health problems was due to a propensity to experience negative emotions (Dubey & Pandey, Citation2013) so it is possible that anhedonia may explain to some extent associations between alexithymia and pain medication dependence.
The findings suggest that factors related to alexithymia could potentially be targets for psychological interventions for patients with chronic back pain. Studies of people with depression (Spek et al., Citation2008) and cancer (Tulipani et al., Citation2010) have shown that interventions can reduce alexithymia. In a study of people with chronic nonmalignant pain at a pain rehabilitation clinic, an intervention that specifically targeted understanding and expression of feelings and emotions reduced alexithymia and increased quality of life, although it did not reduce self-rated pain (Melin et al., Citation2010).
Alexithymia might also influence people’s responses to other types of intervention, especially those involving insight, emotional awareness and emotional disclosure (Lumley et al., Citation2008). These include compassionate mind training (CMT), cognitive behavior therapy (CBT) and acceptance and commitment therapy (ACT), which have all been applied for chronic pain patients with problem medication use (Dhokia et al., Citation2020; Guarino et al., Citation2018; Hughes et al., Citation2017; Vowles et al., Citation2020; see also Hruschak et al., Citation2018). These psychological approaches all involve recognition and awareness of emotional states, and alexithymia would be expected to be an obstacle to effective engagement and successful outcomes, so it is possible that CMT, CBT or ACT interventions could be adapted or refined for certain groups of people with chronic pain by incorporating alexithymia-related elements, for example, to help people identify or describe feelings and emotions and help them discriminate between emotions and physical sensations.
Existing recommendations for avoiding pain medication misuse and dependence include screening patients for risks of aberrant behavior related to opioids (Larance et al., Citation2016), and opioid use and dependency were associated with poorer outcomes among spinal pain patients (Dersh et al., Citation2008; Schoenfeld et al., Citation2017, Citation2018). In our findings, borrowing pain medication and needing to make an emergency phone call or clinical visit, which are included in checklists of opioid use risk behaviors (e.g., Butler et al., Citation2007) were both significant predictors in block 1 of the linear regression, but only borrowing medication was significant in block 2 of the linear and logistic regression analyses. None of the other risk behaviors for pain medication misuse included in the regression analyses (feeling addicted to pain medication, using pain medication for other problems, taking more pain medication than prescribed, worrying about use of pain medication, and feeling that others worried) had significant independent associations with psychological dependence, despite having significant bivariate associations with psychological dependence. Also, none of the pain medication variables included in the regression analyses (prescription medication, strong opioids and anticonvulsant medication) had significant independent associations with psychological dependence, despite their significant bivariate associations with psychological dependence, and feeling disabled by pain was the only clinical factor that was a significant independent predictor.
It might be argued that the risk behaviors for medication misuse that were included as covariates are very similar to symptoms of dependence, so we re-ran the regression analyses without including those variables. This produced an almost identical pattern of findings. In block 1 of the linear regression, being disabled by pain (β = 0.359, p < 0.001) and pain intensity (β = 0.351, p < 0.001) were both significant independent predictors, as in the main analysis. In block 2, Difficulty Identifying Feelings (β = 0.234, p = 0.004), Rumination (β = 0.260, p = 0.004) and the pain intensity x rumination interaction (β = 0.199, p = 0.003) were all significant independent predictors, as in the main analysis. In the logistic regression, pain intensity (B = 0.810, p = 0.001) was the only significant predictor in block 1, and the pain intensity x high alexithymia interaction (B = 2.165, p = 0.003) was the only significant predictor in block 2, as in the main analysis. Including known risk behaviors for medication misuse therefore did not affect the pattern of independent associations between emotion regulation factors and psychological dependence on pain medication.
The rationale for exploring all potential interactions within a single regression model was to obtain the simplest and most parsimonious model and to identify interaction effects that were associated with psychological dependence on pain medication independently of other interactions. However, we also ran regression analyses in which the interaction terms were each added separately. This showed that both the pain intensity x rumination and the pain intensity x Difficulty Identifying Feelings (DIF) interactions but none of the other interactions tested were significant when added to the model separately. When the pain intensity x rumination and pain intensity x DIF interactions were added together, only the intensity x rumination interaction was significant, as in the main analysis. Full details of those tests and simple slopes analysis of the pain intensity x DIF interaction are given in the Supplementary Material.
Previous research showed that psychological factors like personality and depression were associated with pain medication misuse among spinal pain patients (Clark et al., Citation2017; Heinemann et al., Citation1992; Krause et al., Citation2015), and examined emotion regulation factors in relation to pain medication dependence among other populations of people with chronic pain (Elander et al., Citation2014). Previous research also showed how emotion regulation factors affected outcomes such as depression and anxiety among people with pain (Soleymani et al., Citation2020). However, there was not much evidence about emotion regulation and psychological dependence on pain medication among spinal pain patients. The present findings add to existing knowledge by showing that emotion regulation factors moderate the strength of the association between pain intensity and psychological dependence, which was their specific proposed mode of action among this specific clinical sample of people with chronic spinal pain. This suggests they could be specifically targeted by initiatives to reduce pain medication use and dependence among people at risk, so that preventative and treatment approaches could go beyond focusing on aberrant drug use behaviors or other previously known risk factors.
The study limitations include the fact that the participants were predominantly of a White British ethnic background and from two UK hospitals, so findings might reflect local patterns or treatment approaches. Also, because of the focus on psychological dependence the sample included participants taking non-opioid pain medication as well as those taking opioids. However, the sample was similar to other samples in previous reports of medication use among people with spinal pain (eg., Clark et al., Citation2017; Heinemann et al., Citation1992; Krause et al., Citation2015). In fact, in Heinemann et al.’s (Citation1992) study, only 43% reported prescription medication use, compared with 88.7% in the present study, and the present sample had more frequent pain and greater pain intensity than in Krause et al.’s (Citation2015) study. However, future research might benefit from focusing on samples defined by their use of prescribed, opioid medications.
The data were all based on participants’ self-reports and, because of the need to use the measures in the form they were validated, the time frames they referred to differed. Also, for the logistic regression analyses, the pain acceptance measures were dichotomized using median splits, which can be controversial and should be avoided where possible (DeCoster et al., Citation2011). However, we used recognized cut-points wherever they existed and used median splits only where these were not available. The very large confidence intervals for the significant odds ratios in the logistic regression also likely reflect that the study was relatively underpowered for multivariate logistic analyses, so the results of the logistic regression should be treated as early-stage findings to be followed up with further research.
Finally, the study was cross-sectional, which makes all interpretations of the findings in terms of causal sequences problematic. Future work would benefit from using prospective and/or experimental designs to determine the temporal/causal nature of the observed associations between constructs.
To conclude, the findings showed that rumination and alexithymia played roles in psychological dependence on pain medication, with effects that underline how the experience of chronic pain is moderated by psychological factors. The results suggest that helping people to reduce rumination about pain and improve their ability to identify and describe feelings and emotions and discriminate between emotions and physical sensations could have potential value as part of interventions to help people with chronic pain avoid or reduce their dependence on pain medications.
Supplemental Material
Download MS Word (41.2 KB)Acknowledgements
The authors would like to thank all the participants in the study, and all the staff at the Spinal Outpatients Department, Royal Derby Hospital, and the Physiotherapy Department, London Road Community Hospital, Derby, for helping facilitate data collection, especially Lucy Vale, Sharon Falconbridge, Dominic Wetherall and Bill Wilsdon. The authors also wish to thank Emma Borrie for helping with pain medication coding, and the anonymous journal reviewers and associate editor for their helpful comments on a previous draft.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Aaron, R. V., Finan, P. H., Wegener, S. T., Keefe, F. J., & Lumley, M. A. (2020). Emotion regulation as a transdiagnostic factor underlying co-occurring chronic pain and problematic opioid use. The American Psychologist, 75(6), 796–810. https://doi.org/10.1037/amp0000678
- Adams, E. H., Breiner, S., Cicero, T. J., Geller, A., Inciardi, J. A., Schnoll, S. H., Senay, E. C., & Woody, G. E. (2006). A comparison of the abuse liability of tramadol, NSAIDs, and hydrocodone in patients with chronic pain. Journal of Pain and Symptom Management, 31(5), 465–476. https://www.sciencedirect.com/science/article/pii/S0885392406001916 https://doi.org/10.1016/j.jpainsymman.2005.10.006
- Aiken, L. S., West, S. G., & Reno, R. R. (1991). Multiple regression: Testing and interpreting. Sage.
- Andersson, G. B. (1999). Epidemiological features of chronic low-back pain. Lancet (London, England), 354(9178), 581–585. https://doi.org/10.1016/S0140-6736(99)01312-4
- Antony, M. M., Bieling, P. J., Cox, B. J., Enns, M. W., & Swinson, R. P. (1998). Psychometric properties of the 42-item and 21-item versions of the Depression Anxiety Stress Scales in clinical groups and a community sample. Psychological Assessment, 10(2), 176–181. https://doi.org/10.1037//1040-3590.10.2.176
- Arteta, J., Cobos, B., Hu, Y., Jordan, K., & Howard, K. (2016). Evaluation of how depression and anxiety mediate the relationship between pain catastrophizing and prescription opioid misuse in a chronic pain population.Pain Medicine (Malden, Mass.), 17(2), 295–303. https://doi.org/10.1111/pme.12886
- Bagby, R. M., Parker, J. D., & Taylor, G. J. (1994). The twenty-item Toronto Alexithymia Scale—I. Item selection and cross-validation of the factor structure. Journal of Psychosomatic Research, 38(1), 23–32. https://doi.org/10.1016/0022-3999(94)90005-1
- Bagby, R. M., Taylor, G. J., & Parker, J. D. (n.d). Toronto Alexithymia Scale (TAS-20). https://contextualscience.org/TAS_Measure
- Bonnet, U., Strasser, J. C., & Scherbaum, N. (2019). Screening for physical and behavioral dependence on non-opioid analgesics in a German elderly hospital population. Addictive Behaviors, 90, 265–271. https://www.sciencedirect.com/science/article/pii/S0306460318309055?casa_token=29_Pbv8rN-MAAAAA:gBA8aKqiv9lbnfcBXuGxMWcOFqteZS6jFHivb2XMwtFlZRByJYFGTZybqcHIg7MnbONUzLL0_A https://doi.org/10.1016/j.addbeh.2018.11.009
- Butler, S. F., Budman, S. H., Fernandez, K. C., Houle, B., Benoit, C., Katz, N., & Jamison, R. N. (2007). Development and validation of the current opioid misuse measure. Pain, 130(1–2), 144–156. https://doi.org/10.1016/j.pain.2007.01.014
- Clark, J. M., Cao, Y., & Krause, J. S. (2017). Risk of pain medication misuse after spinal cord injury: The role of substance use, personality, and depression. The Journal of Pain, 18(2), 166–177. https://doi.org/10.1016/j.jpain.2016.10.011
- Compton, P. A., Wu, S. M., Schieffer, B., Pham, Q., & Naliboff, B. D. (2008). Introduction of a self-report version of the Prescription Drug Use Questionnaire and relationship to medication agreement noncompliance. Journal of Pain and Symptom Management, 36(4), 383–395. https://doi.org/10.1016/j.jpainsymman.2007.11.006
- Corbelli, I., Caproni, S., Eusebi, P., & Sarchielli, P. (2012). Drug-dependence behaviour and outcome of medication-overuse headache after treatment. The Journal of Headache and Pain, 13(8), 653–660. https://doi.org/10.1007/s10194-012-0492-z
- DeCoster, J., Gallucci, M., & Iselin, A. M. R. (2011). Best practices for using median splits, artificial categorization, and their continuous alternatives. Journal of Experimental Psychopathology, 2(2), 197–209. https://doi.org/10.5127/jep.008310
- Dersh, J., Mayer, T. G., Gatchel, R. J., Polatin, P. B., Theodore, B. R., & Mayer, E. A. (2008). Prescription opioid dependence is associated with poorer outcomes in disabling spinal disorders. Spine, 33(20), 2219–2227. https://doi.org/10.1097/BRS.0b013e31818096d1
- Dhokia, M., Elander, J., Clements, K., & Gilbert, P. (2020). A randomized-controlled pilot trial of an online compassionate mind training intervention to help people with chronic pain avoid analgesic misuse. Psychology of Addictive Behaviors: Journal of the Society of Psychologists in Addictive Behaviors, 34(7), 726–733. https://doi.org/10.1037/adb0000579
- Ding, N., Yang, J., Liu, Y., & Yuan, J. (2015). Paying less but harvesting more: The effect of unconscious acceptance in regulating frustrating emotion. Science China. Life Sciences, 58(8), 799–809. https://doi.org/10.1007/s11427-015-4875-7
- Dubey, A., & Pandey, R. (2013). Mental health problems in alexithymia: Role of positive and negative emotional experiences. Journal of Projective Psychology & Mental Health, 20(2), 128–136.
- Elander, J., Duarte, J., Maratos, F. A., & Gilbert, P. (2014). Predictors of painkiller dependence among people with pain in the general population. Pain Medicine (Malden, Mass.), 15(4), 613–624. https://doi.org/10.1111/pme.12263
- Elander, J., Kapadi, R., & Bateman, A. H. (2021). Patient-reported factors associated with degree of pain medication dependence and presence of severe dependence among spinal outpatients. Pain Management, 12(3), 291–300. https://doi.org/10.2217/pmt-2021-0055
- Etcheverrigaray, F., Grall-Bronnec, M., Blanchet, M. C., Jolliet, P., & Victorri-Vigneau, C. (2014). Ibuprofen dependence: A case report. Pharmacopsychiatry, 47(3), 115–117. https://doi.org/10.1055/s-0034-1371868
- Field, A. P. (2009). Discovering statistics using IBM SPSS statistics (3rd ed.). Sage.
- Fish, R. A., McGuire, B., Hogan, M., Morrison, T. G., & Stewart, I. (2010). Validation of the Chronic Pain Acceptance Questionnaire (CPAQ) in an Internet sample and development and preliminary validation of the CPAQ-8. Pain, 149(3), 435–443. https://doi.org/10.1016/j.pain.2009.12.016
- Garland, E. L., Trøstheim, M., Eikemo, M., Ernst, G., & Leknes, S. (2020). Anhedonia in chronic pain and prescription opioid misuse. Psychological Medicine, 50(12), 1977–1988. https://doi.org/10.1017/S0033291719002010
- Gaudiano, B. A. (2011). A review of acceptance and commitment therapy (ACT) and recommendations for continued scientific advancement. The Scientific Review of Mental Health Practice, 8(2), 5–22.
- Ghandehari, O., Gallant, N. L., Hadjistavropoulos, T., Williams, J., & Clark, D. A. (2020). The relationship between the pain experience and emotion regulation in older adults. Pain Medicine (Malden, Mass.), 21(12), 3366–3376. https://doi.org/10.1093/pm/pnaa135
- Godersky, M. E., Vercammen, L. K., Ventura, A. S., Walley, A. Y., & Saitz, R. (2017). Identification of non-steroidal anti-inflammatory drug use disorder: A case report. Addictive Behaviors, 70, 61–64. https://www.sciencedirect.com/science/article/abs/pii/S0306460317300709?casa_token=joefWs4XqoQAAAAA:7eFG_yIVGvxEO2u5G7TFHxjwbN0FM4dhn8-CVUYZj9Y4V46V1CsMjvBlDysu3ZiZMnNv5I9i7w https://doi.org/10.1016/j.addbeh.2017.02.008
- Gross, J. J. (2001). Emotion regulation in adulthood: Timing is everything. Current Directions in Psychological Science, 10(6), 214–219. https://doi.org/10.1111/1467-8721.00152
- Guarino, H., Fong, C., Marsch, L. A., Acosta, M. C., Syckes, C., Moore, S. K., Cruciani, R. A., Portenoy, R. K., Turk, D. C., & Rosenblum, A. (2018). Web-based cognitive behavior therapy for chronic pain patients with aberrant drug-related behavior: Outcomes from a randomized controlled trial. Pain Medicine (Malden, Mass.), 19(12), 2423–2437. https://doi.org/10.1093/pm/pnx334
- Hayes, A. F. (2022). Introduction to mediation, moderation, and conditional process analysis: A regression-based approach (3rd ed.). Guilford Press.
- Heinemann, A. W., McGraw, T. E., Brandt, M. J., Roth, E., & Dell’Oliver, C. (1992). Prescription medication misuse among persons with spinal cord injuries. The International Journal of the Addictions, 27(3), 301–316. https://doi.org/10.3109/10826089209068744
- Hong, J., Reed, C., Novick, D., & Happich, M. (2013). Costs associated with treatment of chronic low back pain: An analysis of the UK general practice research database. Spine, 38(1), 75–82. https://doi.org/10.1097/BRS.0b013e318276450f
- Hruschak, V., Cochran, G., & Wasan, A. D. (2018). Psychosocial interventions for chronic pain and comorbid prescription opioid use disorders: A narrative review of the literature. Journal of Opioid Management, 14(5), 345–358.
- Hughes, L. S., Clark, J., Colclough, J. A., Dale, E., & McMillan, D. (2017). Acceptance and Commitment Therapy (ACT) for chronic pain. The Clinical Journal of Pain, 33(6), 552–568. https://doi.org/10.1097/AJP.0000000000000425
- Jamison, R. N., & Edwards, R. R. (2013). Risk factor assessment for problematic use of opioids for chronic pain. The Clinical Neuropsychologist, 27(1), 60–80. https://doi.org/10.1080/13854046.2012.715204
- Kano, M., & Fukudo, S. (2013). The alexithymic brain: The neural pathways linking alexithymia to physical disorders. BioPsychoSocial Medicine, 7(1), 1. https://doi.org/10.1186/1751-0759-7-1
- Kapadi, R., Elander, J., & Bateman, T. (2021). A systematic review of evidence about the role of alexithymia in chronic back pain. Health Psychology Update, 30(1), 3–13. https://doi.org/10.53841/bpshpu.2021.30.1.3
- Kelly, J. F., Magill, M., Slaymaker, V., & Kahler, C. (2010). Psychometric validation of the Leeds Dependence Questionnaire (LDQ) in a young adult clinical sample. Addictive Behaviors, 35(4), 331–336. https://doi.org/10.1016/j.addbeh.2009.11.005
- Koechlin, H., Coakley, R., Schechter, N., Werner, C., & Kossowsky, J. (2018). The role of emotion regulation in chronic pain: A systematic literature review. Journal of Psychosomatic Research, 107, 38–45. https://doi.org/10.1016/j.jpsychores.2018.02.002
- Kratz, A. L., F Murphy, J., Kalpakjian, C. Z., & Chen, P. (2018). Medicate or meditate? Greater pain acceptance is related to lower pain medication use in persons with chronic pain and spinal cord injury.The Clinical Journal of Pain, 34(4), 357–365. https://doi.org/10.1097/AJP.0000000000000550
- Krause, J. S., Clark, J. M. R., & Saunders, L. L. (2015). Pain medication misuse among participants with spinal cord injury. Spinal Cord, 53(8), 630–635. https://doi.org/10.1038/sc.2015.42
- Larance, B., Bruno, R., Lintzeris, N., Degenhardt, L., Black, E., Brown, A., Nielsen, S., Dunlop, A., Holland, R., Cohen, M., & Mattick, R. P. (2016). Development of a brief tool for monitoring aberrant behaviours among patients receiving long-term opioid therapy: The Opioid-Related Behaviours in Treatment (ORBIT) scale. Drug and Alcohol Dependence, 159, 42–52. https://doi.org/10.1016/j.drugalcdep.2015.11.026
- Loas, G., Fremaux, D., & Boyer, P. (1997). Anhedonia and alexithymia: Distinct or overlapping constructs. Perceptual and Motor Skills, 84(2), 415–425. https://doi.org/10.2466/pms.1997.84.2.415
- Lovibond, P. F., & Lovibond, S. H. (1995a). The structure of negative emotional states: Comparison of the Depression Anxiety Stress Scales (DASS) with the Beck Depression and Anxiety Inventories. Behaviour Research and Therapy, 33(3), 335–343. https://doi.org/10.1016/0005-7967(94)00075-u
- Lovibond, S. H., & Lovibond, P. F. (1995b). Manual for the depression anxiety & stress scales (2nd ed.). Psychology Foundation.
- Lumley, M. A., Beyer, J., & Radcliffe, A. (2008). Alexithymia and physical health problems: A critique of potential pathways and a research agenda. In A. Vingerhoets, I. Nyklíček, & J. Denollet (Eds.), Emotion regulation: Conceptual and clinical issues (pp. 43–68). Springer Science + Business Media.
- Lumley, M. A., Stettner, L., & Wehmer, F. (1996). How are alexithymia and physical illness linked? A review and critique of pathways. Journal of Psychosomatic Research, 41(6), 505–518. https://doi.org/10.1016/s0022-3999(96)00222-x
- Manchikanti, L., Singh, V., Datta, S., Cohen, S. P., & Hirsch, J. A. (2009). Comprehensive review of epidemiology, scope, and impact of spinal pain. Pain Physician, 12(4), E35–E70. https://doi.org/10.36076/ppj.2009/12/E35
- Martel, M. O., Dolman, A. J., Edwards, R. R., Jamison, R. N., & Wasan, A. D. (2014). The association between negative affect and prescription opioid misuse in patients with chronic pain: The mediating role of opioid craving.The Journal of Pain, 15(1), 90–100. https://doi.org/10.1016/j.jpain.2013.09.014
- McDonough, M., Johnson, J. L., White, J. M., & Buisman-Pijlman, F. T. (2019). Measuring opioid dependence in chronic pain patients: A comparison between addiction clinic and pain clinic patient populations. Journal of Opioid Management, 15(4), 285–293. https://doi.org/10.5055/jom.2019.0514
- Melin, E. O., Thulesius, H. O., & Persson, B. A. (2010). Affect School for chronic benign pain patients showed improved alexithymia assessments with TAS-20. BioPsychoSocial Medicine, 4(1), 5. https://doi.org/10.1186/1751-0759-4-5
- Oberleitner, L. M. S., Lumley, M. A., Grekin, E. R., M Z Smith, K., Loree, A. M., Carty, J. N., & Valentino, D. (2019). Problematic prescription opioid use in a chronic pain treatment facility: The role of emotional processes. Substance Use & Misuse, 54(3), 495–505. https://doi.org/10.1080/10826084.2018.1521426
- Osman, A., Barrios, F. X., Kopper, B. A., Hauptmann, W., Jones, J., & O’neill, E. (1997). Factor structure, reliability, and validity of the Pain Catastrophizing Scale. Journal of Behavioral Medicine, 20(6), 589–605. https://doi.org/10.1023/a:1025570508954
- Parker, J. D., Michael Bagby, R., Taylor, G. J., Endler, N. S., & Schmitz, P. (1993). Factorial validity of the 20‐item Toronto Alexithymia Scale. European Journal of Personality, 7(4), 221–232. https://doi.org/10.1002/per.2410070403
- Parker, J. D., Taylor, G. J., & Bagby, R. M. (2003). The 20-Item Toronto Alexithymia Scale: III. Reliability and factorial validity in a community population. Journal of Psychosomatic Research, 55(3), 269–275. https://doi.org/10.1016/s0022-3999(02)00578-0
- Raistrick, D., Bradshaw, J., Tober, G., Weiner, J., Allison, J., & Healey, C. (1994). Development of the Leeds Dependence Questionnaire (LDQ): A questionnaire to measure alcohol and opiate dependence in the context of a treatment evaluation package. Addiction (Abingdon, England), 89(5), 563–572. https://doi.org/10.1111/j.1360-0443.1994.tb03332.x
- Raistrick, D., Tober, G., Sweetman, J., Unsworth, S., Crosby, H., & Evans, T. (2014). Measuring clinically significant outcomes–LDQ, CORE-10 and SSQ as dimension measures of addiction. Psychiatric Bulletin (2014), 38(3), 112–115. https://doi.org/10.1192/pb.bp.112.041301
- Safavi, S., Farmani, F., & Sadr, A. R. (2016). The efficacy of the emotion regulation intervention on coping styles in patients with alexithymia. International Journal of Applied Behavioral Sciences, 3(4), 26–34.
- Said, O., Elander, J., & Maratos, F. (2019). An international study of analgesic dependence among people with pain in the general population. Substance Use & Misuse, 54(8), 1319–1331. https://doi.org/10.1080/10826084.2019.1577457
- Schoenfeld, A. J., Belmont, P. J., Jr, Blucher, J. A., Jiang, W., Chaudhary, M. A., Koehlmoos, T., Kang, J. D., & Haider, A. H. (2018). Sustained preoperative opioid use is a predictor of continued use following spine surgery. The Journal of Bone and Joint Surgery. American Volume, 100(11), 914–921. https://doi.org/10.2106/JBJS.17.00862
- Schoenfeld, A. J., Nwosu, K., Jiang, W., Yau, A. L., Chaudhary, M. A., Scully, R. E., Koehlmoos, T., Kang, J. D., & Haider, A. H. (2017). Risk factors for prolonged opioid use following spine surgery, and the association with surgical intensity, among opioid-naive patients. The Journal of Bone and Joint Surgery. American Volume, 99(15), 1247–1252. https://doi.org/10.2106/JBJS.16.01075
- Schreiber, L. R., Grant, J. E., & Odlaug, B. L. (2012). Emotion regulation and impulsivity in young adults.Journal of Psychiatric Research, 46(5), 651–658. https://doi.org/10.1016/j.jpsychires.2012.02.005
- Sheppes, G., Suri, G., & Gross, J. J. (2015). Emotion regulation and psychopathology. Annual Review of Clinical Psychology, 11(1), 379–405. https://doi.org/10.1146/annurev-clinpsy-032814-112739
- Smeets, R. J., Vlaeyen, J. W., Kester, A. D., & Knottnerus, J. A. (2006). Reduction of pain catastrophizing mediates the outcome of both physical and cognitive-behavioral treatment in chronic low back pain. The Journal of Pain, 7(4), 261–271. https://doi.org/10.1016/j.jpain.2005.10.011
- Soleymani, A., Arani, A. M., Raeissadat, S. A., & Davazdahemami, M. H. (2020). Rumination-Focused cognitive-behavioral therapy for chronic low back pain: A randomized controlled trial. Galen Medical Journal, 9, e1722. https://doi.org/10.31661/gmj.v9i0.1722
- Spek, V., Nyklícek, I., Cuijpers, P., & Pop, V. (2008). Alexithymia and cognitive behaviour therapy outcome for subthreshold depression. Acta Psychiatrica Scandinavica, 118(2), 164–167. https://doi.org/10.1111/j.1600-0447.2008.01199.x
- Stubbs, B., Koyanagi, A., Thompson, T., Veronese, N., Carvalho, A. F., Solomi, M., Mugisha, J., Schofield, P., Cosco, T., Wilson, N., & Vancampfort, D. (2016). The epidemiology of back pain and its relationship with depression, psychosis, anxiety, sleep disturbances, and stress sensitivity: Data from 43 low-and middle-income countries. General Hospital Psychiatry, 43, 63–70. https://doi.org/10.1016/j.genhosppsych.2016.09.008
- Sturgeon, J. A. (2014). Psychological therapies for the management of chronic pain. Psychology Research and Behavior Management, 7, 115–124. https://doi.org/10.2147/PRBM.S44762
- Sullivan, M. J. L. (2009). The Pain Catastrophising Scale: User manual. McGill University. https://centerforinsightmedicine.com/wp-content/uploads/2022/02/PCSManual.pdf
- Sullivan, M. J. L., Bishop, S. R., & Pivik, J. (1995). The pain catastrophizing scale: Development and validation. Psychological Assessment, 7(4), 524–532. https://doi.org/10.1037/1040-3590.7.4.524
- Swift, L., Stephenson, R., & Royce, J. (2006). The 20‐item Toronto Alexithymia Scale: Validation of factor solutions using confirmatory factor analysis on physiotherapy out‐patients. Psychology and Psychotherapy, 79(Pt 1), 83–88. https://doi.org/10.1348/147608305X42875
- Taylor, G. J., Bagby, R. M., & Parker, J. D. (1992). TAS-20 package. http://www.gtaylorpsychiatry.org/tas.htm
- Tulipani, C., Morelli, F., Spedicato, M. R., Maiello, E., Todarello, O., & Porcelli, P. (2010). Alexithymia and cancer pain: The effect of psychological intervention. Psychotherapy and Psychosomatics, 79(3), 156–163. https://doi.org/10.1159/000286960
- Vest, N., Reynolds, C. J., & Tragesser, S. L. (2016). Impulsivity and risk for prescription opioid misuse in a chronic pain patient sample. Addictive Behaviors, 60, 184–190. https://doi.org/10.1016/j.addbeh.2016.04.015
- Vowles, K. E., Witkiewitz, K., Cusack, K. J., Gilliam, W. P., Cardon, K. E., Bowen, S., Edwards, K. A., McEntee, M. L., & Bailey, R. W. (2020). Integrated behavioral treatment for veterans with co-morbid chronic pain and hazardous opioid use: A randomized controlled pilot trial. The Journal of Pain, 21(7–8), 798–807. https://doi.org/10.1016/j.jpain.2019.11.007
- Walid, M. S., Hyer, L., Ajjan, M., Barth, A. C., & Robinson, J. S.Jr, (2007). Prevalence of opioid dependence in spine surgery patients and correlation with length of stay. Journal of Opioid Management, 3(3), 127–132. https://doi.org/10.5055/jom.2007.0050
- Watkins, E. R., & Roberts, H. (2020). Reflecting on rumination: Consequences, causes, mechanisms and treatment of rumination. Behaviour Research and Therapy, 127, 103573. https://doi.org/10.1016/j.brat.2020.103573
- Wheeler, C. H., Williams, A. C. D. C., & Morley, S. J. (2019). Meta-analysis of the psychometric properties of the Pain Catastrophizing Scale and associations with participant characteristics. Pain, 160(9), 1946–1953. https://doi.org/10.1097/j.pain.0000000000001494
- Wong, W. S., & Fielding, R. (2013). Suppression of emotion expression mediates the effects of negative affect on pain catastrophizing: A cross-sectional analysis. The Clinical Journal of Pain, 29(10), 865–872. https://doi.org/10.1097/AJP.0b013e31827da3b5