Abstract
The traditional Van herby cheese incorporates herbs mainly consisting of Allium species as antimicrobial and flavouring aids. The total antioxidant capacities of 16 different herbs (8 species) collected from different locations were assayed for the first time by CUPRAC, ABTS/persulfate, FRAP, and Folin methods. The assay results correlated well among each other, because all were electron-transfer assays. The highest results were obtained with Folin having the highest redox potential. The second highest results were with CUPRAC, especially for the allium sp., because the sulfur containing antioxidants in allium could be best assayed with CUPRAC, whereas FRAP was nonresponsive to thiol-type compounds. The order of CUPRAC antioxidant capacities as trolox equivalents was thymus sp. > chaerophyllum sp. > allium sp. > prangos sp. ≥ ferula sp. On the other hand, the order of Folin findings was thymus sp. > allium sp. > chaerophyllum sp. > ferula sp. ≥ prangos sp.
INTRODUCTION
When natural antioxidant defences of the organism (of enzymatic, non-enzymatic, or dietary origin) are overwhelmed by an excessive generation of reactive oxygen species, a situation of oxidative stress occurs, in which cellular and extracellular macromolecules (proteins, lipids and nucleic acids) can suffer oxidative damage, causing tissue injury.[Citation1,Citation2] Consumption of foods naturally bearing antioxidant activity (e.g., various food plants, fruits, and vegetables) is the most efficient way of combating such tissue injuries, undesired transformations and health risks.
In many studies about plant antioxidant research, it has been indicated that the measured antioxidant activity was dependent on the type of assay selected,[Citation3–5] and the observed antioxidant activity (or capacity) was not fully correlated to total polyphenolics content of the plant extracts.[Citation3,Citation5,Citation6] However, electron transfer (ET)- based antioxidant capacity assays involving a redox reaction with the oxidant (also the probe for monitoring the reaction) as an indicator of the reaction end-point:
where the change in color of the probe is proportional to the total antioxidant concentration, may yield results that are compatible with polyphenolic contents. In general, the antioxidant capacities reported by ET-based assays show acceptable correlations.[Citation7,Citation8] In this regard, Folin (FCR), ABTS/TEAC, FRAP, and CUPRAC assays are all classified as electron-transfer (ET) based assays, and it is emphasized that the reaction rate differences between antioxidants and oxidants are not reflected in the ABTS/TEAC values because the TEAC assay is an end-point assay.[Citation7] The diverse antioxidant activity/capacity assay methods existing in literature depending on the consumption of chromogenic radicals, i.e., ABTS[Citation9] and DPPH,[Citation10] oxygen radical absorption capacity: ORAC,[Citation11] or ferric reducing ability: FRAP[Citation12] have been extensively criticized for their inadequacies.[Citation13] Ou et al.[Citation14] concluded that there is no “total antioxidant” as a nutritional index available for food labeling because of the lack of standard quantification methods. Therefore the selected chromogenic redox reagent for the assay of plant material should be easily accessible, stable, selective, respond to all types of known antioxidants regardless of chemical type or hydrophilicity; the concerned redox reaction should be rapid and the resulting colour should be stable for a reasonable period of time. Such a photometric reagent fitting the above purposes, bis(neocuproine) copper(II) chloride, which had been introduced by our research group for various reducing agents as a mild oxidant,[Citation15] and used to determine the biochemically important reductants such as cysteine[Citation16] and vitamin E,[Citation17] and ascorbic acid,[Citation18] has been recently developed by our group for measuring total antioxidant capacity in plant extracts[Citation13] and human serum[Citation19] via cupric ion reducing potency, and this method–named as the CUPRAC method– was applied to the measurement of antioxidant capacities of apricots[Citation20] and herbal teas.[Citation21] This work aims to measure the antioxidant capacities of various herbal plants used in the manufacture of traditional Van herby cheese, called ‘otlu peynir’ in Eastern Anatolia of Turkey, using the electron transfer–based antioxidant assays of CUPRAC,[Citation13] ABTS/persulfate,[Citation22] Folin,[Citation23] and FRAP.[Citation12] The results expressed as trolox equivalent antioxidant capacities were compared among themselves to produce meaningful results.
The herby cheese produced in Van is known as ‘otlu peynir’ in East Anatolia, and is also produced in other cities such as Diyarbakir, Mus, and Bitlis. It is known that Van has semi-arid Mediterranean climate, while Mus and Bitlis have little rainy Mediterranean climate, both with cold winters.[Citation24] The local production has continued for more than 200 years. The herbs mainly consisting of wild garlic (allium) species are belived to be added to Van cheese as antimicrobial preservatives and flavouring aids[Citation25] as well as vitamin-rich sources to be eaten under severe winter conditions when fresh vegetables are no longer available to the mountain villagers, raising the resistance of the population to diseases.[Citation26] Although antibacterial activities of the extracts of some herbs used in Van herby cheese were investigated,[Citation27] their total antioxidant capacities were not studied aside from their ascorbic acid content.[Citation26] In the traditional way of herby cheese making, the fresh milk is filtered and renneted at its natural temperature. After coagulation, it is cut into small pieces and whey is removed. The herbs in sliced form are added to curd at a ratio of 0.5–3.0 kg per curd obtained from 100 kg milk, and the curd is mixed well to get a homogenous distribution of herbs inside the curd. The cheese making is completed by pressing, breaking down into blocks by hand, salting, filling into containers by forcing air out, and final ripening for 3 months.[Citation26]
The objectives of each operation at this stage can be defined as follows: (i) pressing: accelerating the separation of water from the curd;[Citation28] (ii) salting: used as brine in the form of 20–25 % salt solution, with the purpose of improving taste, inhibiting salt-sensitive microorganisms, restricting acidity, insolubilizing caseine, and regulating dehydration via osmotic pressure;[Citation28] (iii) filling into containers by forcing air out: minimizing the air-carried pathogen bacteria. After the first week of ripening, just one side of the cases was opened and placed onto a sterile cloth, without removing cases. They all were placed in sterilized fine sand by making the open side of cases down so the remained whey can easily drain;[Citation29] (iv) Ripening: The cheeses were ripened at approximately 9°C for 90 days. The benefits in biochemical parameters expected from cheese ripening are proteolysis (enzymatic protein degradation into free amino acids with concomitant increase of dissolved N), lipolysis (breakdown of fat to release fatty acids), and stabilization of microbial counts.[Citation29] Proteolysis during ripening of cheese may lead to changes in elasticity and viscosity.[Citation30] The process of ripening may also improve the physical properties and equilibrated salt migration from the curd,[Citation31] while the textural and sensorial attributes of cheese were significantly affected by the ripening period.[Citation32] Rheological studies suggested that the most important factors influencing the texture of the cheese is the level of total solids, and the extent of protein degradation recorded as soluble nitrogen during the ripening period.[Citation33]
MATERIALS AND METHODS
Materials and Instrumentation
The basic herbal plants used in the manufacture of Van herby cheese—as collected in varying periods of the year 2005, pressed, dried according to herbarium techniques, and identified by biologist Dr. Ozgokce with respect to Flora of Turkey,[Citation34,Citation35] and tested in this work for antioxidant capacity—were the following (given in the format; botanical name, “local name,” geographical location of collection, date of Van Yuzuncuyil University Herbarium entry, Herbarium index code of collector).
-
Allium vineale L. (Liliaceae), “sirmo,” Van Erciş- downwards from Sor Village, meadow, 1850 m above sea level, May 25, 2005, F 12 773: Plant height may reach 40–60 cm in humid areas; parts of plant other than bulb and flowers are used in herby cheese making. This plant is used exclusively in herby cheese though some plants may not be used by some manufacturers. The above-ground parts of the plant are collected while budding, sliced into 2–3 cm pieces, and added to herby cheese composition.
-
Allium schoenoprasum L. (Liliaceae), “sirmo,” Van- Çaldıran exit, meadow, 2000 m, May 23, 2005, F 12 774: This multi-annual plant may reach up to 60 cm, have oblong cylindirical bulbs, the flower community have hollow stems, 1–2 leaves, flower stems of different length, flowers pink or pinkish purple. The above-ground parts are collected from the young plant, and sliced into 1–2 cm pieces before being added to herby cheese composition. Also used in the region as vegetable and spice.
-
Allium schoenoprasum L. (Liliaceae), “sirmo,” Van- back of Erek Mountain, Keşişgöl, meadow, 2100 m, May 23, 2005, F 11 775: Properties same as 2.
-
Allium vineale L. (Liliaceae), “sirmo,” Van- Gürpınar, Norduz Plateau, meadow, 2000 m, May 23, 2005, F 11 776: Properties same as 1.
-
Allium atroviolaceum Boiss. (Liliaceae), “sirmo,” Van- between Gürpınar and Çatak, hill side, 1800 m, May 23, 2005, F 11 777: Plant at a height of 50–100 cm, have 3–5 leaves of 2–10 mm width, flower stems of different lengths, flower dark pink or blackish colored, multi-annual plants with bulbs of 1.0–2.5 cm diameter. The above-ground parts of the plant are collected while budding, sliced into 2–3 cm pieces, and added to herby cheese composition.
-
Ferula rigidula DC. (Apiaceae), “siyabu,” Van- Alacabük Mountain, upper sides of Doluca Village, hill side, 2000 m, June 25, 2005, F 12 770: The plant may reach up to a height of 70–120 cm, base leaves 5–6 segmented, fruit stems 7–12 mm, fruits obovate-oblong. The above-ground parts of the young plant are collected, sliced into 0.5–1.5 cm pieces, and added to herby cheese. The plant is also consumed as food in the region as uncooked, boiled, or fried with eggs.
-
Prangos ferulacea (L.) Lindl. (Apiaceae), “heliz,” Van-Alacabük Mountain, upper sides of İn Village, steppe, 2000 m, June 25, 2005, F 12 771: Multi-annual, 50–150 cm height plant, base, and lower leaves 60–80 cm long, thread-like leaves six-segmented, flowers yellow-colored, and nonhairy. Fresh stems and leaves of the plant are incorporated in herby cheese. Also used in the region as vegetable and spice.
-
Chaerophyllum macropodum Boiss. (Apiaceae), “mendi,” Van- Alacabük Mountain, Palandiz Hills, hill side, 2400 m, June 23, 2005, F 12 772: The plant may reach a height of 40–120 cm within 2 years; it has a thick root and stem, straight branches, long white feathers, lower leaves at a length of 20–30 cm. Flowers white colored, and fruit linear oblong. Fresh stem and leaves incorporated in herby cheese. Also used in the region as vegetable and spice.
-
Thymus transcaucasicus Ronniger (Lamiaceae), “kekik, catir, catri,” Van- Özalp, Beyazıt Mountain, steppe, 2680 m, June 23, 2003, F 3810: This multi-annual plant is branched from the base and grows up to 5–10 cm, leaves 7–10 mm, about 7 mm flower with colors ranging from white to pink, stem and leaves stained by secretion. Its leaves are used in herby cheese making, and the plant is also used as spice.
-
Allium vineale L. (Liliaceae), “sirmo,” Van- Alacabük Mountain, Aydınocak Village, meadow, 2000 m, June 23, 2003, F 11 178 : Properties same as 1.
-
Chaerophyllum macropodum Boiss. (Apiaceae), “mendi,” Van- Alacabük Mountain, Palandiz Hills, hill side, 2400 m, June 23, 2003, F 11 176 : Properties same as 8.
-
Prangos ferulacea (L.) Lindl. (Apiaceae), “heliz,” Van- Alacabük Mountain, upper side of İn Village, steppe, 2000 m, June 25, 2003, F 11 175: Properties same as 7.
-
Chaerophyllum macropodum Boiss. (Apiaceae), “mendi,” Van- Alacabük Mountain, Palandiz Hills, hill side, 2400 m, May 23, 2005, F 11 778: Properties same as 8.
-
Prangos ferulacea (L.) Lindl. (Apiaceae), “heliz,” Van-Alacabük Mountain, upper side of İn Village, steppe, 2000 m, May 25, 2005, F 11 779: Properties same as 7.
-
Thymus kotschyanus Boiss. & Hohen subsp. kotschyanus (Lamiaceae) “kekik, catir, catri,” Bitlis/Van- Alacabük Mountain, southeastern hills, climbing from Yıldöndü to the mountain peak, 2900 m, June 16, 2002, F 10 864: Multi-annual plant branched from base, reaching a height of 3–10 cm; leaves about 9–13 mm with brown-to-red colored small oily purses, flowers 6–8 mm with colors ranging from white to faint pink, plant having numerous excretion purses in the stem and leaves. The young leaves of the plant are used for herby cheese making. Above-ground parts of the plant are also used for spice and herbal tea (kekik cayi) making as a cure for stomach ache.
-
Chaerophyllum macropodum Boiss. (Apiaceae), “mendi,” Van- Alacabük Mountain, Palandiz Hills, hill side, 2400 m, June 23, 2003, F 11 176: Properties same as 8.
Neocuproine (2,9-dimethyl-1,10-phenanthroline) and Folin-Ciocalteau phenol reagent were purchased from Sigma Chem., trolox ((±)-6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid) from Aldrich Chem., ABTS (2,2′-azinobis(3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt) and TPTZ (2,4,6-tripyridyl-S-triazine) from Fluka Chem., ammonium acetate, copper(II) chloride, potassium persulfate, sodium hydroxide, copper(II) sulfate, sodium carbonate, sodium potassium tartarate, 96% ethyl alcohol, methanol, glacial acetic acid, and ferric chloride hexahydrate from E. Merck. CuCl2 solution, 1.0x10−2 M, was prepared by dissolving 0.4262 g CuCl2.2H2O in water, and diluting to 250 mL. Ammonium acetate buffer at pH 7.0, 1.0 M, was prepared by dissolving 19.27 g NH4Ac in water and diluting to 250 mL. Neocuproine (Nc) solution, 7.5 × 10−3 M, was prepared daily by dissolving 0.039 g Nc in 96% ethanol, and diluting to 25 mL with ethanol. Trolox 1.0 × 10−3 M was prepared in 96% ethanol. The chromogenic radical reagent ABTS, at 7.0 mM concentration, was prepared by dissolving 0.1920 g of the compound in water, and diluting to 50 mL. To this solution K2S2O8 (as an oxidant to convert the ABTS reagent to the ABTS.+ radical cation) was added to yield a final persulfate concentration of 2.45 mM, as described by Re et al.[Citation22] The resulting ABTS radical cation solution was left to mature at room temperature in the dark for 12–16 h, and then used for TEAC assays.
The solutions used in the Folin assay of polyphenolics were prepared as follows: Lowry A: 2% aqueous Na2CO3 in 0.1 M NaOH; Lowry B: 0.5% CuSO4 aqueous solution in 1% NaKC4H4O6 solution; Lowry C: prepared freshly as mixture (50 mL Lowry A + 1 mL Lowry B); Folin-Ciocalteau reagent was diluted with H2O at a volume ratio of 1:3 prior to use. All percentages are given as (w/v), and distilled and deaerated (N2-bubbled) water was used throughout. The FRAP solutions were prepared as folllows: A suitable mass of FeCl3.6H2O was weighed so that the final concentration of Fe(III) in solution would be 2.0 × 10−2 M; 1 mL of 1 M HCl solution was added, dissolved in some water and diluted to 50 mL with H2O. A suitable mass of TPTZ was weighed such that its final concentration would be 1.0 × 10−2 M, dissolved in 96% EtOH, and diluted to 50 mL. In order to prepare 0.3 M CH3COOH/CH3COONa buffer solution at pH 3.6, 3.1 g of CH3COONa.3H2O was weighed and 16 mL glacial acetic acid was added, diluted with water to 1 L. The FRAP reagent was prepared as follows: The pH 3.6 acetic acid buffer, 1.0 × 10−2 M TPTZ solution, and 2.0 × 10−2 M FeCl3.6H2O solution were mixed in this order at a volume ratio of 10:1:1. The FRAP reagent was prepared and used freshly.
All spectrophotometric measurements were made with a pair of matched quartz cuvettes using a Varian CARY 1E UV-Vis spectrophotometer. The pH measurements were made with the aid of a E512 Metrohm Herisau pH-meter using a glass electrode; the centrifugations were performed with an Adams Dynac Centrifuge apparatus. Ultra-Turrax CAT X620 apparatus was used for extraction.
Procedures
Solvent Extraction of Plant Materials
The moisture of plant materials were estimated by drying in an oven at 105°C for 2 h. The dry plant specimens were crushed in a mill, and 2-g samples were taken for each plant species. These samples were soaked in 80% MeOH overnight, and homogenized in an Ultra-Turrax apparatus by gradually increasing the number of cycles per unit time. The obtained extracts were transfered to centrifuge tubes and centrifuged for 10 min, and subsequently filtered through a filter paper into 100 mL flasks. The same procedure was repeated 3 times with 25 mL portions of 80% MeOH on the remaining part of the plants. All filtered extracts were combined, and diluted to 100 mL using the same solvent. Each extraction procedure was run thrice in parallel.[Citation20,Citation36] The obtained extracts were analyzed for their antioxidant capacities on the next day after preserving the N2 bubbled and stoppered extracts in a refrigerator at + 4°C. The antioxidant capacities of plant samples were reported based on dry matter content.
CUPRAC Assay of Total Antioxidant Capacity
One mL of CuCl2 solution (1.0 × 10−2 M), 1 mL of neocuproine alcoholic solution (7.5 × 10−3 M), and 1 mL NH4Ac buffer solution were mixed; 0.5 mL of dilute plant extract (previously diluted with MeOH at a volume ratio of 1:10) followed by 0.6 mL of water were added (total volume = 4.1 mL), and mixed well in stoppered tubes. Absorbance against a reagent blank was measured at 450 nm after 30 min. Since the calibration curve for pure trolox is a line passing through the origin, the trolox equivalent molar concentration of the plant extract sample in final solution may be found by dividing the observed absorbance to the ϵ for trolox (optical cuvette thickness = 1 cm). The trolox equivalent antioxidant capacity (TEAC) may be traced back to the original extract considering all dilutions, and proportionated to the initial mass of plant sample taken to find a capacity in the units of micromoles TR/g dry matter. The recommended technique was applied thrice to three different 0.5 mL aliquots of each plant extract. If the above practice is followed, then:
where the molar absorptivity of trolox in the CUPRAC method is ϵTR = 1.67 × 104 Lmol−1cm−1.
ABTS/Persulfate Assay of Total Antioxidant Capacity
The matured ABTS radical solution of blue-green colour was diluted with ethanol at a ratio of 1:10. The absorbance of the 1:10 diluted ABTS·+ radical cation solution was 1.28 ± 0.04 at 734 nm. To one mL of the radical cation solution, 4 mL of ethanol were added, and the absorbance at 734 nm was read at the end of the first and sixth minute. The procedure was repeated for the unknown plant extract by adding 1 mL of the radical cation solution to 1 mL of dilute plant extract (previously diluted with MeOH at a volume ratio of 1:10) and 3 mL of ethanol, and recording the absorbance readings at the end of first and sixth min. The absorbance difference (△A) was found by subtracting the extract absorbance from that of the reagent blank (pure radical solution), and this was correlated to trolox equivalent antioxidant concentration with the aid of a linear calibration curve (usually the absorbance decrease at the 6th min was used for calculations). The recommended technique was applied thrice to three different 1.0 mL aliquots of each plant extract:
where the molar absorptivity of trolox in the ABTS method is ϵTR = 2.6 × 104 Lmol−1cm−1.
Folin Method of Total Phenolics Assay
To 0.5 mL of the dilute plant extract (previously diluted with MeOH at a volume ratio of 1:10) was added 1.5 mL H2O. An aliquot of 2.5 mL of Lowry C solution was added, and the mixture was let to stand for 10 min. At the end of this period, 0.25 mL of Folin reagent was added, and 30 more min was allowed for stabilization of the blue color formed. The absorbance against a reagent blank was measured at 750 nm. The recommended technique was applied thrice to three different 0.5 mL aliquots of each plant extract.
where the molar absorptivity of trolox in the Folin method is ϵTR = 4.65 × 103 Lmol−1cm−1.
FRAP Assay of Total Antioxidant Capacity
3 mL of the FRAP reagent was added to 0.3 mL H2O. Then 25, 50, and 100 μL aliquots of the plant extracts were taken, and 96% EtOH was added to make the final volume 3.4 mL. The absorbance at 595 nm (A595 ) was read against a reagent blank at the end of 6 min:
where the molar absorptivity of trolox in the FRAP method is ϵTR = 4.63 ×104 Lmol-1cm-1 .
RESULTS AND DISCUSSION
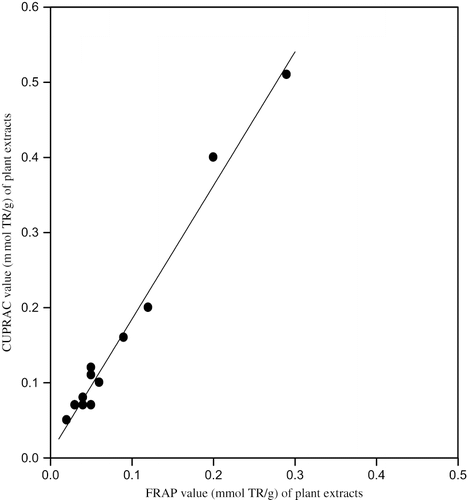
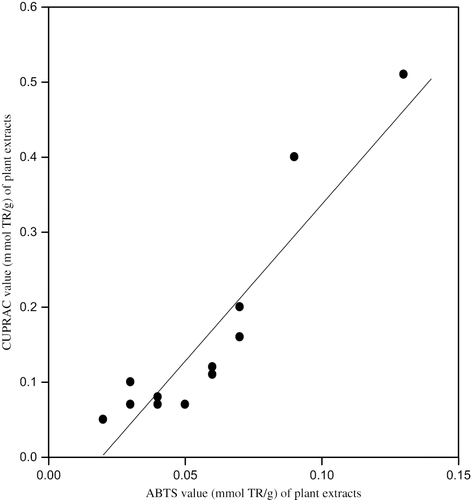
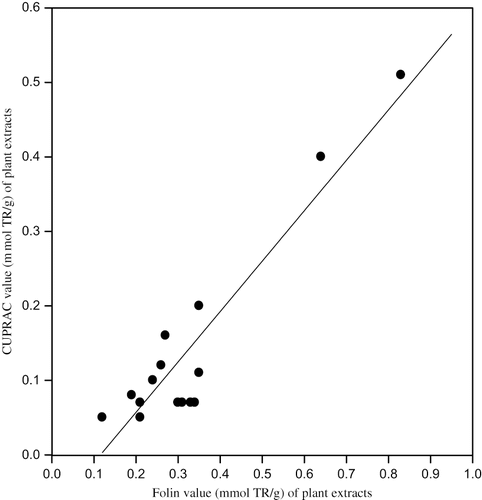
The total antioxidant capacities in trolox (TR) equivalents of 16 different plant samples collected from different geographical locations of Van (8 different species) used in the manufacture of Van herby cheese: ‘otlu peynir,’ as assayed by CUPRAC, ABTS/persulfate, FRAP, and Folin methods, are depicted in . Generally the assay results correlated well among each other, because all were electron transfer based assays[Citation7,Citation8] having similar mechanism. For example, the CUPRAC results as a function of ABTS (), of FRAP (), and of Folin () are shown in the corresponding figures, with the squared linear correlation coefficients (r2) of 0.837, 0.984, and 0.866, respectively. When these results (i.e., 16 pairs of analytical findings for all combinations) were subjected to statistical analysis,[Citation37] the following linear equations were obtained:
Table 1 The total antioxidant capacities in trolox (TR) equivalents of 16 different plant samples collected from different geographical locations of Van (8 different species) used in the manufacture of Van herby cheese: ‘otlu peynir’, as assayed by CUPRAC, ABTS/persulfate, FRAP, and Folin methods
Statistical analysis with two-tailed t-tests using the computed t-data using the equation: t = │r│√(n−2) / √(1−r2) for (n−2) degrees of freedom[Citation37] confirmed that the ‘null hypothesis’ (stating that there is no correlation between the used x and y values) was rejected at 95% confidence level, meaning that a significant correlation does exist between the tested variables. Thus the experimentally found t-values for CUPRAC-ABTS, CUPRAC-FRAP, and Folin-CUPRAC binary correlations were 8.48, 29.7, and 9.51, respectively, while the theoretical value was t.05 = 2.14 for (n−2) = 14 degrees of freedom.
The highest correlation of CUPRAC was obtained with FRAP, because these are Cu(II)– and Fe(III)– reducing antioxidant assays with similar redox potentials,[Citation13,Citation19,Citation38] though CUPRAC is capable of measuring a greater variety of antioxidant compounds –regardless of their hydrophilicity– than FRAP.[Citation13,Citation19] It is also noteworthy that the CUPRAC-Folin correlation was better than those of most other antioxidant tests with Folin, because CUPRAC also enabled the oxidation of phenolic hydroxyl groups to the corresponding quinones like the Folin assay.[Citation13] The highest results of antioxidant capacity were obtained with the Folin test, because the molybdato-phospho-tungstate heteropoly acid reagent of this test had the highest redox potential (among all applied assays) in alkaline medium where most phenolic compounds are deprotonated and open to oxidative attack, and thus Folin measured all phenolic species nonselectively, as well as antioxidants.[Citation7,Citation8] The second highest results were obtained with CUPRAC especially for the wild garlic (allium), because the sulfur-containing antioxidant compounds present in allium vegetables[Citation39] can be best assayed with the CUPRAC method, whereas methods like FRAP method do not respond efficiently to glutathione-type compounds.[Citation13,Citation19,Citation40,Citation41] A detailed study of iron(III)-based antoxidant assay methods (including FRAP) carried out in our laboratory[Citation38] revealed that high-spin Fe(III) with half-filled d-orbitals (d5) indeed showed a chemical inertness toward thiol-type antioxidants as well as toward certain hydroxycinnamic acids abundant in the plant kingdom, while the Cu(II)-neocuproine reagent in CUPRAC[Citation13,Citation19] reacted much faster with those compounds. Thus, this should explain the higher CUPRAC results with the Allium species, as on the average, the CUPRAC antioxidant capacities for the tested plants were 1.78 times those of FRAP. A major garlic compound present in Allium species, S-allylcysteine, was shown to minimize intracellular oxidative stress of LDL.[Citation39]
The weakest correlation with the CUPRAC results—although existent at 95 % confidence level—was obtained with the ABTS/persulfate test (r2 = 0.837). As for hydroxycinnamic acids which are almost the most abundant phenolic components in the citrus family and in some other fruits, the TEAC coefficients with respect to the CUPRAC method (and with respect to the ABTS assay, as shown in parantheses) were as follows: caffeic acid 2.9 (1.4), chlorogenic acid 2.5 (1.2), ferulic acid 1.2 (2.2), and p-coumaric acid 0.6 (1.6).[Citation13] The Trolox equivalent capacity order for these phenolic acids was just the opposite of that of the most widely used ABTS assay.[Citation42] Thiol type antioxidants (e.g., glutathione) showed a TEAC value of 1.5 in ABTS compared to the TEAC value of 0.5 in CUPRAC.[Citation19] Moreover, the ABTS method may be considered as both an electron transfer (ET) and a hydrogen atom transfer (HAT) assay, comprising both mechanisms, which is somewhat different from the CUPRAC assay which is purely ET-based.[Citation43] Thus the correlation with CUPRAC was weakest for ABTS assay results among the three reference methods of analysis (i.e., ABTS, FRAP, and Folin). Structural properties of hydroxycinnamic acids would normally dictate that two –OH bearing caffeic and chlorogenic acids should exhibit higher TEAC coefficients than one –OH bearing ferulic and p-coumaric acids. Furthermore, ferulic acid having an electron-donating methoxy group in ortho-position relative to the phenolic –OH, thereby allowing increased stabilization of the resulting aryloxy radical through electron delocalization after H-atom donation by the hydroxyl group, should show a higher TEAC coefficient than para-coumaric acid which lacks such a group. Thus structural requirements dictate that hydroxycinnamic acids should have a TEAC order as measured by the CUPRAC and not by the ABTS assay. Moreover, the order of peroxyl radical scavenging ability of hydroxycinnamic acids, and thus the order for their ability to enhance the resistance of LDL to oxidation, was measured as caffeic acid > chlorogenic acid > ferulic acid > p-coumaric acid,[Citation44] again entirely consistent with the results of the CUPRAC method. Since it was shown that the TEAC order of hydroxycinnamic acids and the value attributed to glutathione (G-SH) reflected the superiority of CUPRAC over ABTS,[Citation43] the discrepancies between the CUPRAC and ABTS results for 16 plant species tested in this work () may be accounted for.
The order of reported CUPRAC antioxidant capacities—as trolox equivalents—in was thymus sp. > chaerophyllum sp. > allium sp. > prangos sp. ≥ ferula sp. On the other hand, the order of Folin findings was thymus sp. > allium sp. > chaerophyllum sp. > ferula sp. ≥ prangos sp. Both orders of antioxidant status revealed that Thymus sp. (Lamiaceae) showed the greatest capacity, due to its higher phenolic content. Generally thyme species, both domestic and wild, were shown before to have considerable antioxidant activity[Citation45] due to carvacrol and/or thymol type of phenolics.[Citation46] Both Thymus transcaucasicus Ronniger oil and Thymus kotschyanus Boiss. & Hohen oil were shown to contain thymol (33–35%), carvacrol (11–12%), as well as other phenols, e.g., linanol and p-cymol.[Citation47] Allium species had higher phenolic content but less antioxidant capacity than chaerophyllum sp. Previously, the higher antibacterial activity of Allium vineale (than either Prangos ferulacea or Chaerophyllum macropodum) was explained by the higher phenolic content of the former species in MeOH extracts.[Citation27] In regard to antioxidant properties of Allium sp., Allium vineale L. (wild), Allium atroviolaceum Boiss., and Allium schoenoprasum L. were shown to contain the antioxidant constituents of GSH (nmol/mg-protein) as 0.108, 0.399, and 0.669; flavonoids (mg/g) as 259, 59, and 432; and vitamin C (mg/g) as 0.066, 0.157, and 0.122, respectively.[Citation48] As indicated, there is a great variety of herbal plants used for herby cheese making in the region, but Allium sp. is included in every practise as a first preference, because these wild garlic types act as natural antioxidant[Citation49] and antibacterial[Citation27] agents. It was also observed that identical species had great variability in antioxidant content with respect to geographical location and altitude.
CONCLUSION
Although the antimicrobial activity of certain herbs used in herby cheese making was studied before, the total antioxidant capacities in trolox (TR) equivalents of 16 different plant samples collected from different geographical locations of Van (8 different species) used in the manufacture of Van herby cheese (‘otlu peynir’) were assayed for the first time in this work by CUPRAC, ABTS/persulfate, FRAP, and Folin methods. There was very good linear correlations among the assay results. The highest results of antioxidant capacity were obtained with the Folin test because of its highest redox potential in alkaline medium. The second highest results were obtained with CUPRAC especially for the wild garlic (allium), because the sulfur-containing antioxidant compounds present in allium vegetables could be best assayed with the CUPRAC method, whereas methods like FRAP method did not respond to glutathione-type compounds. The weakest correlation with CUPRAC assay results—though existent at 95% confidence level—was obtained for ABTS/persulfate test, because the Trolox equivalent antioxidant capacities (TEAC values) of hydroxycinnamic acids and of –SH compounds were quite different in these two assays. The order of reported CUPRAC antioxidant capacities—as trolox equivalents—was thymus sp. > chaerophyllum sp. > allium sp. > prangos sp. ≥ ferula sp. On the other hand, the order of Folin findings was thymus sp. > allium sp. > chaerophyllum sp. > ferula sp. ≥ prangos sp. Both orders of antioxidant status revealed that Thymus sp. (Lamiaceae) showed the greatest antioxidant capacity, due to their higher phenolic content. Allium sp. is included in every practise of herby cheese making in East Anatolia as a first preference.
ACKNOWLEDGMENTS
The authors would like to express their gratitude to Istanbul University Research Fund, Bilimsel Arastirma Projeleri Yurutucu Sekreterligi, for the funding of Projects YOP-4/27052004 and 2724, and to State Planning Organization of Turkey for the Advanced Research Project of Istanbul University (2005K120430). The authors would like to extend their gratitude to TUBITAK (Turkish Scientific and Technical Research Council) for the Research Project 105T402, and to TUBITAK (Turkish Scientific and Technical Research Council) for the Research Project 106T514.
REFERENCES
- Halliwell , B. and Gutteridge , J.M.C. 1989 . Free Radicals in Biology and Medicine , Oxford, , U.K. : Oxford University Press .
- Halliwell , B. and Aruoma , O.I. 1991 . DNA damage by oxygen-derived species: its mechanisms and measurement in mammalian systems . FEBS Letters , 281 : 9 – 19 .
- Dorman , H.J. , Peltoketo , A. , Hiltunen , R. and Tikkanen , M.J. 2003 . Characterisation of the antioxidant properties of de-odourised aqueous extracts from selected Lamiaceae herbs . Food Chemistry , 83 : 255 – 262 .
- Trouillas , P. , Calliste , C.-A. , Allais , D.-P. , Simon , A. , Marfak , A. , Delage , C. and Duroux , J.-L. 2003 . Antioxidant, anti-inflammatory and antiproliferative properties of sixteen water plant extracts used in the Limousin countryside as herbal teas . Food Chemistry , 80 : 399 – 407 .
- Miliauskas , G. , Venskutonis , P.R. and van Beek , T.A. 2004 . Screening of radical scavenging activity of some medicinal and aromatic plant extracts . Food Chemistry , 85 : 231 – 237 .
- Kahkonen , M.P. , Hopia , A.I. , Vuorela , H.J. , Rauha , J.P. , Pihlaja , K. , Kujala , T.S. and Heinonen , M. 1999 . Antioxidant activity of plant extracts containing phenolic compounds . Journal of Agricultural and Food Chemistry , 47 : 3954 – 3962 .
- Huang , D. , Ou , B. and Prior , R.L. 2005 . The chemistry behind antioxidant capacity assays . Journal of Agricultural and Food Chemistry , 53 : 1841 – 1856 .
- Prior , R.L. , Wu , X. and Schaich , K. 2005 . Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements . Journal of Agricultural and Food Chemistry , 53 : 4290 – 4302 .
- Miller , N.J. , Rice-Evans , C.A. , Davies , M.J. , Gopinathan , V. and Milner , A. 1993 . A novel method for measuring antioxidant capacity and its application to monitoring the antioxidant status in premature neonates . Clinical Science , 84 : 407 – 412 .
- Sanchez-Moreno , C. , Larrauri , J.A. and Saura-Calixto , F. 1998 . A procedure to measure the antiradical efficiency of polyphenols . Journal of the Science of Food and Agriculture , 76 : 270 – 276 .
- Cao , G. , Verdon , C.P. , Wu , A.H.B. , Wang , H. and Prior , R.L. 1995 . Automated oxygen radical absorbance capacity assay using the COBAS FARA II . Clinical Chemistry , 4 : 1738 – 1744 .
- Benzie , I.F.F. and Strain , J.J. 1996 . The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay . Analytical Biochemistry , 239 : 70 – 76 .
- Apak , R. , Güçlü , K. , Özyürek , M. and Karademir , S.E. 2004 . Novel total antioxidant capacity index for dietary polyphenols, vitamin C and E, using their cupric ion reducing capability in the presence of neocuproine: CUPRAC method . Journal of Agricultural and Food Chemistry , 52 : 7970 – 7981 .
- Ou , B. , Huang , D. , Hampsch-Woodill , M. , Flanagan , J.A. and Deemer , E.K. Analysis of antioxidant activities of common vegetables employing oxygen radical absorbance capacity (ORAC) and ferric reducing antioxidant power (FRAP) assays: A comparative study . Journal of Agricultural and Food Chemistry 2002, 50 , 3122 – 3128 .
- Tütem , E. , Apak , R. and Baykut , F. 1991 . Spectrophotometric determination of trace amounts of copper(I) and reducing agents with neocuproine in the presence of copper(II) . Analyst , 116 : 89 – 94 .
- Tütem , E. and Apak , R. Simultaneous spectrophotometric determination of cystine and cysteine in amino acid mixtures using copper(II)-neocuproine reagent . Analytica Chimica Acta 1991 , 255 121 – 125 .
- Tütem , E. , Apak , R. , Günaydı , E. and Sözgen , K. 1997 . Spectrophotometric determination of vitamin E (alpha-tocopherol) by the copper(II)-neocuproine reagent . Talanta , 44 : 249 – 255 .
- Güçlü , K. , Sözgen , K. , Tütem , E. , Özyürek , M. and Apak , R. 2005 . Spectrophotometric determination of ascorbic acid using copper(II)-neocuproine reagent in beverages and pharmaceuticals . Talanta , 65 : 1226 – 1232 .
- Apak , R. , Güçlü , K. , Özyürek , M. , Karademir , S.E. and Altun , M. 2005 . Total antioxidant capacity assay of human serum using copper(II)-neocuproine as chromogenic oxidant: The CUPRAC method . Free Radical Research , 39 : 949 – 961 .
- Güçlü , K. , Altun , M. , Özyürek , M. , Karademir , S.E. and Apak , R. 2006 . Antioxidant Capacity of Fresh, Sun- and Sulfited-Dried Malatya Apricot (Prunus Armeniaca) Assayed by CUPRAC, ABTS/TEAC and Folin Methods . International Journal of Food Science and Technology , 41 : 76 – 85 .
- Apak , R. , Güçlü , K. , Özyürek , M. , Karademir , S.E. and Ercag , E. 2006 . The Cupric Ion Reducing Antioxidant Capacity and Polyphenolic Content of Some Herbal Teas . International Journal of Food Science & Nutrition , 57 : 292 – 304 .
- Re , R. , Pellegrini , N. , Proteggente , A. , Pannala , A. , Yang , M. and Rice-Evans , C. 1999 . Antioxidant activity applying an improved ABTS radical cation decolorization assay . Free Radical Biology and Medicine , 26 : 1231 – 1237 .
- Singleton , V.L. , Orthofer , R. and Lamuela-Raventos , R.M. 1999 . Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent . Methods in Enzymology , 299 : 152 – 178 .
- Hamzaoglu , E. 2006 . Phytosociological studies on the steppe communities of East Anatolia . Ekoloji , 15 : 29 – 55 .
- Kucukoner , E. , Tarakci , Z. and Sagdic , O. 2006 . Physicochemical and microbial characteristics and mineral content of herby cacik, a traditional Turkish dairy product . Journal of the Science of Food and Agriculture , 86 : 333 – 338 .
- Coskun , H. and Öztürk , B. 2000 . Vitamin C contents of some herbs used in Van herby cheese (Van otlu peyniri) . Nahrung , 44 : 379 – 380 .
- Sagun , E. , Durmaz , H. , Tarakci , Z. and Sagdic , O. 2006 . Antibacterial activities of the extracts of some herbs used in Turkish herby cheese against Listeria Monocytogenes Serovars . International Journal of Food Properties , 9 : 255 – 260 .
- Coşkun , H. 2005 . Otlu Peynir, Gıda Teknolojisi Yayınları , Vol. No: 31 , Ankara, , Turkey : Bizim Büro Basımevi .
- Coşkun , H. 1998 . Microbial and biochemical changes in herby cheese during ripening . Nahrung , 42 : 309 – 313 .
- Venugopal , V. and Muthukumarappan , K. 2003 . Rheological properties of cheddar cheese during heating and cooling . International Journal of Food Properties , 6 : 99 – 114 .
- Guizani , N. , Kasapis , S. , Al-Attabi , Z.H. and Al-Ruzeiki , M.H. 2002 . Microbial, physicochemical, and biochemical changes during ripening of camembert cheese made of pasteurized cow's milk . International Journal of Food Properties , 5 : 483 – 494 .
- Topcu , A. and Saldamli , I. 2006 . Proteolytical, chemical, textural and sensorial changes during the ripening of Turkish white cheese made of pasteurized cows' milk . International Journal of Food Properties , 9 : 665 – 678 .
- Guizani , N. , Al-Attabi , Z. , Kasapis , S. and Gaafar , O.M. 2006 . Ripening profile of semi-hard standard goat cheese made from pasteurized milk . International Journal of Food Properties , 9 : 523 – 532 .
- Davis , P.H. , Mill , R.R. and Tan , K. , eds. 1988 . Flora of Turkey and the East Aegean Islands , Vol. 10 , Edinburgh, , U.K. : Edinburgh University Press . (supplement)
- Güner , A. , Özhatay , N. , Ekim , T. and Baser , K.H.C. , eds. 2000 . Flora of Turkey and the East Aegean Islands , Vol. 11 , Edinburgh, , U.K. : Edinburgh University Press . (supplement 2)
- Garcia-Alonso , M. , Pascual-Teresa , S. , Santos-Buelga , C. and Rivas-Gonzalo , J.C. 2004 . Evaluation of the antioxidant properties of fruits . Food Chemistry , 84 : 13 – 18 .
- Miller , J.C. and Miller , J.N. 1993 . “ Satatistics for Analytical Chemistry ” . London, , U.K. : Ellis Horwood Series in Analytical Chemistry, Prentice Hall .
- Berker , K.I. , Güçlü , K. , Tor , I. and Apak , R. 2007 . Comparative evaluation of Fe(III) reducing power- based antioxidant capacity assays in the presence of phenanthroline, batho-phenanthroline, tripyridyltriazine (FRAP), and ferricyanide reagents . Talanta , 72 : 1157 – 1165 .
- Park , E.J. and Pezzuto , J.M. 2002 . Botanicals in cancer chemoprevention . Cancer and Metastasis Reviews , 21 : 231 – 255 .
- Gorinstein , S. , Leontowicz , M. , Leontowicz , H. , Najman , K. , Namiesnik , J. , Park , Y.-S. , Jung , S.-T. , Kang , S.-G. and Trakhtenberg , S. 2006 . Supplementation of garlic lowers lipids and increases antioxidant capacity in plasma of rats, Nutrition Research . 26 : 362 – 368 .
- Mazor , D. , Greenberg , L. , Shamir , D. , Meyerstein , D. and Meyerstein , N. 2006 . Antioxidant properties of bucillamine: Possible mode of action . Biochemical and Biophysical Research Communications , 349 : 1171 – 1175 .
- Rice-Evans , C.A. , Miller , N.J. and Paganga , G. 1997 . Antioxidant properties of phenolic compounds . Trends Plant Science , 2 : 152 – 159 .
- Apak , R. , Güçlü , K. , Demirata , B. , Özyürek , M. , Celik , S.E. , BektaşoGGGlu , B. , Berker , K.I. and Özyurt , D. 2007 . Comparative evaluation of total antioxidant capacity assays applied to phenolic compounds, and the CUPRAC assay . Molecules-Phenolics special issue , 12 : 1496 – 1547 .
- Kanski , J. , Aksenova , M. , Stoyanova , A. and Butterfield , D.A. 2002 . Ferulic acid antioxidant protection against hydroxyl and peroxyl radical oxidation in synaptosomal and neuronal cell culture systems in vitro: structure-activity studies . Journal Nutritional Biochemistry , 13 : 273 – 281 .
- Zheng , W. and Wang , S.Y. 2001 . Antioxidant activity of phenolic compounds in selected herbs . Journal of Agricultural and Food Chemistry , 49 : 5165 – 5170 .
- Kulevanova , S. and Panovska , T.K. 2001 . Antioxidant activity of essential oils of different wild Thymus L. Species . Bulletin of the Chemists and Technologists of Macedonia , 20 : 61 – 66 .
- Sefidkon , F. , Dabiri , M. and Rahimi-Bidgoly , A. 1999 . The effect of distillation methods and stage of plant growth on the essential oil content and composition of Thymus kotschyanus Boiss. & Hohen . Flavour and Fragrance Journal , 14 : 405 – 408 .
- Stajner , D. , Milic , N. , Canadanovic-Brunet , J. , Kapor , A. , Stajner , M. and Popovic , B.M. 2006 . Exploring Allium species as a source of potential medicinal agents . Phytotherapy Research , 20 : 581 – 584 .
- Stajner , D. , Canadanovic-Brunet , J. and Pavlovic , A. 2004 . Allium schoenoprasum L., as a natural antioxidant . Phytotherapy Research , 18 : 522 – 524 .