ABSTRACT
The objective of the present study was to analyze and compare the phenolic compounds and their antioxidant capacities of new lines of Dacus carota. The selected cultivars showed high variation in the contents of total phenolics (30.26–65.39 mg/100 g FW) and total ascorbic acid (41.12–58.36 mg/100 g FW). Analysis on RP-HPLC revealed that hydroxycinnamic acids and its derivatives were major phenolic compounds present in D. carota extracts, whereas 5-caffeolquinic acid was a major hydroxycinnamic acid (ranged from 30.26 to 65.39 mg/100 g FW). DCP cultivar showed high total antioxidant capacity (77.69 mg/100 g), 2,2-diphenyl-1-picrylhydrazyl (DPPH) scavenging capacity (52.36 mg/100 g), superoxide radical scavenging capacity (53.69 mg/100 g), and hydroxyl radical scavenging capacity (51.91 mg/100 g). A linear relationship was found between total phenolic acid contents and antioxidant capacity. Both phenolic compounds and antioxidant capacities varied significantly (ρ < 0.05) among cultivars. DCP cultivar was found to be a rich source of phenolics and ascorbic acid with high antioxidant activity.
Introduction
Daily consumption of fruits and vegetables in diet offers several human health benefits. Fruits and vegetables have defensive action against several diseases which might be attributed to the presence of compounds especially flavonoids, anthocyanin, flavones, isoflavone, catechin, isocatechin, vitamin E, ascorbic acid, and β-carotene owing the potential as antioxidants.[Citation1]
Phenolics have a remarkable range of biological and pharmacological properties such as anti-inflammatory antioxidant, anti-proliferative, antiviral, and anti-allergic effects.[Citation2] Chemical composition is one of the utmost significant quality criteria for fruit products regarding their nutritional and health attributes. Fruits contain appreciable amounts of phenolic antioxidants, a large heterogeneous group of biologically active non-nutrient compounds recognized for their therapeutic properties. These therapeutic properties of fruits and vegetables are due to the presence of effective antioxidant and free-radical quenching possessions of phenolic compounds.[Citation3]
Currently, the phenolic constituents are used in food and therapeutic medication due to their antioxidant activity and other health endorsing effects.[Citation4] Increasing demand of natural antioxidants due to their health reimbursement criterion is attracting the scientific focus to search more and more plants as harmless phenolic compounds. Recognizing the nutritional importance of natural antioxidant and other bioactive compounds present in Dacus carota and significant demand of consumers, enormous private seed companies and research institutes have started refinement to grow high-yielding bright-shaded cultivars particularly for red and black D. carota. Many times local genotypes of D. carota are crossed with genotypes of western origin to develop cultivars which have adapted to Indian climatic conditions in healthier way.[Citation5] As a result, now the Indian market has high-yielding cultivars of D. carota with different colors. However, scanty evidence is available on level of antioxidant potentiality and antioxidant compounds of Indian cultivars.[Citation6]
Recently, Ayub Agriculture Research Institute (AARI), Faisalabad, Pakistan, has grown some new lines of D. carota cultivars as an effort to provide the healthy ingredients to the consumers in the form of vegetable. In the present study, various phenolic compounds and antioxidant activity of newly developed commercial cultivars of D. carota were investigated. Detailed information regarding the phenolic profile and antioxidant properties of these cultivars with desired genetic architecture will serve as a reference material to develop more D. carota cultivars rich in medicinally important ingredients in addition to their other nutritional attributes. Generation of such information will benefit both breeders as well as general and health-conscious consumers.
Materials and methods
Collection and preparation sample preparation
Root part of seven approved cultivars of D. carota, DCW, DCY, DCP, T29, DCR, DC3, and DC90, were collected from fields of vegetable section of AARI in the month of January 2015. The cultivation was done in winter season (October 2014 to January 2015). The maximum and minimum temperature (°C) for the months of October 2014, November 2014, December 2014, and January 2015 were 35.7 ± 3.2 and 20.2 ± 3.0, 25.5 ± 1.9 and 14.2 ± 2.4, 21.4 ± 2.3 and 8.3 ± 1.1, and 19.3 ± 1.5 and 8.0 ± 1.2 (°C), respectively. The average relative humidity (%) and total rain fall (mm) of the months of October 2014, November 2014, December 2014, and January 2015 were 48.0 ± 9.5 and 1.26, 68.0 ± 14.4 and 2.74, 79.0 ± 18.5.0 and 8.10, and 67.0 ± 13.6 and 0.01, respectively. The samples were further authenticated and verified by Dr. Qasim Ali, assistant professor of botany, Government College University Faisalabad. All the samples were wrapped in polythene bags and stored at −10°C.
Preparation of extracts
Extractions were carried out following the previously developed method[Citation7] with a slight modification. A sample of 25 g of each cultivar was homogenized with 50 mL of 75% ethanol in a juice blender at 4°C separately. Then, the homogenized samples were carefully transferred to 100 mL volumetric flask (each) and 75% ethanol was added to make up the volume. Each sample was further homogenized using orbital shaker (KJ-201BD, Jiangsu Kangjian Medical Apparatus Co., Ltd., China) for 20 min at 200 rpm and filtered with muslin cloth. The clear filtrate of each sample was analyzed in three replicates for different assays within 2 days.
Sample preparation for HPLC analysis
Phenolic compounds were extracted and purified according to the methods of Mayen[Citation8] with slight modification. Homogenization of 100 g grated D. carota was done in 100 mL of cold ethanol (80%) with 0.5% sodium metabisulfite and leaving it for 30 min and repeating this experiment three times alternately. Filtration of this homogenized material was done by strata of cheesecloth. Three consecutive extractions were carried out with 50 mL of same solvent. The filtered solution was centrifuged at 7000 rpm for 15 min. Ethanol was evaporated at 35–40°C in vacuum, and pigments were removed by using petroleum (2:1, v/v). Then ammonium sulfate (20%) and metaphosphoric acid (2%) were added to the aqueous phase, and then for the extraction of phenolics, ethyl acetate (1:1, v/v) was added. Ethyl acetate evaporated to dryness under vacuum at 35–40°C. Finally, 10 mL of methanol was added to the residue and homogenized to clear solution and stored at −20°C prior to high-performance liquid chromatographic (HPLC) analysis.
Exploration of phenolic compounds by HPLC
The exploration of phenolic compounds was done by HPLC equipped with pump (L-6200A), diode array detector L-4500 (Hitachi Ltd., Tokyo, Japan), and computer Elonex pc 466/I (Elonex, Aspley method, London, UK). After filtration through 0.45 µm filter, a 20 µL sample was once injected. Column (Alltima C18 250 mm, 4.6 mm I.D., Alltech Acquaintances Utilized Science, Ltd., Carnforth, UK) was used for gradient separation of the phenolic compounds. Mobile phase consisted of solvent A (2% acetic acid) and solvent B methanol/acetonitrile (15:10, v/v). Flow rate of 1.0 mL/min was attained with the help of pump. The best separation was completed by the use of the following gradient elution: at 0.0 min, A – 90% and B – 10%; at 10.0 min, A – 80% and B – 20%; at 15.0 min, A – 70% and B – 30%; at 25.0 min, A – 60% and B – 40%; at 30.0 min, A – 50% and B – 50%; and at 40.0 min, A – 50% and B – 50%. The diode array detector was used at wavelength of 330 nm for separation and monitoring of phenolic compounds.[Citation9]
Total phenolic (TP)
Amount of TP was measured using Folin–Ciocalteu reagent procedure as described by Singleton.[Citation10] D. carota dried extract (50 mg) was mixed with 7.5 mL deionized water and 0.5 mL of Folin–Ciocalteu reagent. The mixture was preserved at ambient temperature for 10 min, and then 1.5 mL of 20% sodium carbonate (w/v) was added. The mixture was heated at 40°C on water bath for 20 min and then was transferred to ice bath for cooling. The absorbance was measured by spectrophotometer at 755 nm. With the help of calibration curve of gallic acid (R2 = 0.9986), amount of TP was calculated. The results were described as gallic acid in milligrams per 100 g of fresh weight. All samples were analyzed individually in triplicate, and results were averaged and the calculation was done on fresh weight basis.
Total ascorbic acid (TAA)
Indophenols method was used for the determination of ascorbic acid contents, in accordance with Nielsen,[Citation11] and the results were described as milligram of ascorbic acid per 100 g of fresh weight. From each cultivar, 100 g of the sample was accurately weighed separately and extraction of juice was done by juice extractor, and 20 mL of metaphosphoric acid–acetic acid was added to this mixture and made the volume up to 100 mL. Then, 2.0 mL of the samples extract was pipette out into three of the 50 mL Erlenmeyer flask followed by 5.0 mL of the metaphosphoric acid–acetic acid solution. The titration of samples was done by indophenol dye solution until a light rose-pink color persisted for 5 s. The ascorbic acid contents were measured from amount of dye used in the titration.
Total antioxidant activity
The evaluation of total antioxidant activity was governed by coupled oxidation of β-carotene and linoleic acid as described by Taga et al.[Citation12] with slight modification. β-carotene (10.0 mg) was dissolved in 50.0 mL of chloroform. The sample solution (3.0 mL) was pipette out into a conical flask containing 400 μL Tween 20 and 40 μL linoleic acid. Removal of chloroform was done with the help of rotary evaporator under vacuum at 35°C. Oxygenated distilled water (100 mL) was added to the β-carotene emulsion and mixed well. Then, 0.2 mL of the diluted extract and 3.0 mL of aliquot of the β-carotene emulsion were poured in a test tube and mixed well. The test tubes were immediately incubated at 50°C in a controlled water bath. Spectrophotometer was used for monitoring the oxidation of β-carotene emulsion by assessing absorbance at 470 nm. Absorbance of samples was measured at 10, 20, 30, 40, 50, and 60 min. A control sample was prepared by adding 0.2 mL distilled water instead of the extract. First-order kinetics was used for the calculation of degradation rate of the extracts.
where t is time (min), a is initial absorbance at time 0, b is absorbance at 10, 20, 30, 40, 50, and 60 min, and ln is natural log. The extent antioxidant activity (AA) was measured as inhibition (%) relative to the control by using following equation. The results were reported as milligrams per 100 g of FW after calculations.
Superoxide radical scavenging activity
Superoxide radical scavenging activity of cultivars of D. carota was determined using the method described by Nishimiki[Citation13] with slight modifications, briefly, 1.0mL of nitro-blue-tetrazolium (NBT) solution (phosphate buffer pH~7.4, 156 μM NBT/100 mM) was mixed with 1.0 mL of 468 μM NADH solution in 100 mM phosphate buffer, pH 7.4, then 1.0 mL of sample solution of carrot extract (30-50 μg/mL) in ethanol was added and mixed thoroughly. 100 μl of phenazine-methosulphate (PMS) solution was added to the above mixture to start the reaction. After that reaction mixture was incubated at 25°C for five minutes and the absorbance was measured at 560 nm against blank sample. Decreased in absorbance indicated increased superoxide anion scavenging activity.
Hydroxyl radical scavenging capacity
Metal-chelating activity of antioxidants in the conditions of Fenton-like reactions using a Co (II) complex and henceforth the shielding aptitude against the formation of hydroxyl radical was dignified by hydroxyl radical averting capacity (HORAC) assay established previously.[Citation14] Briefly, 0.55 M solution of H2O2 was prepared in distilled water, and 15.7 mg of CoF2·4H2O was dissolved for the preparation of 4.6 mM Co (II), and picolinic acid (20 mg) was added in 20 mL of distilled water (60 nM, final concentration). Sample (10 µL) was placed directly in the plate reader and incubated at 37°C for 10 min. After the completion of incubation period, 10 µL of Co (II) and 10 µL of H2O2 (27.5 mM, final concentration) solutions were added consequently. After shaking the prepared samples, fluorescence was measured after every minute. Phosphate buffer solution was used as blank sample. Standard antioxidant solutions (100, 200, 600, 800, and 1000 µM) in phosphate buffer (75 mM, pH 7.4) were used for construction of standard curve. Regression equation was used for the calculation of final HORAC values between the area under the curve and antioxidant standards. One HORAC unit was consigned to the net fortification area providing 1.0 µM antioxidant standard, and the sample activity was expressed as milligrams per 100 g of FW.
DPPH radical scavenging assay
For the determination of free radical scavenging activities of D. carota extracts, a previously developed procedure[Citation15] was used with slight modification. Freshly prepared solution of 2,2-diphenyl-1-picrylhydrazyl (DPPH) was added to 1.0 mL of D. carota extract containing 25 µg/mL of dry matter in methanol. Absorbance of the prepared samples was measured by spectrophotometer at 0, 0.5, 1, 2, 5, and 10 min by setting wavelength at 515 nm. The calibration curve was used to measure the remaining amounts of DPPH radical. Comparison of radical scavenging activity of D. carota extracts was done by using absorbance taken at fifth minute. The results were reported as DPPH scavenging capacity percent.
Results and discussion
Phenolic composition
Phenlolics are present in vegetable foods as non-essential dietary components, commonly known as secondary plant metabolites. These are anti-nutritive due to the adverse effect on protein digestibility. On the other hand, they are too much important due to their inhibition against atherosclerosis and cancer.[Citation16] HPLC chromatogram of the standard compounds is given in . (A–G) shows the typical chromatograms of phenolic compounds extracted from selected cultivars of D. carota. All the cultivars contained hydroxycinnamicacid derivatives namely 3-caffeoylquinic acid, cis-3-caffeoylquinic acid, 5-caffeoylquinic acid, caffeic acid, 3-p-coumaroylquinic acid, 3-feruloyquinic acid, 3,4-dicaffeoylquinic acid, 5-feruloyquinic acid, cis-5-caffeoylquinic acid, 5-p-coumaroylquinic acid, 4-feruloyquinic acid, 3,5-dicaffeoylquinic acid, 3,4-diferuloylquinic acerbic, and 3,5-diferuloylquinic acid. These derivatives were identified and quantified by comparing retention times and peak areas with standards.
Figure 1. HPLC chromatograph mixture of standard phenolic compounds. Peak identification: (1) 3-caffeoylyquinic acid, (2) cis-3-caffeoylquinic acid, (3) 5-caffeoylquinic acid, (4) caffeic acid, (5) 3-p-coumaroylquinic acid, (6) 3-feruloyquinic acid, (7) 3,4-dicaffeoylquinic acid, (8) 5-feruloquinic acid, (9) cis-5-caffeoylquinic acid, (10) 5-p-coumaroylquinic acid, (11) 4-feruloyquinic acid, (12) 3,5-dicaffeoylquinic acid, (13) 3,4-diferuloylquinic acid, and (14) 3,5-diferuloylquinic acid.
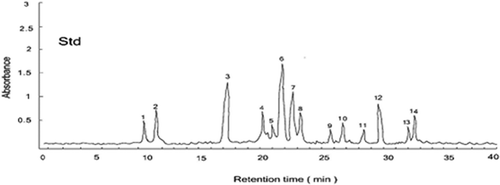
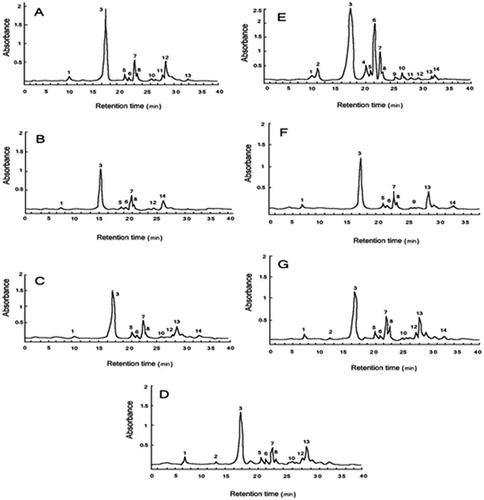
Figure 2. HPLC chromatograms of phenolic compounds of seven cultivars of Daucus carota. Chromatogram identification: (A) T29, (B) DCY, (C) DCR, (D) DC90, (E) DCP, (F) DCW, and (G) DC3. Peak identification: (1) 3-caffeoylquinic acid, (2) cis-3-caffeoylquinic acid, (3) 5-caffeoylquinic acid, (4) cafeic acid, (5) 3-p-coumaroylquinic acid, (6) 3-feruloyquinic acid, (7) 3,4-dicaffeoylquinic acid, (8) 5-feruloyquinic acid, (9) cis-5-caffeoylquinic acid, (10) 5-p-coumaroylquinic acid, (11) 4-feruloyquinic acid, (12) 3,5-dicaffeoylquinic acid, (13) 3,4-diferuloyquinic acid, and (14) 3,5-diferuloyquinic acid.
revealed the quantitative values of total detectable and individual phenolic compounds. 5-caffeoylquinic acid was the major hydroxylcinnamic acid present in all the cultivars, ranging from 6.98 to 33.23 mg/100 g of total phenolic compounds. The total amount of phenolic compounds in DCP cultivar was 54.62 mg/100 g FW, whereas the corresponding values in other cultivars ranged from 12.8 to 20.29 mg/100 g FW.
Table 1. Phenolic acids profile in different cultivars of D. carota.
Higher contents (ρ < 0.05) of 3-caffeoylquinic acid, cis-3-caffeoylquinic acid, 5-caffeoylquinic acid, caffeic acid, 3-p-coumaroylquinic acid, 3-feruloyquinic acid, 5-feruloyquinic acid, cis-5-caffeoylquinic acid, 5-p-coumaroylquinic acid, 4-feruloyquinic acid, and 3,5- diferuloylquinic acid were the major phenolic compounds identified in DCP cultivar. The compounds 3,4-dicaffeoylquinic acid, 3,5-dicaffeoylquinic acid, and 3,4- diferuloylquinic acid were the major compounds present in DC3, T29, and DCW cultivars. DCP cultivar was the only one which contained caffeic acid (1.93 mg/100 g FW) among all the selected cultivars, which is comparable to the result (2.42 mg/100 g FW) reported by Alasalvar et al.[Citation9] in different colored carrots.
In the present study, some phenolic compounds were not detected like isocoumarins 6- hydroxymellein and 6-methoxymellein as described by Harding and Heale,[Citation17] and para hydroxyl benzoic acid as described by Babic et al.[18] In total, 14 types of phenolic acids were detected in the present work, but Alasalvar et al.[Citation9] reported that 9–11 hydroxycinnamic derivatives were present in different colored carrots. The difference in phenolic acid composition may differ between cultivars, as well as among parts within the individual plant.[Citation19]
revealed that the Folin–Ciocalteu assay was performed for the determination of total phenolics (TP) of the extracts of different cultivars. In the DCP cultivar, TP contents were 65.39 mg/100 g FW, which is higher than other cultivars. Kahkonen et al.[Citation20] investigated the gallic acid equivalent phenolic compounds in D. carota bark and dermis, which comprised of 6.6 and 0.6 mg/g of dry weight. Vinsonn et al.[Citation21] investigated that 46.4 mg catechin equivalent/100 g of fresh weight was present in D. carota. The diversity of results in the studies may be due to altered extraction procedures and the ways to express the results. The concentrations of phenolic compounds in different cultivars detected by the Folin–Ciocalteu and HPLC methods declined in the similar order. Nevertheless, greater levels of total phenolics were determined by Folin–Ciocalteu assay as compared with HPLC in D. carota cultivars. Howard et al.[Citation22] reported the same variance among two approaches used for analyses. This may be possible owing to the excessive revealing of insignificant phenolics or superfluous reducing compounds persistent in the extracts. Qualities of D. carota made it popular in fast-food servings and prepared salads. We endorsed that the greater level of phenolic compounds present in the DCP cultivar could be used in the production of phenolic compounds supplemented diets in the food-processing industry.
Table 2. Total ascorbic acid and total phenolics of selected cultivars of D. carota.
Total ascorbic acid (TAA)
Ascorbic acid is effective against heart diseases, high blood pressure, and endothelial dysfunction. Ascorbic acid in the vegetal fleshy tissue is responsible for dynamic growth, and the total extent of ascorbic acid varies among species and cultivars.[Citation23] also showed the TAA of selected cultivars of D. carota. TAA levels ranged from 41.12 to 58.36 mg/100 g FW (ρ < 0.05) which were comparable to the result (54.5 mg/100 g FW) reported by Venkatachalam et al.[Citation24] in red carrots. The difference of TAA values in literature may be due to the difference in genotype or methods of determination. As compared with other cultivars, DCP and DC3 showed higher levels of TAA, whereas DCW and DCY had the lower levels of ascorbic acid content. The production of ascorbic acid depends upon the light intensity and fluctuation in growth temperature.[Citation25]
Total antioxidant capacity (TAC)
revealed that TAC of the selected cultivars of D. carota ranged from 41.56 to 77.69 mg/100 g FW (ρ < 0.05). DCP cultivar showed the higher levels of TAC with 77.69 mg/100 g FW. This result is not in agreement with Venkatachalam et al.,[Citation24] who reported TAC in red carrots only, but describing TAC as 47.65 ± 1.20 mg/100 g is comparable to DCR, T29, and DC90 cultivars described in the present study. also revealed that with the increase of phenolic compounds and ascorbic acid contents, the value of TAC gone to higher side. The polyphenols contributed for the supreme multi-functionality of the natural antioxidants.[Citation26] Antioxidant capacity of D. carota was also reviewed in various studies. The difference of TAC values in these studies may be due to the difference in genotype or methods used for the determination of TAC.
Table 3. Antioxidant activities of the extracts from different D. carota cultivars.
Free radical scavenging capacities
showed that the selected cultivars contained significant levels of DPPH scavenging activity and ranged from 27.55% to 52.36%. This was not in agreement with previously published results of Zhang and Hamauzu.[Citation27] They found higher value of DPPH scavenging capacity in carrot peel (75.8%) than xylem and phloem. The difference in results in the present study is because of the use of whole root of D. carota in sample preparation. The order of decreasing level of DPPH scavenging activity of cultivars in the present study was DCP > DC3 > T29 > DC90 > DCR > DCW > DCY. Due the presence of dissimilar phenolic compounds in the respective extracts, the results are in diverse response to the testing technique.
shows that the superoxide radical scavenging activity in the selected cultivars of D. carota ranged from 26.55 to 53.69 mg/100 g FW. Among DCW, DCY, DCP, T29, DCR, DC3, and DC90 cultivars, the DCP showed the higher superoxide radical scavenging activity with 53.69 mg/100 g FW (ρ < 0.05). Lu et al.[Citation28] and Maheswari et al.[Citation29] reported that mostly polyphenols contributed to antioxidant activities of various fruits and vegetables. In the present research, the higher value of superoxide radical scavenging activity in DC-purple cultivar is due to the presence of high concentration of phenolics and its derivatives.
shows that the hydroxyl radical scavenging activity of the selected cultivars of D. carota ranged from 28.12 to 51.91 mg/100 g FW (ρ < 0.05). The trend was same as that of DPPH scavenging capacity, that is, DCP > DC3 > T29 > DC90 > DCR > DCW > DCY. However, the antioxidant activity of selected fruits and vegetables in this study () was related with their phenolic contents (). This was in agreement with previously published results reported by Alasalvar et al.[Citation9]
Liaison between phenolic content and antioxidant properties in D. carota
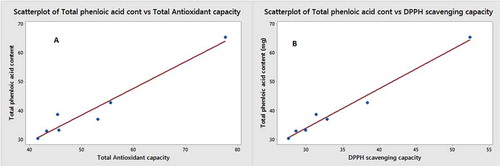
shows that antioxidant activity and radical scavenging activity correlated well with total phenolic contents in D. carota. These results were consistent with the results reported by Lee et al. in which a rectilinear relationship between phenolic concentration and antioxidant activity in capsicum was prominent. Various researches could not invent considerable correspondence among the total phenolic content and antioxidant activity of the vegetal extracts. This might be because fruits and vegetables contain numerous different antioxidant constituents including carotenes, victual-vitamins, phenolic compounds, and bioflavonoids.[Citation27] This could affect the measurements of antioxidant action. Afterward, carotenes and vitamin C in D. carota could affect the measurement of antioxidant activity of phenolics.
Conclusion
Characterization, identification, and quantification of phenolic compounds isolated from cultivars of D. carota were done prior to antioxidant activity. The hydroxycinnamic acids and its derivatives were found in significant extent. Concentration of phenolic compounds differed from cultivar to cultivar, while a decrease in the antioxidant and radical scavenging activities was observed in same order as the phenolic content decreased, and a linear correlation was observed in total phenolic contents and activities. Therefore, the present research concludes that the antioxidant properties of D. carota depend upon the amount of phenolic compounds in the respective extracts. DCP cultivar could be considered for value-added utilization in the processing industry due to the presence of higher level of phenolic acids and antioxidant properties as compared with other cultivars.
Acknowledgments
We extend our gratitude to Dr. Muhammad Ikram, vegetable section of Ayub Agricultural Research Institute (AARI), Faisalabad, Pakistan, for providing us the samples of available D. carota cultivars. This work is a part of Ph.D. project. This article does not contain any studies with human participants or animals performed by any of the authors.
References
- Liu, R.H. Health Benefits of Fruit and Vegetables are from Additive and Synergistic Combinations of Phytochemicals. The American Journal of Clinical Nutrition 2003, 78(3), 517S–520S.
- Strack, F.; Mussweiler, T. Explaining the Enigmatic Anchoring Effect: Mechanisms of Selective Accessibility. Journal of Personality and Social Psychology 1997, 73(3), 437.
- Datta, S.; Karsch, F.; Petreczky, P.; Wetzorke, I. Behavior of Charmonium Systems after Deconfinement. Physical Review D 2004, 69(9), 094507.
- Craig, W.J. Health-promoting Properties of Common Herbs. The American Journal of Clinical Nutrition 1999, 70(3), 491s–499s.
- Koley, T.K.; Singh, S.; Khemariya, P.; Sarkar, A.; Kaur, C.; Chaurasia, S.N.S.; Naik, P.S. Evaluation of Bioactive Properties of Indian Carrot (D. carota L.): A Chemometric Approach. Food Research International 2014, 60, 76–85.
- Tarwadi, K.; Agte, V. Antioxidant and Micronutrient Potential of Common Fruits Available in the Indian Subcontinent. International Journal of Food Sciences and Nutrition 2007, 58(5), 341–349.
- Lim, Y.Y.; Lim, T.T.; Tee, J.J. Antioxidant Properties of Several Tropical Fruits: A Comparative Study. Food Chemistry 2007, 103(3), 1003–1008.
- Mayen, M.; Barón, R.; Mérida, J.; Medina, M. Changes in Phenolic Compounds During Accelerated Browning in White Wines from cv. Pedro Ximenez and cv. Baladi Grapes. Food Chemistry 1997, 58(1), 89–95.
- Alasalvar, C.; Grigor, J.M.; Zhang, D.; Quantick, P.C.; Shahidi, F. Comparison of Volatiles, Phenolics, Sugars, Antioxidant Vitamins, and Sensory Quality of Different Colored Carrotvarieties. Journal of Agricultural and Food Chemistry 2001, 49(3), 1410–1416.
- Singleton, V.L.; Orthofer, R.; Lamuela-Ranventos, R.M. Analysis of Total Phenols Other Oxidation Substrates and Antioxidants by Means of Folin–Ciocalteureagent. Methods in Enzymology 1999, 299, 152–178.
- Nielsen, S. Food Analysis; Aspen Publishers. Inc.: Gaithersburg, Maryland United State Department of Agriculture Nutrient Base, Aspen Publishers, Inc., 1998, 2004.
- Taga, M.S.; Miller, E.E.; Pratt, D.E. Chia Seeds as a Source of Natural Lipid Antioxidants. Journal of the American Oil Chemists’ Society 1984, 61(5), 928–931.
- Liu, F.; Ooi, V.E.C.; Chang, S.T. Free radical scavenging activities of mushroom polysaccharide extracts. Life sciences 1997, 60(10), 763–771.
- Ou, B.X.; Huang, D.J.; Hampsch-Woodill, M.; Flanagan, J.A.; Deemer, E.K. Analysis of Antioxidant Activities of Common Vegetables Employing Oxygen Radical Absorbance Capacity (ORAC) and Ferric Reducing Antioxidant Power (FRAP) Assays: A Comparative Study. Journal of Agriculture and Food Chemistry 2002, 50(11), 3122–3128.
- Iqbal, S.; Bhanger, M.I.; Anwar, F. Antioxidant Properties and Components of Some Commercially Available Varieties of Rice Bran in Pakistan. Food Chemistry 2005, 93, 265–272.
- Martínez-Valverde, I.; Periago, M.J.; Ros, G. Nutritional Importance of Phenolic Compounds in the Diet. Archivoslatinoamericanos de nutricion, 2000, 50(1), 5–18.
- Harding, V.K.; Heale, J.B. Isolation and Identification of the Antifungal Compounds Accumulating in the Induced Resistance Response of Carrot Root Slices to Botrytis cinerea. Physiological Plant Pathology, 1980, 17(3), 277–289.
- Babic, I.; Amiot, M.J.; Nguyen-The C.; Aubert S. Changes in Phenolic Content in Fresh Ready-To-Use Shredded Carrots During Storage. Journal of Food Science 1993, 58(2), 351–356.
- Francisco, M.; Velasco, P.; Moreno, D.A.; Garcia-Viguera, C.; Cartea, M.E. Cooking Methods of Brassuca Rapa Affect the Preservation of Glucosinolates, Phenolics and Vitamin C. Food Research International 2010, 43, 1455–1463.
- Kahkonen, M.P.; Hopia, A.I.; Vuorela, H.J.; Rauha, J.P.; Pihlaja, K.; Kujala, T.S.; Heinonen, M. Antioxidant Activity of Plant Extracts Containing Phenolic Compounds. Journal of Agricultural and Food Chemistry 1999, 47(10), 3954–3962.
- Vinsonn, J. A.; Hao, Y.; Su, X.; Zubik, L. Phenol antioxidant quantity and quality in foods: vegetables. Journal of Agricultural and Food Chemistry, 1998; 46(9), 3630–3634.
- Howard, L.R.; Talcott, S.T.; Brenes, C.H.; Villalon, B. Changes in Phytochemical and Antioxidant Activity of Selected Pepper Cultivars (Capsicum Species) as Influenced by maturity. Journal of Agricultural and Food Chemistry 2000, 48(5), 1713–1720.
- Lee, S.K.; Kader, A.A. Preharvest and Postharvest Factors Influencing Vitamin C Content of Horticultural Crops. Postharvest Biology and Technology 2000, 20(3), 207–220.
- Venkatachalam, K.; Rangasamy, R.; Krishnan, V. Total Antioxidant Activity and Radical Scavenging Capacity of Selected Fruits and Vegetables from South India. International Food Research Journal, 2014; 21(3).
- Harris, R.S. Effects of Agricultural Practices on the Composition of Foods. Nutritional Evaluation of Food Processing 1975, 2, 33–57.
- Slusarczyk, S.; Hajnos, M.; Skalicka-Woźniak, K.; Matkowski, A. Antioxidant Activity of Polyphenols from Lycopuslucidus Turcz. Food Chemistry, 2009, 113(1), 134–138.
- Zhang, D.; Hamauzu, Y. Phenolic Compounds and Their Antioxidant Properties in Different Tissues of Carrots (D. carota L.). Journal of Food Agriculture and Environment 2004, 2, 95–100.
- Lu, X.; Wang, J.; Al-Qadiri, H.M.; Ross, C.F.; Powers, J.R.; Tang, J.; Rasco, B.A.Determination of Total Phenolic Content and Antioxidant Capacity of Onion (Allium cepa) and Shallot (Allium oschaninii) Using Infrared Spectroscopy. Food Chemistry 2011, 129(2), 637–644.
- Maheswari, S.U.; Mohankumar, J.B.; Uthira, L. Comparitive Study On Antioxidant Activity of Organic and Conventionally Grown Roots and Tubers Vegetables of India. Electronic Journal of Environmental, Agricultural and Food Chemistry 2012, 11(2), 136–147.