ABSTRACT
Introduction: We hypothesized that chest compressions located directly over the left ventricle (LV) would improve hemodynamics, including coronary perfusion pressure (CPP), and return of spontaneous circulation (ROSC) in a swine model of cardiac arrest. Methods: Transthoracic echocardiography (echo) was used to mark the location of the aortic root and the center of the left ventricle on animals (n = 26) which were randomized to receive chest compressions in one of the two locations. After a period of ten minutes of ventricular fibrillation, basic life support (BLS) with mechanical cardiopulmonary resuscitation (CPR) was initiated and performed for ten minutes followed by advanced cardiac life support (ACLS) for an additional ten minutes. During BLS the area of maximal compression was verified using transesophageal echo. CPP and other hemodynamic variables were averaged every two minutes. Results: Mean CPP was not significantly higher in the LV group during all time intervals of resuscitation; mean CPP was significantly higher in the LV group during the 12–14 minute interval of BLS and during minutes 22–30 of ACLS (p < 0.05). Aortic systolic and diastolic pressures, right atrial systolic pressures, and end-tidal CO2 (ETCO2) were higher in the LV group during all time intervals of resuscitation (p < 0.05). Nine of the left ventricle group (69%) achieved ROSC and survived to 60 minutes compared to zero of the aortic root group (p < 0.001). Conclusions: In our swine model of cardiac arrest, chest compressions over the left ventricle improved hemodynamics and resulted in a greater proportion of animals with ROSC and survival to 60 minutes.
Introduction
Since cardiac arrest is one of the leading causes of death among adults over the age of forty, small incremental improvements in survival can translate into thousands of lives saved each year.Citation1–3 Current cardiopulmonary resuscitation (CPR) guidelines, place an emphasis on “pushing hard” (a depth of at least 5cm in adults), “pushing fast” (a rate of at least 100 min−1), and allowing for full recoil during compressions since these maneuvers increase survival.Citation4–13 However, the optimum location for chest compressions during CPR is unknown.
The current recommended location for chest compressions during CPR is on “the center of the victim's chest.”Citation12,13 There may be a more ideal location. The outcome of cardiac arrest victims is related to the vital organ perfusion generated by chest compressions, however, under ideal laboratory conditions myocardial and cerebral blood flow generated during standard closed chest compressions are only 10–40% of normal.Citation14–16 Previous authors have reported that direct compression of the ventricles is the most effective way to generate blood flow during CPR; however, standard chest compressions usually do not directly compress the heart.Citation13,17 Several human radiologic studies have proposed that the recommended location for chest compressions may be suboptimal because it usually over the ascending thoracic aorta or the left ventricular outflow tract rather than centered over the heart.Citation18–22 These findings suggest that the current site of compressions may inhibit antegrade blood flow; however, there are currently no published reports to support this assumption.
We are unaware of any literature that reports a difference in vital organ perfusion when chest compressions are performed directly over the heart rather than the standard location. We hypothesized that compressions located directly over the left ventricle (LV) would increase mean coronary perfusion pressure (CPP), the principal determinant of myocardial perfusion, over a 20 minute period of resuscitation when compared to standard compressions in a swine model of cardiac arrest; in our model we used the aortic root (AR) to represent the location of standard compressions based on the human radiologic studies which have shown that standard chest compressions are usually located over the ascending thoracic aorta. Secondary analyses included an evaluation of the rate of return of spontaneous circulation (ROSC) between the two groups as well as an assessment of hemodynamic and laboratory variables.
Methods
Study Design
We conducted a prospective, randomized comparative investigation approved by our Institutional Animal Care and Use Committee. All procedures involving animals complied with the regulations and guidelines of the Animal Welfare Act, the National Institutes of Health Guide for the Care and Use of Laboratory Animals, and the American Association for Accreditation of Laboratory Animal Care. The housing of animals and the performance of the study took place in the Animal Care Facility at our institution. Reporting adheres to the Animals in Research Reporting In Vivo Experiments (ARRIVE) guidelines.Citation23
Population and Setting
Twenty-six female Yorkshire swine weighing 25–32kg were obtained 2–4 days before experimentation to allow acclimation to our facility. Per the vendor, the animals were free from viral, bacterial, and parasitic pathogens.
Animals were housed individually in 4 × 4 foot cages with rubberized textured flooring in a temperature and humidity controlled building with a 12 hour light/dark cycle set on a timer. Animals were allowed free access to water and were provided a maintenance diet (PMI Nutrition International, LLC, Brentweed, MO, USA). Within 48 hours of arrival to the facility a physical exam of each animal was performed to evaluate for lesions and to ensure normal heart and lung sounds. A complete blood cell count and a blood chemistry analysis were also performed. No pre-treatment with any medications was performed.
All experiments were initiated during the morning hours. Animals were initially sedated with 20 mg/kg intramuscular ketamine; general anesthesia was subsequently induced and mechanical ventilation initiated (Fabius GS; Draeger-Siemens, New York, NY) with a mixture of 60% oxygen and isoflurane (1–2.5%) with a tidal volume of 10 mL/kg at a respiratory rate of 12 min−1. End-tidal CO2 (ETCO2) was monitored by in-line waveform capnography, and the respiratory rate was adjusted to maintain an ETCO2 between 38 and 42 mm Hg prior to induction of cardiac arrest. Continuous cardiac rhythm and heart rate were monitored by electrocardiography (ECG) using standard limb leads. Peripheral capillary pulse oximetry (SpO2) was also monitored continuously. The anesthesia used in this experiment is standard for swine models. All other drugs, routes of administration, and timing of administration are those outlined in CPR guidelines.Citation12,13 Standard weight based doses were used.
Experimental Protocol
High fidelity, solid state micromanometer-tipped pressure transducers (Millar MPC-500; Millar Inc., Houston, TX) were advanced through right internal jugular vein and right femoral artery into thoracic locations to measure continuous aortic and right atrial pressures, respectively. Unfractionated heparin (100 u/kg) was provided to prevent catheter clotting. Near infrared spectroscopy (NIRS) sensors were adhered to the scalp and the right flank to continuously monitor and record cerebral and renal regional oximetry (rSO2), respectively (INOVS 5100C Cerebral/Somatic Oximeter; Covidien, Minneapolis, MN). Once all catheters and sensors were in place, the animals were allowed to acclimate for 10 minutes, and each animal received a bolus of 15 mL/kg of 0.9% saline intravenously to replace overnight fasting fluid deficits.
Animals were placed in a v-shaped trough to eliminate lateral movements during chest compressions. During the 10 minute acclimatization period, transthoracic echocardiography (TTE) (z.one ultra sp; Zonare Medical Systems, Inc., Mountain View, CA) was used to locate the AR and the center of the LV in two orthogonal planes (the parasternal long axis and parasternal short axis). The animals' skin was marked in the mid-sternum at the level of the AR to represent the standard compression location and at the center of the LV to represent the LV compression location. A multiplane transesophageal (TEE) transducer (P8–3TEE; Zonare Medical Systems, Inc., Mountain View, CA) was used to obtain a mid-esophageal long axis (ME LAX) view of the heart. The TEE transducer was left in place for recording the area of maximal compression (AMC) during compressions.
Animals were randomly allocated to LV or standard chest compression groups. Allocation was performed using a commonly employed computer-generated randomization program (http://www.randomization.com). Randomization was performed for all animals prior to the beginning of the study and the results for each animal were kept sealed until ventricular fibrillation (VF) had been induced. A graphic display of the general protocol is presented in , and a more detailed outline of the ACLS portion of the protocol is presented in .
Figure 1. (a) Experimental protocol timeline; (b) Detailed ACLS protocol timeline. *rhythm check; BLS: Basic Life Support; ALCS: Advanced Cardiac Life Support; Post Resusc: Post Resuscitation; VF: ventricular fibrillation; ROSC: return of spontaneous circulation; J: joules; CPR: cardiopulmonary resuscitation; FiO2: fraction of inspired oxygen; mg: milligram; kg: kilogram; Epi: epinephrine; Amio: amiodarone; prn: as needed; gtt: infusion.
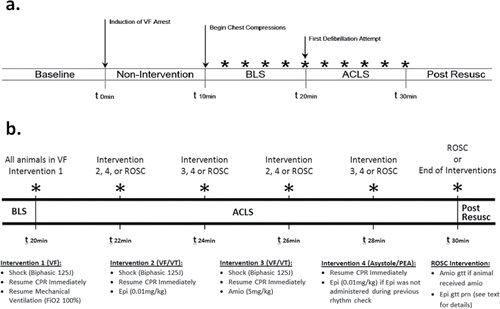
Ventricular fibrillation was induced with a 3-second 60Hz 100 mA electric current delivered across the thorax (Model 1745A Power Supply; B&K Precision Corporation, Yorba Linda, CA) as previously described.Citation24,25 VF was confirmed by ECG, the sudden loss of arterial pulsations, and an abrupt reduction of the systolic blood pressure to less than 25 mmHg. The induction of cardiac arrest represented time zero during the experiment; mechanical ventilation and anesthesia were simultaneously discontinued at time zero. All animals remained in VF arrest without any intervention for a period of 10 minutes. During the 10 minute arrest period, the allocation to either the standard or LV compression groups was unblinded, and the center of the piston on the automatic mechanical compression device (AMCD) (Thumper 407CC; Michigan Instruments, Grand Rapids, MI) was lowered into place over the corresponding skin marking. Defibrillation pads were placed over the right and left lateral chest and connected to a biphasic electronic defibrillator/monitor (Lifepak 20; Physio Control Inc., Redmond, WA).
Basic Life Support
After 10 minutes of cardiac arrest, Basic Life Support (BLS), was initiated using the AMCD over the allocated position; compressions were delivered at a rate of 100 min−1, at a depth of 5cm, with a 50% duty cycle and a compression-ventilation ratio of 30:2. Compressions were briefly interrupted every two minutes to perform a rhythm analysis that lasted 2–5 seconds. During BLS, a 10-second video clip of the ME LAX view was saved for future review. Although the use of automated external defibrillators (AED) has been incorporated into BLS algorithms, a 10-minute interval of BLS without defibrillation was used as a practical approach because this duration of CPR is necessary to adequately compare CPR techniques.Citation25 Additionally, most bystanders do not have access to an AED and emergency medical services (EMS) response times can approach or exceed 10 minutes, which allowed our BLS period to approximate bystander BLS prior to the arrival of ACLS qualified personnel.Citation3,27,28
Advanced Cardiac Life Support
After 10 minutes of BLS, advanced cardiac life support (ACLS), was initiated with a 125 Joule (J)(approximately 4J/kg) biphasic waveform defibrillation attempt, resumption of mechanical ventilation with 100% oxygen, and continuous compressions at the same rate and depth (). Every two minutes compressions were interrupted for a rhythm analysis that lasted 2–5 seconds. If the rhythm was VF or ventricular tachycardia (VT), another 125J defibrillation attempt was provided and compressions were re-initiated. If the animal was in asystole or an organized rhythm, no defibrillation attempt was made and compressions were re-initiated; if an organized rhythm was present at a second consecutive rhythm analysis, compressions were only re-initiated if the animal did not meet criteria for ROSC. At the second and fourth ACLS rhythm analyses, epinephrine (0.01 mg/kg) followed by a 10-mL normal saline flush was administered if the animal had not met criteria for ROSC. During the third and fifth ACLS rhythm analyses, amiodarone (5 mg/kg) followed by a 10-mL normal saline flush was administered if the animal had not met criteria for ROSC and was in a defibrillation-appropriate rhythm (VF or VT).
Return of Spontaneous Circulation and Post-Resuscitation Care
Return of spontaneous circulation was defined as an organized rhythm with a sustained aortic systolic blood pressure greater than 60 mm Hg without any intervention for one minute during a scheduled rhythm check. If ROSC was attained, the animals were supported in a simulated intensive care setting until termination of the protocol at minute 60. After ROSC, mechanical ventilation was provided with the initial ventilator settings and 100% oxygen. Respiratory rate was adjusted to maintain an ETCO2 of 38–42 mmHg. Inhaled isoflurane was administered as necessary.
An epinephrine infusion was started as needed, at a rate of 0.1 mcg/kg/min and titrated by 0.1 mcg/kg/min every two minutes to a maximum of 2.0 mcg/kg/min, to maintain an aortic systolic blood pressure (SBP) greater than 90 mmHg. If the SBP rose above 120 mmHg the epinephrine was titrated down by 0.1 mcg/kg/min every two minutes. An amiodarone infusion (5 mg/kg/hr) was started if the animal had received amiodarone during ACLS.
Termination of the Protocol
Animals were considered expired if the aortic SBP was less than 60 for 10 minutes after minute 30. Expired animals were euthanized with 100 mg/kg sodium pentobarbital, and mechanical ventilation was terminated. Animals that did attain ROSC were supported until minute 60, to ascertain short-term viability; at this time all life support, including medication infusions and mechanical ventilation, were terminated and the remaining animals were euthanized. No post-care was performed as all animals were euthanized at the end of the study.
Measurements
Hemodynamic data (aortic systolic and diastolic blood pressure, right atrial systolic and diastolic blood pressure, SpO2, ETCO2, cerebral and renal regional oximetry) were continuously monitored and averaged for two minute intervals at baseline and from minutes 10 to 30 of the protocol. Baseline for hemodynamic measurements was defined as the two-minute interval immediately prior to time zero. CPP was calculated as the difference between the end-diastolic aortic pressure and the simultaneous end-diastolic right atrial pressure. Mean CPP was derived for successive 2 minute intervals during the resuscitative period.
Arterial blood gas specimens were obtained from the left carotid artery at baseline (immediately prior to time zero), and at 10, 20, and 30 minutes during the protocol.
The number of animals that attained ROSC in each group and the number of ROSC animals that survived to 60 minutes was subsequently recorded. The total amount of epinephrine and amiodarone that each animal received were also recorded.
The ten second TEE video recordings were randomly compiled into a file that was independently assessed at the conclusion of all data collection by an investigator who is certified in TEE and blinded to the remainder of the data. This investigator rated the AMC in each video as being over the LV or AR.
Key Outcome Measures
The primary outcome was the difference in mean CPP between the standard and LV groups over the 20 minute resuscitation period (minutes 10–30). Secondary outcomes included the difference in: 1) ROSC, 2) the remaining hemodynamic variables, and 3) arterial blood gas variables between the standard and LV groups. All time intervals during the resuscitation period were included in the primary analysis regardless of ROSC since the effect that compressions over the LV would have on hemodynamics was unknown. CPP between ROSC and non-ROSC animals was also determined, and the total amount of epinephrine and amiodarone in each experimental group was determined as well.
Analytical Methods
The experimental unit for analysis was an individual animal. No animals or data were excluded. Means and standard deviations were calculated for measured variables of each treatment group across all resuscitation time intervals, baseline and end-of-study. Test for normality was performed by Shapiro-Wilk test.Citation29 To control for within subject variation across time intervals a repeated measures (within-subject) analysis of variance was used.Citation30 The Bonferroni method was used to correct the level of significance for multiple comparisons. For the primary outcome (CPP), a Welch two sample t-test was used to compare differences within measurement times between treatment groups. A non-weighted Cohen's Kappa (κ) was used to calculate the agreement between AMC rating by TEE and AMCD placement on the thorax. Statistical analysis was performed using R Version 3.0.3 (Vienna Austria).
Sample Size Determinations
CPP was used to estimate a priori power. Based on prior, unpublished work by our group, an effect size, f, of 0.57 was estimated to detect a 1-SD difference in CPP between the treatment groups. To demonstrate the equivalency of the treatments, a β of 0.05 was used to avoid a false negative. Based on this design and an expected mortality of 75%, a total of 26 subjects were required (13 subjects × 2 groups × 7 repeated measures) to produce a sample size of 182. We tested the sensitivity of the power to a reduction in the number of subjects.
Results
There was no difference in the size of the animals or the baseline hemodynamic and laboratory measures between the standard and LV groups (p > 0.05 for all measures) (). There was 100% agreement between the AMC by TEE review and location of AMCD placement (κ = 1.0, 95% CI 1.0–1.0).
Table 1. Baseline characteristics of animals
Coronary Perfusion Pressure
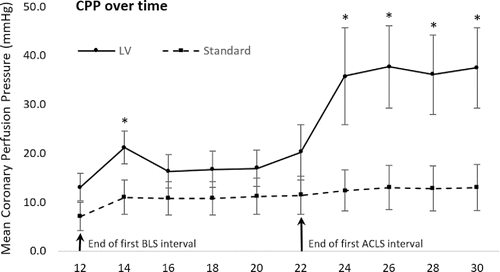
The primary outcome of mean CPP was not significantly higher in the LV group during all time intervals of resuscitation (). Mean CPP was significantly higher in the LV group during the 12–14 minute interval of BLS and during minutes 22–30 of ACLS (p < 0.05), however there was no difference in CPP during the 14–22 minute interval (p > 0.05) ().
Table 2. Repeated-measures analysis of variance for hemodynamic and arterial blood gas variables
Figure 2. Difference in mean coronary perfusion pressure between treatment groups. Standard error bars represent standard error of the mean. * signifies a significant difference between LV and Standard treatment groups (p < 0.05).
The mean CPP among animals that attained ROSC was higher than the mean CPP of non-ROSC animals (p < 0.0001) (). The mean CPP for ROSC animals during the BLS period was 18.0 (±13.5 SD) and the CPP for non-ROSC animals during BLS was 11.1 (±11.3 SD) (p = 0.002). During the ACLS period, the mean CPP for ROSC animals was 42.3 (± 30.7 SD) and the CPP for non-ROSC animals was 12.9 (±14.0 SD) (p < 0.001). Among the non-ROSC animals there was no significant difference in CPP between the LV and standard groups (p > 0.05) ().
Table 3. Mean coronary perfusion pressure of animals that did and did not attain return of spontaneous circulation in each treatment group
Return of Spontaneous Circulation and Short-term Survival
The number of animals that attained ROSC was significantly higher in the LV group (p < 0.001) (). All animals that attained ROSC did so during the ACLS period (two at minute 26, five at minute 28, and two at minute 30), and all animals that attained ROSC survived to 60 minutes.
Table 4. Return of spontaneous circulation and short-term survival in each treatment group
Hemodynamic and Laboratory Variables
The difference in the hemodynamic and mean blood gas variables between the LV and standard experimental groups are demonstrated in . Aortic systolic and diastolic pressures, right atrial systolic pressures, and end-tidal CO2 (ETCO2) were higher in the LV group during all time intervals of resuscitation (p < 0.05). Detailed graphical representation of hemodynamic and ABG values over time during the resuscitation period are presented in Supplements 1 and 2, respectively. The mean total dose of epinephrine was similar between the LV group (0.54 mg ± 0.15 mg SD) and the standard group (0.60 mg ± 0.06 mg SD) (p > 0.05). The mean total dose of amiodarone was also similar between the LV group (228 mg ± 93.9 mg SD) and the standard group (240 mg ± 71.6 mg SD) (p > 0.05).
Adverse Events
There were no adverse events to report, and no modifications to the protocol were required to reduce adverse events.
Discussion
In our study we detected a difference in hemodynamic variables and ROSC when chest compressions were performed over the LV rather than the aortic root in our swine model of VF cardiac arrest. Our primary outcome variable, mean CPP, was higher in the LV group during the final eight minutes of ACLS, and the incidence of ROSC was higher in the LV group (69% vs 0.0%). Although mean CPP in the LV group was higher during the BLS period as well, the difference was not significant except during the 12–14 minute interval. Aortic systolic and diastolic pressures, right atrial systolic pressures, and ETCO2 were higher in the LV group during all time intervals of resuscitation. Secondary outcomes were exploratory in nature and will be further investigated in future studies.
Recent radiologic and clinical studies have reported findings that suggest the current standard location for chest compressions on “the center of the victim's chest” is not optimal because the heart is not located beneath this location. The thorax is more compressible caudally, and hemodynamic measures including peak arterial pressure and ETCO2 increase when compressions are performed at the lower end of the sternum, more directly over the heart, rather than at the standard location.Citation12,13,21,31–33 The increase in ETCO2 with distal compressions is of particular interest since increases in ETCO2 have repeatedly been shown to be correlated with ROSC, and ETCO2 has been proposed as a surrogate measure for blood flow during CPR.Citation34–40 The most significant change detected in our study was an increase in ETCO2 during every time interval of resuscitation (). Our study is the first to demonstrate that ETCO2 and ROSC can be improved by placing compressions directly over the left ventricle.
In our experiment the higher rate of ROSC among the LV group may be due to an increase in the number of animals meeting a threshold CPP during the BLS interval. illustrates that the difference in CPP between the standard and LV groups was not significant during the BLS period except for the 12–14 minute interval; however, analysis of ROSC animals demonstrated that the mean CPP of the ROSC animals was significantly higher than non-ROSC animals during both BLS and ACLS. This observation highlights the importance of meeting a minimum threshold CPP to attain ROSC. Both human and animal studies have demonstrated that failure to generate a CPP of at least 15–20 mm Hg during CPR is rarely associated with successful resuscitation.Citation5–7,23,41,42 Our findings indicate that the threshold CPP for ROSC may be met more often when compressions are performed directly over the LV.
The increased CPP in the LV group during ACLS may be due to the increased presence of ROSC among the LV group in our experiment. demonstrates a divergence in mean CPP between treatment arms during the ACLS period; however, the significant difference in CPP did not remain between arms when the ROSC animals were removed from the analysis (). Two possible explanations for the divergence in CPP during ACLS are that the LV animals received different quantities of hemodynamically active medications or that the animals which attained ROSC were able to generate higher CPPs once they had synchronized cardiac activity. Medication administration is not likely the etiology, since the amount of epinephrine and amiodarone that the two groups received was not different. Therefore, the difference in CPP during ACLS may be due to the animals regaining spontaneous cardiac activity.
Our investigation is the first to demonstrate a difference in ROSC between two different chest compression locations in an animal model. This difference in ROSC and any subsequent clinical importance should be established in humans before accepting this approach. If future human research demonstrates that an increase in ROSC can be attained with chest compressions over the LV, these findings must still be made applicable to the lay rescuer, or to the first providers who are performing CPR, during the most time-sensitive period of CPR to become strong enough to support a change in practice. There may be a more optimal landmark than the “center of the chest” for the lay rescuer. Many emergency physicians and some prehospital providers are already facile with limited ultrasound modalities and could likely reproduce the same simple TTE techniques we used to locate the left ventricle in this animal study.Citation43 More scientific work is needed to aid laypersons and more advanced providers in identifying the optimal location for chest compressions.
Limitations
Our laboratory study had a few limitations. First, although swine are commonly used in cardiac arrest studies due to anatomic and physiologic similarities to humans, differences still remain; the swine heart is more vertically oriented, there is an extra lobe of lung in the left hemithorax and there are differences in chest wall anatomy which somewhat alter compression mechanics.Citation44–47 In the average human, compression directly over the left ventricle would likely occur even more laterally on the chest that in swine. None of these anatomic differences diminish the importance of the finding that chest compressions in different locations on the chest alter CPP and the rate of ROSC. Additionally, we analyzed young healthy swine which may have physiology that is not typical of cardiac arrest patients. Second, we were unable to completely blind the treatment arms. Preparation for each experiment was performed with all laboratory personnel completely blinded to the treatment arms, however, once the AMCD arm had been lowered into position blinding could no longer be maintained. However, strict adherence to resuscitation and post-resuscitation protocols was upheld to minimize potential treatment bias, and we reported objective data to limit subjectivity in the interpretation of our results. Third, necropsy was not performed, so we cannot report on differences in injuries caused by compressions between the two experimental groups. Currently, there is not enough evidence to support any one location of compressions based on the incidence of injuries.Citation48–53 The risk-benefit ratio in regards to complications from compressions in different locations is unknown and will require further investigation to elucidate; however, the findings of our animal study suggests that compressions as close to the heart as possible may allow cardiac arrest victims the best opportunity at survival regardless of injury pattern. Fourth, the variance of CPP values measured was larger than anticipated, so we may not have had the power required to detect differences that may exist in CPP during the BLS period; however, the differences that were detected are not likely to be due to spurious results due to correction by the Bonferroni method. Furthermore, it is also unclear whether the increased CPP during ACLS was a consequence of higher ROSC or vice versa. Finally, this study only addressed VF as the initial cardiac rhythm.
Conclusions
In summary, closed chest compressions directed over the left ventricle resulted in improved hemodynamics and a higher rate of ROSC and survival to 60 minutes as compared to chest compressions in the standard location in a swine model of cardiac arrest. Although our primary outcome, CPP, was not improved during the entire resuscitation period, CPP was higher during ACLS with LV compressions. Secondary outcomes including aortic systolic and diastolic pressures, right atrial systolic pressures, and ETCO2 were higher in the LV group during all time intervals of resuscitation; ROSC and survival to 60 minutes were also improved in the LV group.
Supplement_2_-_ABG_Values.pdf
Download PDF (227.2 KB)Supplement_1_-_Hemodynamic_Values.pdf
Download PDF (248 KB)References
- Mozaffarian D, Benjamin EJ, Go AS, et al. American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics-2016 update: A report from the American Heart Association. Circulation. 2016;133(4):e38–360.
- Dunne RB, Compton S, Zalenski RJ, Swor R, Welch R, Bock BF. Outcomes from out-of-hospital cardiac arrest in Detroit. Resuscitation. 2007;72(1):59–65.
- Sasson C, Rogers MA, Dahl J, Kellermann AL. Predictors of survival from out-of-hospital cardiac arrest: a systematic review and meta-analysis. Circ Cardiovasc Qual Outcomes. 2010;3(1):63–81.
- Idris AH, Guffey D, Pepe PE, et al. Resuscitation Outcomes Consortium Investigators. Chest compression rates and survival following out-of-hospital cardiac arrest. Crit Care Med. 2015;43(4):840–8.
- Idris AH, Guffey D, Aufderheide TP, et al. Resuscitation Outcomes Consortium Investigators. Relationship between chest compression rates and outcomes from cardiac arrest. Circulation. 2012;125(24):3004–12.
- Abella BS, Sandbo N, Vassilatos P, et al. Chest compression rates during cardiopulmonary resuscitation are suboptimal: a prospective study during in-hospital cardiac arrest. Circulation. 2005;111(4):428–34.
- Edelson DP, Abella BS, Kramer-Johansen J, et al. Effects of compression depth and pre-shock pauses predict defibrillation failure during cardiac arrest. Resuscitation. 2006;71(2):137–45.
- Stiell IG, Brown SP, Christenson J, et al. Resuscitation Outcomes Consortium Investigators. What is the role of chest compression depth during out-of-hospital cardiac arrest resuscitation? Crit. Care Med. 2012;40(4):1192–8.
- Stiell IG, Brown SP, Nichol G, et al. Resuscitation Outcomes Consortium Investigators. What is the optimal chest compression depth during out-of-hospital cardiac arrest resuscitation of adult patients? Circulation. 2014;130(22):1962–70.
- Babbs CF, Kemeny AE, Quan W, Freeman G. A new paradigm for human resuscitation research using intelligent devices. Resuscitation. 2008;77(3):306–15.
- Paradis NA, Martin GB, Rivers EP, et al. Coronary perfusion pressure and the return of spontaneous circulation in human cardiopulmonary resuscitation. JAMA. 1990;263(8):1106–13.
- Perkins GD, Handley AJ, Koster RW, et al. Adult Basic Life Support and Automated External Defibrillation Section Collaborators. European Resuscitation Council Guidelines for Resuscitation 2015 Section 2. Adult basic life support and automated defibrillation. Resuscitation. 2015;95(Oct):81–99.
- Kleinman ME, Brennan EE, Goldberger ZD, et al. Part 5: Adult basic life support and cardiopulmonary resuscitation quality: 2015 American Heart Association Guidelines Update for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2015;132(18 Suppl 2):S414–35.
- Voorhees WD, Babbs CF, Tacker WA. Regional blood flow during cardiopulmonary resuscitation in dogs. Crit Care Med. 1980;8(3):134–6.
- Michael JR, Guerci AD, Koehler RC, et al. Mechanism by which epinephrine augments cerebral and myocardial perfusion during cardiopulmonary resuscitation in dogs. Circulation. 1984;69(4):822–35.
- Halperin HR, Tsitlik JE, Guerci AD, Mellits ED, Levin HR, Shi AY, Chandra N Weisfeldt ML. Determinants of blood flow to vital organs during cardiopulmonary resuscitation in dogs. Circulation. 1986;73(3):539–50.
- Benson DM, O'Neill B, Erpelding J, et al. Open-chest CPR improves survival and neurologic outcome following cardiac arrest. Resuscitation. 2005;64(2):209–17.
- Pickard A, Darby M, Soar J. Radiological assessment of the adult chest: implications for chest compressions. Resuscitation. 2006;71(3):387–90.
- Shin J, Rhee JE, Kim K. Is the inter-nipple line the correct hand position for effective chest compression in adult cardiopulmonary resuscitation? Resuscitation. 2007;75(2):305–10.
- You Y. Optimum location for chest compressions during two-rescuer infant cardiopulmonary resuscitation. Resuscitation. 2009;80(12):1378–81.
- Cha KC, Kim YJ, Shin HJ, et al. Optimal position for external chest compression during cardiopulmonary resuscitation: an analysis based on chest CT in patients resuscitated from cardiac arrest. Emerg Med J. 2013;30(8):615–9.
- Hwang SO, Zhao PG, Choi HJ, et al. Compression of the left ventricular outflow tract during cardiopulmonary resuscitation. Acad Emerg Med. 2009;16(10):928–33.
- Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol. 2010;8(6):e1000412.
- Reynolds JC, Salcido DD, Manegazzi JJ. Coronary perfusion pressure and return of spontaneous circulation after prolonged cardiac arrest. Prehosp Emerg Care. 2010;14(1):78–84.
- Reynolds JC, Salcido DD, Manegazzi JJ. Conceptual models of coronary perfusion pressure and their relationship to defibrillation success in a porcine model of prolonged out-of-hospital cardiac arrest. Resuscitation. 2012;83(7):900–6.
- Friess SH, Sutton RM, Bhalala U, et al. Hemodynamic directed cardiopulmonary resuscitation improves short-term survival from ventricular fibrillation cardiac arrest. Crit Care Med. 2013;41(12):2698–704.
- Deakin CD, Shewry E, Gray HH. Public access defibrillation remains out of reach for most victims of out-of-hospital cardiac arrest. Heart. 2014;100(8):619–23.
- Meaney PA, Nadkarni VM, Kern KB, Indik JH, Halperin HR, Berg RA. Rhythms and outcomes of adult in-hospital cardiac arrest. Crit Care Med. 2010;38(1):101–8.
- Razali NM, Wah YB. Power comparisons of Shapiro-Wilk, Kolmogorov-Smirnov, Lilliefors and Anderson-Darling tests. J Stat Model Analy. 2011;2(1):21–33.
- Gibbons RD, Hedeker D, DuToit S. Advances in Analysis of Longitudinal Data. Ann Rev Clin Psychol. 2010;6:79–107.
- Orlowski JP. Optimum position for external cardiac compression in infants and children. Ann Emerg Med. 1986;15(6):667–73.
- Qvigstad E, Kramer-Johansen J, Tomte O, et al. Clinical pilot study of different hand positions during manual chest compressions monitored with capnography. Resuscitation. 2013;84(9):1203–7.
- Cha KC, Kim HJ, Shin HJ, et al. Hemodynamic effect of external chest compressions at the lower end of the sternum in cardiac arrest patients. J Emerg Med. 2013;44(3):691–7.
- Sanders AB, Ewy GA, Atlas M, Kern KB. Expired PCO2 as a prognostic indicator of successful resuscitation from cardiac arrest. Ann Emerg Med. 1985;14(10):948–52.
- Falk JL, Rackow EC, Weil MH. End-tidal carbon dioxide concentration during cardiopulmonary resuscitation. N Engl J Med. 1988;318(10):607–11.
- Callaham M, Barton C. Prediction of outcome of cardiopulmonary resuscitation from end-tidal carbon dioxide concentration. Crit Care Med. 1990;18(4):358–62.
- Wayne MA, Levine RL, Miller CC. Use of end-tidal carbon dioxide to predict outcome in prehospital cardiac arrest. Ann Emerg Med. 1995;25(6):762–7.
- Cantineau JP, Lambert Y, Merckx P, et al. End-tidal carbon dioxide during cardiopulmonary resuscitation in humans presenting mostly with asystole: a predictor of outcome. Crit Care Med. 1996;24(5):791–6.
- Kolar M, Krizmaric M, Klemen P, Grmec S. Partial pressure of end-tidal carbon dioxide successful predicts cardiopulmonary resuscitation in the field: a prospective observational study. Crit Care. 2008;12(5):R115.
- Sheak KR, Wiebe DJ, Leary M, et al. Quantitative relationship between end-tidal carbon dioxide and CPR quality both in-hospital and out-of-hospital cardiac arrest. Resuscitation. 2015;89:149–54.
- 41. Niemann JT, Criley JM, Rosborough JP, Niskanen RA, Alferness C. Predictive indices of successful cardiac resuscitation after prolonged arrest and experimental cardiopulmonary resuscitation. Ann Emerg Med. 1985;14(6):521–8.
- Sanders AB, Kern KB, Atlas M, Bragg S, Ewy GA. Importance of the duration of inadequate coronary perfusion pressure on resuscitation from cardiac arrest. J Am Coll Cardiol. 1985;6(1):113–8.
- Aichinger G, Zechner PM, Prause G, et al. Cardiac movement identified on prehospital echocardiography predicts outcome in cardiac arrest patients. Prehosp Emerg Care. 2012;16(2):251–5.
- Idris AH, Becker LB, Ornato JP, et al. Utstein-style guidelines for uniform reporting of laboratory CPR research. A statement for healthcare professionals from a task force of the American Heart Association, the American College of Emergency Physicians, the American College of Cardiology, the European Resuscitation Council, the Heart and Stroke Foundation of Canada, the Institute of Critical Care Medicine, the Safar Center for Resuscitation Research, and the Society for Academic Emergency Medicine Writing Group. Circulation. 1996;94(9):2324–36.
- Crick SJ, Sheppard MN, Ho SY, Gebstein L, Anderson RH. Anatomy of the pig heart: comparisons with normal human cardiac structure. J Anat. 1998;193(Pt 1):105–19.
- Neurauter A, Nysaether J, Kramer-Johansen J, et al. Comparison of mechanical characteristics of the human and porcine chest during cardiopulmonary resuscitation. Resuscitation. 2009;80(4):463–9.
- Cherry BH, Nguyen AQ, Hollrah RA, Olivencia-Yurvati AH, Mallet RT. Modeling cardiac arrest and resuscitation in the domestic pig. World J Crit Care Med. 2015;4(1):1–12.
- Orlowski JP. Optimum Position for external cardiac compression in infants and children. Ann Emerg Med. 1986;15(6):667–73.
- Thaler MM, Krause VW. Serious trauma in children after external cardiac massage. N Engl J Med. 1962;267:500–1.
- Meron G, Kurkciyan I, Sterz F, et al. Cardiopulmonary resuscitation-associated major liver injury. Resuscitation. 2007;75(3):445–53.
- Krischer JP, Fine EG, Davis JH, Nagel EL. Complications of cardiac resuscitation. Chest. 1987;92(2):287–91.
- Black CJ, Busuttil A, Robertson C. Chest wall injuries following cardiopulmonary resuscitation. Resuscitation. 2004;63(3):339–43.
- Bjork RJ, Snyder BD, Campion BC, Loewenson RB. Medical complications of cardiopulmonary arrest. Arch Intern Med. 1982;142(3):500–3.

