Abstract
The opioid crisis is a growing concern for Americans, and it has become the leading cause of injury-related death in the United States. An adjunct to respiratory support that can reduce this high mortality rate is the administration of naloxone by Emergency Medical Services (EMS) practitioners for patients with suspected opioid overdose. However, clear evidence-based guidelines to direct EMS use of naloxone for opioid overdose have not been developed. Leveraging the recent Agency for Healthcare Research and Quality (AHRQ) systematic review on the EMS administration of naloxone for opioid poisonings, federal partners determined the need for a clinical practice guideline for EMS practitioners faced with suspected opioid poisoning. Project funding was provided by the National Highway Traffic Safety Administration, Office of EMS, (NHTSA OEMS), and the Health Resources and Services Administration, Maternal and Child Health Bureau’s EMS for Children Program (EMSC). The objectives of this project were to develop and disseminate an evidence-based guideline and model protocol for administration of naloxone by EMS practitioners to persons with suspected opioid overdose. We have four recommendations relating to route of administration, all conditional, and all supported by low or very low certainty of evidence. We recommend the intravenous route of administration to facilitate titration of dose, and disfavor the intramuscular route due to difficulty with titration, slower time to clinical effect, and potential exposure to needles. We equally recommend the intranasal and intravenous routes of administration, while noting there are variables which will determine which route is best for each patient. Where we are unable to make recommendations due to evidence limitations (dosing, titration, timing, and transport) we offer technical remarks. Limitations of our work include the introduction of novel synthetic opioids after many of the reviewed papers were produced, which may affect the dose of naloxone required for effect, high risk of bias and imprecision in the reviewed papers, and the introduction of new naloxone administration devices since many of the reviewed papers were published. Future research should be conducted to evaluate new devices and address the introduction of synthetic opioids.
Background
Impact of the Opioid Crisis
Rates of opioid overdose (OD) in the United States have increased fourfold since 2000, with data for 2016 indicating that over 42,000 died from opioid overdose that year alone (Citation1). In 2016, opioid overdose deaths (Citation2) overtook traffic crashes (Citation3) as the leading cause of death by traumatic injury in the United States. In recent years, synthetic opioids, predominantly illicitly-manufactured fentanyl and its analogs, have overtaken prescription opioids and heroin as the leading cause of overdose deaths (Citation1). In 2017, the sharpest increase in drug overdose fatalities was related to fentanyl and fentanyl analogs, representing nearly 30,000 overdose deaths (Citation1). As many opioid overdose patients are discovered by family and close friends (Citation4), the US Surgeon General issued an advisory urging more Americans to carry naloxone to combat the opioid crisis (Citation5). The opioid epidemic has widespread impact on the population at large, affecting family and friends, employers and coworkers, and others who know overdose patients. Factors which contribute to the crisis include substance use disorders, mental health disorders such as depression and bipolar disorder, chronic pain, relapse after a period of abstinence during drug treatment or incarceration and polypharmacy (Citation6, Citation7).
A drastic burden is placed on society as more resources and personnel are allocated to combat this epidemic. Between 2012 and 2016, the rate of naloxone administration by EMS increased 75.1%, from 573.6 to 1004.4 per 100,000 EMS events (Citation8). The increased rate of administration of naloxone mirrors the overdose mortality rate (Citation8). A retrospective study which analyzed data from Northern New England revealed that basic life support (BLS) practitioners were as effective as advanced life support (ALS) practitioners in naloxone administration (Citation9). The role of first responders has expanded to include identification and management of the effects of opioid toxicity through supportive management as well as reversal through the administration of naloxone (Citation10).
Management of Opioid Overdose
For EMS practitioners who suspect an opioid overdose, the first step is to evaluate the extent of the patient’s respiratory depression. Overdoses of opioids are associated with both central nervous system (CNS) and respiratory depression, making the primary risk of death inadequate oxygenation and ventilation, which can decompensate to cardiac arrest. In many situations, it may not be readily apparent if a patient is suffering an opioid overdose versus respiratory depression due to other etiologies or co-ingestions. Due to these concerns, the patient’s airway and respiratory mechanics must be assessed immediately upon patient contact and supported with airway maneuvers and ventilation (e.g., bag-valve-mask) as indicated. Even when naloxone is clinically indicated, respiratory support should be given first or at least contemporaneously. Bag-valve-mask ventilation, incorporating oropharyngeal or nasopharyngeal airways to promote a patent airway, should be used to provide adequate oxygenation and ventilation until the patient is able to breathe adequately without support. In cases where the response to naloxone is inadequate, further airway management may be required, such as a supraglottic airway device or endotracheal tube placement (if within applicable scopes of practice). In other cases, respiratory support may result in recovery as accumulated carbon dioxide is purged, and naloxone may not be necessary.
Naloxone
Naloxone is a mu-opioid receptor antagonist effective at reversing the symptoms of opioid toxicity and associated life-threatening respiratory depression. First synthesized in 1961, naloxone was approved for use in 1971 as an opioid reversal agent, and EMS practitioners began administering naloxone shortly thereafter (Citation11). The need for rapid access to naloxone in the community has expanded naloxone use to include both first responders and laypersons in the out of hospital setting (Citation12). Common routes of naloxone administration include intravenous (IV), intramuscular (IM), subcutaneous (SQ), and intranasal (IN). Two Food and Drug Administration (FDA) approved products for layperson use have been developed: an autoinjector for IM administration and a commercial nasal spray with a bioavailability of approximately 50% relative to IM (Citation13).
Naloxone has a rapid onset of action, reaching maximal serum concentration within 2 minutes after IV administration, 10 minutes after IM, and 15–30 minutes after IN (Citation14). Naloxone distributes to the central nervous system and equilibrates with the plasma within minutes (Citation15). Naloxone is extensively metabolized in the liver to inactive metabolites with a serum half-life of 30–90 minutes (Citation15). Naloxone is an extremely safe medication but can precipitate opioid withdrawal symptoms including agitation or irritability, anxiety, body aches, nausea or vomiting, diarrhea, piloerection, rhinorrhea, and sweating. More severe reactions are extremely rare but may include acute respiratory distress syndrome (ARDS), hypertensive emergency, ventricular tachycardia and fibrillation, and sudden death (Citation16). Dosing of naloxone is based on the goal of restoring adequate respiratory function while minimizing the risk of opioid withdrawal symptoms, which is best accomplished with dose titration and careful monitoring when conditions permit.
Presence of Other Substances and Opioids
An apparently inadequate response to initial dosing of naloxone could be the result of co-ingestants, such as ethanol or benzodiazepines. Additionally, some extremely powerful fentanyl analogs, such as carfentanil or acetyl fentanyl, as well as the opioid partial agonist buprenorphine, may require larger than usual or repeat doses of naloxone to achieve adequate respiratory function and are increasingly involved in opioid overdoses.
Occupational Exposure
The potential for occupational exposure to fentanyl and its analogs has created distinct concern among public safety and EMS practitioners. Therefore, it is important that practitioners utilize appropriate practices and personal protective equipment (PPE) when potentially in the presence of opioids in a form that could pose toxicity. In responding to most suspected opioid overdoses, standard PPE medical gloves are sufficient protection. Credible resources exist to advise public safety and EMS professionals and include the American College of Medical Toxicology’s Statement on Fentanyl Exposure (Citation17).
Project Objectives
The objectives of this project were to develop and disseminate an evidence-based guideline and model protocol for administration of naloxone by Emergency Medical Services (EMS) practitioners to persons with suspected opioid overdose. Also included in the objectives were the development of training materials for EMS practitioners in implementing the guideline, the creation of performance measures by which adherence to the clinical practice guideline and its impact could be assessed, and the development of a manuscript for publication in a peer-reviewed scientific journal. This paper describes the process by which the evidence-based guideline was developed.
Methods
The Institute of Medicine report on trustworthy clinical practice guidelines has made clear that the development of recommendations intended to improve care are to be based on a systematic review of the scientific literature (Citation18). While systematic reviews are the optimal means of evaluating and synthesizing the existing scientific evidence to inform clinical questions, this information alone is insufficient. This clinical practice guideline was developed as a follow-up to the Agency for Healthcare Research and Quality (AHRQ) systematic review on the prehospital administration of naloxone for opioid poisonings that occur in the field (Citation10). This evidence was given in-depth consideration by a panel of relevant stakeholders to develop concise recommendations based on GRADE methodology.
In August 2016, the National EMS Advisory Council (NEMSAC) recommended that the National Highway Traffic Safety Administration develop an evidence-based guideline regarding administration of naloxone by EMS clinicians. The advisory was approved and published after the September 2016 National EMS Advisory Council meeting (Citation19). The current project was developed in the fall of 2017 by the Medical Directors Council of the National Association of State EMS Officials (NASEMSO) in collaboration with the National Association of EMS Physicians (NAEMSP) and the EMS Committee of the American College of Emergency Physicians (ACEP). A Technical Expert Panel (TEP or Panel) was assembled, which included experienced EMS field practitioners, EMS physician medical directors, experts in addiction medicine, pain medicine, toxicology/pharmacology, and GRADE methodologies, as well as a patient advocate (see : Members of Expert Panel). Funding was provided by the National Highway Traffic Safety Administration, Office of EMS, and the Health Resources and Services Administration, Maternal and Child Health Bureau’s EMS for Children Program.
Table 1. Members of Technical Expert Panel
The project scope of work focused on translating the systematic review published by the Agency for Healthcare Research and Quality (AHRQ) in November 2017 into an evidence-based guideline and model protocol for administration of naloxone by EMS practitioners to persons suspected of an opioid overdose (Citation10). This was done through review of the Population Intervention Comparison Outcome (PICO) questions addressed in the systematic review and the evidence identified by the searches. The PICO questions addressed by the AHRQ systematic review are listed in . Following PICO question and evidence review, the TEP used the Grading of Recommendations Assessment, Development and Evaluation (GRADE) methodology to summarize the evidence and assess the strength of the literature and develop treatment recommendations. GRADE is emerging as the most widely used system for clinical practice guideline development (Citation20). It has been endorsed as an optimal method for guideline development in the EMS environment (Citation21). The advantages of the GRADE approach include an outcome-centric analysis of the certainty of evidence as well as a transparent and explicit means of conveying judgements and recommendations through evidence profile tables and evidence to decision (EtD) tables. GRADE also establishes clear and reproducible approaches to the assessment of certainty in evidence, and the direction and strength of recommendations.
Table 2. PICO questions in the AHRQ Review (Citation10)
Evidence Review
This work leverages the published AHRQ systematic review on the Management of Suspected Opioid Overdose with Naloxone by Emergency Medical Services Personnel (Citation10). The review synthesized the data from inception of databases to September 2017 on four key areas: (question 1) route of administration of naloxone; (question 2) titration of naloxone dosing to specific therapeutic endpoints (e.g. spontaneous ventilation); (question 3) timing of repeat dosing of naloxone; and (question 4) transportation or non-transport of patients to a healthcare facility after treatment with naloxone. The search strategies and the inclusion and exclusion criteria used were previously described in detail (Citation10). The systematic review identified 1,934 potential abstracts for articles through sources (). A total of 202 full text articles were identified and reviewed, leading to 13 included publications (). Of the included articles, seven addressed route of administration and six addressed transport of patients. There were no articles identified addressing dose titration or repeat dosing of naloxone.
Figure 1. Literature flow diagram from AHRQ systematic review entitled “Management of Suspected Opioid Overdose with Naloxone by Emergency Medical Services Personnel.” Figure reprinted with permission from the AHRQ.
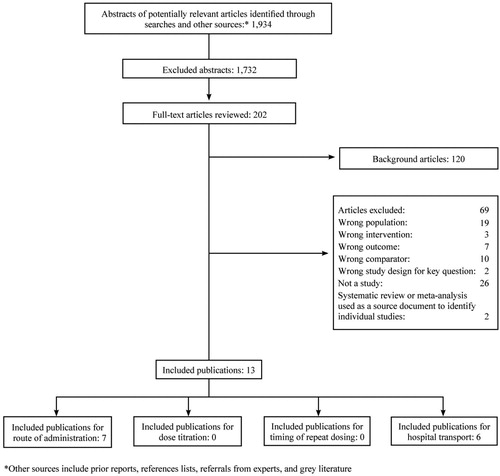
Table 3. Articles identified by the AHRQ systematic review (Citation10) in the four key areas
GRADE Process
Following review of the identified literature, using the GradePro GDT software (Citation22) and concepts for evaluation noted in the GRADE manual (Citation23), summary of evidence and evidence profile tables were generated for each of the interventions for route of administration including intranasal (IN) vs. intravenous (IV); IV vs. intramuscular (IM); and IV vs. subcutaneous (SQ) by the technical expert panel. Each article was evaluated for confidence in the estimate of effect (quality) following evaluation for bias, inconsistency, indirectness, imprecision, and possible confounders. These evidence profile tables were then used to determine the recommendations and their strengths.
Recommendations and Technical Remarks
This evidence-based guideline development process leveraged the systematic evaluation done by AHRQ with the application of GRADE for development of summary of evidence and evidence profile tables for administration of naloxone by EMS practitioners to persons with suspected opioid overdose. Summary of evidence and evidence profile tables were only able to be generated for key question 1, addressing the route of administration of naloxone. Unfortunately, due to the paucity of evidence to review, we were unable to generate tables for key questions 2 (titration of naloxone dosing) and question 3 (timing of repeat dosing). Although six papers were identified for key question 4 (transportation or non-transport of patients to a healthcare facility after treatment), we were unable to generate tables since these manuscripts were evaluations of patient refusal of transport without comparison groups. Listed in the following sections are the recommendations based on the summary of evidence and evidence profile tables as well as the technical remarks for each key question.
PICO Question 1: Routes of Administration
For patients with confirmed or suspected opioid overdose, what are the comparative benefits and harms related to out-of-hospital administration of naloxone by EMS personnel using intravenous, intramuscular, subcutaneous, and intranasal routes of administration?
Recommendation 1: Intranasal (IN) vs. Intramuscular (IM)
View Evidence to Decision details in
Table 4. GRADE Table for Recommendation 1: Intranasal (IN) vs. Intramuscular (IM)
Summary:
IN > IM
Evidence Quality: Very Low
Recommendation Strength: Weak/Conditional
For the comparison of intranasal vs. intramuscular naloxone in the setting of suspected opioid overdose, the panel is in favor of intranasal over intramuscular routes of administration (weak/conditional recommendation/very low certainty in the evidence).
In making this recommendation the panel’s interpretation of the evidence was that the comparison was to some extent driven by dosing considerations. Specifically, very low certainty evidence suggested that intranasal naloxone (2 mg) is similar in efficacy to intramuscular naloxone (2 mg). The recommendation was driven by considerations related to ease of administration and practitioner safety, especially in relation to agitation and adverse opioid withdrawal reactions. There were limited data suggesting that agitation may be more likely with IM administration relative to IN (Citation24). Needlestick injuries might be of particular concern for practitioners who may have less experience with intramuscular injections. Non-transport was taken into consideration as well, and it was felt that intramuscular dosing might carry the greatest risk in this regard due to agitation leading to transport refusal. Intranasal dosing by EMS practitioners is titratable if lower concentrations are used with a syringe and atomizer.
Research is needed on the comparative effectiveness of the FDA-approved naloxone auto-injector (2 mg) and highly concentrated (4 mg/0.1 mL and 2 mg/0.1 mL) IN naloxone formulation, different doses, and dosing strategies.
Recommendation 2: Intranasal (IN) vs. Intravenous (IV)
View Evidence to Decision details in
Table 5. GRADE Table for Recommendation 2 - Intranasal (IN) vs. Intravenous (IV)
Summary:
IN = IV
Evidence Quality: Very Low
Recommendation Strength: Weak/Conditional
Comparing intranasal and intravenous naloxone, the panel equally recommends the intranasal and intravenous routes of administration (weak/conditional recommendation; very low certainty in the evidence).
While the panel favors intranasal and intravenous routes equally, there are many variables which determine the preferred route of administration for an individual patient. EMS practitioners with less training may not be able to obtain intravenous access, making intranasal naloxone the preferred route of administration in those cases. However, if intravenous access can be established with minimal risk of occupational injury, the ability to readily titrate naloxone decreases patient withdrawal symptoms such as agitation, promoting patient and practitioner safety, and also increases the likelihood that the patient will accept transport to hospital (where connections with opiate disorder treatment can be made) making intravenous naloxone preferable.
As immediate respiratory support is applied, intranasal naloxone can be administered while an IV is being placed, but the cumulative effect of both routes should be considered.
In summary, while intravenous access is associated with increased chance of safe management and transport, if intravenous access cannot be safely obtained, intranasal is the recommended route. Otherwise, these two routes of administration are comparable.
Recommendation 3: Intravenous (IV) vs Intramuscular (IM)
View Evidence to Decision details in
Table 6. GRADE Table for Recommendation 3 - Intravenous (IV) vs. Intramuscular (IM)
Summary:
IV > IM
Evidence Quality: Very Low
Recommendation Strength: Weak/Conditional
Both intramuscular and intravenous naloxone are associated with increased risk of needlestick injury when compared with intranasal naloxone (unless needles are used to prepare the dose); therefore, the panel recommends intravenous over intramuscular route of administration (weak/conditional recommendation; very low certainty in the evidence).
The primary rationale for this decision is related to patient outcome. With IV access, the ability to titrate the drug reduces the risk of withdrawal symptoms and patient agitation. This is associated with an increased likelihood of patients consenting to hospital transport. While the intravenous route may be more beneficial, practical considerations may favor another route such as intramuscular when there is greater occupational risk or challenge with the use of the intravenous route.
Research is needed to compare the effects of intramuscular versus intranasal naloxone, in particular related to the FDA-approved autoinjector, as well as dosing strategies and formulation.
Recommendation 4: Intravenous (IV) vs. Subcutaneous (SQ)
View Evidence to Decision details in
Summary:
IV > SQ
Evidence Quality: Very Low
Recommendation Strength: Weak/Conditional
Table 7. GRADE Table for Recommendation 4 - Intravenous (IV) vs Subcutaneous (SQ)
When comparing subcutaneous to intravenous routes of naloxone administration, the panel recommends intravenous naloxone (weak/conditional recommendation; low quality of evidence).
The benefit of intravenous naloxone is the ability to titrate the drug, resulting in reduced risk of complications to both the patient and the practitioner, as well as increased chance of transport to hospital for further care, including connection to opioid disorder treatment options. Subcutaneous delivery of high doses of naloxone can result in over-reversal of the opioid, increasing the risk of patient agitation. While subcutaneous naloxone may be absorbed more slowly than intramuscular naloxone, there does not seem to be a clinically important difference. If IV access is difficult, due to body habitus, comorbidities, or practitioner training, other routes are preferable.
Research comparing intravenous and subcutaneous routes is lacking.
For dosing information, please see Technical Remarks on PICO Question 1a: Doses.
Technical Remarks on PICO Question 1a: Doses
For patients with confirmed or suspected opioid overdose who are administered naloxone in the out-of-hospital setting by EMS practitioners, what are the comparative benefits and harms of different intravenous, intramuscular, subcutaneous, or intranasal doses of naloxone?
Comparative data on initial dosing of naloxone is limited. The standard recommendation for parenteral administration (IV, IM, SQ) is a dose of 0.4 mg, however recommendations range from 0.04 mg up to 2 mg for adults (Citation16, Citation25). The recommended initial pediatric dose of naloxone is 0.1 mg/kg IV, IM, or SQ with a maximum initial dose of 2 mg, which can be repeated every 2–3 minutes as needed. Though weight-based dosing recommendations are lacking for IN use in children, initial doses ranging from 2 mg (>13-year olds) to 4 mg (naloxone nasal spray in infants, children, and adolescents) have been recommended either in the medical literature and/or by manufacturers. Therefore, utilizing 0.1 mg/kg IN naloxone (maximum dose = 4 mg) may be reasonable (Citation26–29).
Guidelines favor lower initial dosing with simultaneous bag-valve-mask ventilation and subsequent dose titration as indicated to avoid severe opioid withdrawal symptoms and facilitate further care (Citation30). Standard initial intranasal dosing recommendation ranges from 2–4 mg when using the commercially available nasal spray or prefilled syringes and atomization devices (Citation31, Citation32). Lower doses may be delivered by drawing up naloxone into a syringe and attaching an atomization device. However, with the advent of high potency opioids, the need for higher initial and repeat doses will need to be evaluated closely. With this in mind, protocols will need to be designed based on local data concerning the type and potency of opioids present in the area.
Technical Remarks on PICO Question 2: Titration
For patients with confirmed or suspected opioid overdose in out-of-hospital settings, what are the comparative benefits and harms of titration of naloxone administered by EMS practitioners until the patient resumes sufficient spontaneous respiratory effort versus until the patient regains consciousness?
As it pertains to managing suspected opioid overdoses, the panel recommends optimal management to be administration of the lowest possible dose at the required frequency to maintain adequate respiratory function without triggering a withdrawal phenomenon. EMS practitioners should titrate to achieve adequate spontaneous respiratory function.
The panel does not recommend initially dosing naloxone to achieve full consciousness. The appropriate dose is one that restores and maintains respiratory function and does not result in return to full consciousness. Restoration of full consciousness is not required for patient safety, and may precipitate withdrawal and agitation, reducing safety for both the patient and the EMS practitioner. Transport, and connection to further treatment, may also be more likely if full consciousness is not restored.
Technical Remarks on PICO Question 3: Timing
For patients with confirmed or suspected opioid overdose in out-of-hospital settings treated with multiple doses of naloxone (including patients who do not improve after an initial dose of intranasal naloxone), what are the effects on benefits and harms of differences in timing of repeat dosing?
After naloxone administration, patients should be monitored closely for restoration of adequate ventilation. Repeat dosing should be administered only if there is an inadequate response to initial dosing or with recrudescence of respiratory depression after an initial response. The exact timing of naloxone repeat administration if there is an inadequate response to an initial dose has not been systematically studied. The onset of action varies by route of administration and the opioid being reversed (Citation16). FDA labeling recommends repeat dosing in 2–3 minutes while some guidelines recommend a repeat dose after 4 minutes (Citation25, Citation27, Citation28). Typically, naloxone begins to have clinical effect within 2–3 minutes of administration regardless of route, with peak effect timing varying according to route. Therefore, we recommend that if there is no improvement within 2–3 minutes, a repeat dose should be administered.
If there is return of significantly slowed breathing or other respiratory compromise after initial response, a repeat dose should be administered, regardless of time since the initial dose. The intravenous route is preferred for subsequent doses, if available, due to the ability to more precisely titrate the dose and avoid withdrawal complications.
If EMS practitioners give repeat doses of naloxone and there is no significant effect, other causes for the patient’s symptoms, including co-ingestants, long-acting agents, head injury, hypoglycemia, or any number of causes for decreased mental status and respiratory depression should be suspected. Reassessment and care including airway management, cardiovascular support, and transport to a hospital should be the next step in those cases.
Awareness of the licit and illicit opioids that are commonly misused and abused is important in selecting the appropriate dose and route of naloxone.
Technical Remarks on PICO Question 4: Transport
For patients with confirmed or suspected opioid overdose in out-of-hospital settings who regain sufficient spontaneous respiratory effort and are alert and oriented after naloxone administration by EMS personnel, what are the benefits and harms of transporting patients to a health care facility versus non-transport?
The AHRQ systematic review identified six papers on patient transport (). These studies focused on the evaluation of patients who refused transport to the emergency department (or were released on scene by a physician) and not the decision making of transport vs. non-transport to a health care facility following a suspected opioid overdose by EMS practitioners. Therefore, no GRADE table could be made since there were no outcome comparisons for transport and non-transport in those papers.
The AHRQ systematic evaluation did summarize data on the possibility of safe release from scene of patients with suspected opioid overdose. In the most recent evaluation (2014 data collection), of the 205 patients who refused transport by EMS following suspected opioid overdose and treatment with naloxone, death within 24 hours occurred in 1/205 (0.49%) patients with 2/205 (0.98%) additional deaths within 30 days (Citation33). The other studies had similar results. However, an important consideration when these data were evaluated by the panel is that these studies occurred prior to the increased presence of high potency opioids, which may change the patient presentation and response to naloxone treatment. Based on these concerns, there is no clear evidence-based guideline for the transport or non-transport of suspected overdose to a health care facility. Furthermore, there are no clear data to guide the duration of observation after an opioid overdose, especially due to varying strengths and half-lives of currently available opioids.
Naloxone has been called the “rescue shot,” and is effective at reversing opioid overdose, but it is not the entire solution. Naloxone should be an initial step in a larger course of evidence-based opioid-use disorder treatment, such as detoxification and medication-assisted treatment, rehabilitation, therapy, support groups, and oversight from primary care providers and therapists. EMS practitioners who treat opioid overdose patients are therefore in contact with a population that is at high risk for subsequent overdoses, since initial overdose is a major predictor of subsequent overdose. EMS practitioners are in a vital position to serve as a bridge between patients and life-saving resources that supplement naloxone treatment.
There are many options for connection to further treatment. Connection to further treatment can include:
Transport to a hospital or other healthcare setting to further interact with healthcare professionals who may connect them with opioid-use disorder treatment.
Leave naloxone on-scene (as permitted by state or local protocol).
Recommend that the patient stock naloxone.
Connect the patient to naloxone sources as applicable (local public health agencies, pharmacies, needle exchange sites, harm reduction organizations, safe consumption spaces).
Connect the patient to further treatment, including but not limited to support groups.
Bring a social worker or other support professionals on-scene.
Provide literature on substance use, mental health, addiction, and/or harm reduction.
If a relevant current community paramedicine program is in place, connect the patient with this program for automatic follow-up after any overdose.
In most EMS systems, the standard protocol is to transport all patients to hospital unless they meet criteria to competently refuse transport. In some systems, transport to an alternative treatment site (mental health or substance abuse treatment facility, for example) is authorized under a state or local protocol. If a system allows EMS practitioners to make the transport decision themselves, the following factors should be considered as reasons to transport the patient to hospital:
The patient is at high risk of experiencing re-occurrence of overdose due to substances ingested.
There are co-morbid psychiatric or medical conditions that would be better addressed in a hospital setting. These could be chronic conditions, injuries, diseases or anything else that would benefit from evaluation and stabilization.
EMS practitioners are unable or lack the resources to refer the overdose patient to treatment resources while in the field.
The hospital has resources for linkage to treatment for overdose patients and/or people who use substances not elsewhere available.
Limitations
Our work was limited by several factors. The number of relevant papers included in the AHRQ review was small, and these papers were limited by a high risk of bias and imprecision. Therefore, our recommendations are based on suboptimal data.
Second, since many of the relevant papers were published, a number of potent synthetic opioids, such as fentanyl and analogs, have appeared in overdose patients. These synthetics, much more potent than heroin or morphine, are presumed to require higher doses of naloxone to achieve clinical effect in an overdose patient (Citation34). There is a need for more current research that incorporates synthetic opioids.
Third, since many of the relevant papers were published, new naloxone delivery devices have appeared on the market. These devices, which deliver different doses of naloxone via several routes, were not included in the identified papers and, therefore, further research is indicated to evaluate the efficacy and effects of these devices.
Fourth, no studies in the AHRQ review addressed pediatric dosing. Therefore, further research is indicated to evaluate optimal routes and doses in age-defined pediatric populations.
Conclusion
In summary, naloxone is an effective agent for reversal of opioid overdose. In conjunction with other treatment, such as respiratory support, naloxone should be available for EMS practitioners at all scope of practice levels. If readily available, use of the IV route is recommended as it allows titration of dose to facilitate optimal effect with reduced likelihood of adverse effects. When the IV route is not readily available, the IN route is favored over the IM route due to ease of use, reduced chance of needlestick injury, and availability to more EMS practitioners. Initial dose selection depends on many factors, including timely local knowledge of opioids in use, but we recommend starting with a dose that results in adequate respiratory function and does not precipitate withdrawal symptoms and full consciousness. Multiple routes may be optimal for some patients, such as an initial intranasal dose while IV access is obtained. Our recommendations are limited by scarcity of relevant research and the advent of more potent synthetic opioids as well as new naloxone delivery devices.
References
- National Institute on Drug Abuse. Overdose death rates [about 7 screens]. Rockville (MD): National Institute on Drug Abuse; 2018 August [cited 2018 Sep 26]. [Internet]. https://www.drugabuse.gov/related-topics/trends-statistics/overdose-death-rates
- Seth P, Scholl L, Rudd RA, Bacon S. Overdose deaths involving opioids, cocaine, and psychostimulants–United States, 2015-2016. MMWR Morb Mortal Wkly Rep. 2018;67:349–58. doi:10.15585/mmwr.mm6712a1.
- National Safety Council. NSC motor vehicle fatality estimates [second page]. Itasca (IL): National Safety Council; 2017 Dec [cited 2018 Sep 26]. [Internet]. https://www.nsc.org/Portals/0/Documents/NewsDocuments/2018/December_2017.pdf
- World Health Organization. Community management of opioid overdose [page 2]. Geneva, Switzerland: World Health Organization; 2014 [cited 2018 Sep 26]. [Internet]. http://www.who.int/substance_abuse/publications/management_opioid_overdose/en/
- United States Office of the Surgeon General. Surgeon general’s advisory on naloxone and opioid overdose [about 3 screens]. Washington (DC): Surgeon General; 2018 Apr 5 [cited 2018 Sep 26]. [Internet]. https://www.surgeongeneral.gov/priorities/opioid-overdose-prevention/naloxone-advisory.html
- Park TW, Lin LA, Hosanagar A, Kogowski A, Paige K, Bohnert A. Understanding risk factors for opioid overdose in clinical populations to inform treatment and policy. J Addict Med. 2016;10(6):369–81. doi:10.1097/ADM.0000000000000245.
- Mueller S, Walley A, Calcaterra S, Glanz J, Binswanger I. A review of opioid overdose prevention and naloxone prescribing: implications for translating community programming into clinical practice. Subst Abus. 2015;36(2):240–53. doi:10.1080/08897077.2015.1010032.
- Cash RE, Kinsman J, Crowe RP, Rivard MK, Faul M, Panchal AR. Naloxone administration frequency during emergency medical service events–United States, 2012–2016. MMWR Morb Mortal Wkly Rep. 2018;67(31):850–3. doi:10.15585/mmwr.mm6731a2.
- Gulec N, Lahey J, Suozzi J, Sholl M, MacLean C, Wolfson D. Basic and advanced EMS providers are equally effective in naloxone administration for opioid overdose in Northern New England. Prehosp Emerg Care. 2018;22(2):163–9. doi:10.1080/10903127.2017.1371262.
- Chou R, Korthuis PT, McCarty D, Coffin P, Griffin J, Davis-O’Reilly C, Grusing S, Daya M. Management of suspected opioid overdose with naloxone by emergency medical services personnel [Internet]. Comparative Effectiveness Review No. 193. (Prepared by the Pacific Northwest Evidence-based Practice Center under Contract No. 290-2015-00009-I.) AHRQ Publication No. 17(18)-EHC025-EF. Rockville (MD): Agency for Healthcare Research and Quality; 2017 November. www.effectivehealthcare.ahrq.gov/reports/final.cfm. doi:10.23970/AHRQEPCCER193.
- Department of Transportation, National Highway Traffic Safety Administration. National training course, emergency medical technician, paramedic, instructor’s lesson plans, Vol. 16. Washington, DC: US Government Printing Office Stock No. 050-003-00279-8; 1977 [cited 2018 Nov 15]. https://babel.hathitrust.org/cgi/pt/id=mdp.39015011479147;view=1up;seq=1
- American Chemical Society. Molecule of the Week: naloxone [about 2 screens]. Washington (DC): American Chemical Society; 2016 May 23 [cited 2018 Sep 26]. [Internet]. https://www.acs.org/content/acs/en/molecule-of-the-week/archive/n/naloxone.html
- Ryan SA, Dunne RB. Pharmacokinetic properties of intranasal and injectable formulations of naloxone for community use: a systematic review. Pain Manag. 2018;8(3):231–45. doi:10.2217/pmt-2017-0060.
- McDonald R, Lorch U, Woodward J, Bosse B, Dooner H, Mundin G, Smith K, Strang J. Pharmacokinetics of concentrated naloxone nasal spray for opioid overdose reversal: phase I healthy volunteer study. Addiction. 2018;113(3):484–93. doi:10.1111/add.14033.
- Rzasa LR, Galinkin JL. Naloxone dosage for opioid reversal: current evidence and clinical implications. Ther Adv Drug Saf. 2017;9(1):63–88. doi:10.1177/2042098617744161.
- Kim HK, Nelson LS. Reducing the harm of opioid overdose with the safe use of naloxone: a pharmacologic review. Expert Opin Drug Saf. 2015;14(7):1137–46. doi:10.1517/14740338.2015.1037274.
- American College of Medical Toxicology. ACMT and AACT position statement: preventing occupational fentanyl and fentanyl analog exposure to emergency responders [about 2 screens]. Phoenix (AZ): American College of Medical Toxicology; 2017 Jul 12 [cited 2018 Sep 26]. [Internet]. https://www.acmt.net/cgi/page.cgi/_zine.html/The_ACMT_Connection/ACMT_Statement_on_Fentanyl_Exposure
- Institute of Medicine. Clinical practice guidelines we can trust. Washington (DC): The National Academies Press; 2011. 290 p. doi:10.17226/13058.
- EMS.gov. National EMS Advisory Council (NEMSAC). Washington (DC): NHTSA Office of EMS; 2017 [cited 2018 Sep 26]. [Internet]. https://www.ems.gov/nemsac.html
- The GRADE Working Group. What is GRADE? [about 1 screen]. The GRADE Working Group; 2004-2018 [cited 2018 Sep 26]. [Internet]. http://www.gradeworkinggroup.org/
- Lang ES, Spaite DW, Oliver ZJ, Gotschall CS, Swor RA, Dawson DE, Hunt RC. A national model for developing, implementing, and evaluating evidence-based guidelines for prehospital care. Acad Emerg Med. 2012;19(2):201–9. doi:10.1111/j.1553-2712.2011.01281.x.
- GRADEpro GDT: GRADEpro Guideline Development Tool [Software]. McMaster University (developed by Evidence Prime, Inc.); 2015 [cited 2018 Sep 26]. https://gradepro.org
- Schünemann H, Brożek J, Guyatt G, Oxman A, editors. GRADE handbook for grading quality of evidence and strength of recommendations [Internet]. The GRADE Working Group; 2013 Oct [cited 2018 Sep 26]. https://gdt.gradepro.org/app/handbook/handbook.html
- Kelly AM, Kerr D, Dietze P, Patrick I, Walker T, Koutsogiannis Z. Randomised trial of intranasal versus intramuscular naloxone in prehospital treatment for suspected opioid overdose. Med J Australia. 2005;182(1):24–7. Epub: 2005 January 3 [cited 2018 Oct 30]. https://www.mja.com.au/journal/2005/182/1/randomised-trial-intranasal-versus-intramuscular-naloxone-prehospital-treatment
- DailyMed. Product Information: NALOXONE HCl intravenous, intramuscular, subcutaneous injection (Mylan Institutional LLC) [about 2 screens]. U.S. National Library of Medicine; 2018 Jan 17 [cited 2018 Sep 26]. [Internet]. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm/setid=d96ebf2e-1051-4b5b-b1c6-2543dbbb2d04
- Lexicomp Online. Pediatric and Neonatal Lexi-Drugs Online. Hudson (OH): Wolters Kluwer Clinical Drug Information, Inc.; 2019.
- Kleinman ME, Chameides L, Schexnayder SM, Samson RA, Hazinski MF, Atkins DL, Berg MD, de Caen AR, Fink EL, Freid EB, et al. Pediatric advanced life support: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Pediatrics. 2010;126(5):e1361–99.
- Hegenbarth MA; American Academy of Pediatrics Committee on Drugs. Preparing for pediatric emergencies: drugs to consider. Pediatrics. 2008;121(2):433–43. <https://www.ncbi.nlm.nih.gov/pubmed/18245435>. doi:10.1542/peds.2007-3284.
- Barton ED, Colwell CB, Wolfe T, Fosnocht D, Gravitz C, Bryan T, Dunn W, Benson J, Bailey J. Efficacy of intranasal naloxone as a needleless alternative for treatment of opioid overdose in the prehospital setting. J Emerg Med. 2005;29(3):265–71. <https://www.ncbi.nlm.nih.gov/pubmed/16183444>.
- Vanden Hoek TL, Morrison LJ, Shuster M, Donnino M, Sinz E, Lavonas EJ, Jeejeebhoy FM, Gabrielli A. Part 12: cardiac arrest in special situations: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2010;122(18 Suppl 3):S829–S61. http://www.ahajournals.org/doi/abs/10.1161/CIRCULATIONAHA.110.971069. doi:10.1161/CIRCULATIONAHA.110.971069.
- Lavonas EJ, Drennan IR, Gabrielli A, Heffner AC, Hoyte CO, Orkin AM, Sawyer KN, Donnino MW. Part 10: special circumstances of resuscitation: 2015 American Heart Association Guidelines Update for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2015;132(18 Suppl 2):S501–S18. https://www.ahajournals.org/doi/abs/10.1161/CIR.0000000000000264. doi:10.1161/CIR.0000000000000264.
- DailyMed. Product Information: NARCAN(R) nasal spray, naloxone HCl nasal spray (Adapt Pharma, Inc) [about 2 screens]. U.S. National Library of Medicine; 2018 Jul 20 [cited 2018 Sep 26]. [Internet]. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm/setid=724df050-5332-4d0a-9a5f-17bf08a547e1
- Levine M, Sanko S, Eckstein M. Assessing the risk of prehospital administration of naloxone with subsequent refusal of care. Prehosp Emerg Care. 2016;20(5):566–9. doi:10.3109/10903127.2016.1142626.
- O’Donnell JK, Halpin J, Mattson CL, Goldberger BA, Gladden RM. Deaths involving fentanyl, fentanyl analogs, and U-47700–10 States, July-December 2016. MMWR Morb Mortal Wkly Rep. 2017;66(43):1197–202. doi:10.15585/mmwr.mm6643e1.

