ABSTRACT
In the present study, phenolic content and antioxidant capacities including cellular antioxidant activity of eight Chinese sweet corn varieties were investigated. The total phenolic content revealed significant varietal differences and phenolics existed mainly in free form. HPLC analysis showed that ferulic and p-coumaric acids were the dominating phenolics in sweet corn. The DPPH scavenging capacity, ferric reducing antioxidant power, and cellular antioxidant activity demonstrated distinct varietal differences, which were positively correlated with their phenolic content, although the orders of antioxidant activity of eight varieties determined by three different methods were not consistent. Sweet corn could be a better substitute for many commonly consumed vegetables in terms of phenolics and antioxidant activity.
Introduction
Sweet corns (Zea mays saccharata Sturt.) are special varieties of maize with high sugar content due to altered endosperm starch synthesis. According to the level of sugar in the corn kernel, four groups of sweet corn have been developed for commercial production including standard, supersweet, sugary enhanced, and high sugar sweet corn.[Citation1] Supersweet corn is the most commonly planted variety due to its higher sugar content and relatively longer shelf life. Sweet corn is mainly planted for fresh consumption or canned production as a vegetable rather than a grain. Nowadays, it is one of the most important vegetables on the dining table in America, China, and many other countries by virtue of its high nutritional value and good taste. Recently, sweet corn has become increasingly more popular in China and it was reported that the planting area of sweet corn in China accounted for over 20% of global planting area in 2010. Furthermore, South China is the main production area because of the warm climate. The planting area of sweet corn in the Guangdong province accounts for over 50% of the whole country. Despite the relatively late initiation of sweet corn breeding program, many new varieties with good eating quality have been developed now in China. Breeders have been devoted mainly to the improvement of yield, sweetness, and other flavor characteristics. Whereas, less attention has been paid to the nutritional quality and health benefits of sweet corn compared to other vegetables.
The epidemiological evidence has shown that increased consumption of fresh vegetables is associated with reduced risk of many chronic disorders such as diabetes, cancers, and cardiovascular diseases.[Citation2–Citation4] A number of studies indicate that oxidative stress arising from oxidant/antioxidant imbalance plays a paramount role in the initiation and progress of these chronic diseases. The health benefits of fresh vegetables are mainly attributed to the high abundance of non-nutrient phytochemicals, especially phenolics, and their potent antioxidant activity. Phenolic compounds contained in vegetables can scavenge excessive free radicals produced in the human body by donating hydrogen, quenching singlet oxygen or chelating metal ions, etc. Furthermore, phenolics can also improve the redox status indirectly by increasing the expression and activity of antioxidant enzymes. Many of the available methods for evaluation of antioxidant activity including DPPH radical scavenging capacity and ferric reducing antioxidant power are performed in a chemical environment. These chemical-based methods may mislead us (at least to some degree) in predicting the in vivo antioxidant activity due to the nonphysiological reaction system and failure to take bioavailability of antioxidants into consideration. Cellular antioxidant activity (CAA) assay, however, is a cell-based method that can mimic the complex biological system to some extent.[Citation5] Therefore, the CAA assay has been widely used for screening the antioxidant activity of varieties of samples.[Citation6,Citation7]
It has been found that phenolic compounds in plants occur in free and bound forms. A large portion of phenolics present in grains were proved to exist in bound form, while this phenomenon is quite opposite in fruits and vegetables.[Citation8] The phenolic profiles of common corn have been reported previously. Adom et al. found that corn had much higher total phenolic and flavonoid content than other whole grains including rice, wheat, and oats.[Citation9] Several phenolic compounds were identified and quantified from corn including ferulic, vanillic, protocatechuic, and syringic acids.[Citation9,Citation10] Although the information regarding the phenolic profile of common corn can provide a clue to the phenolics of sweet corn, there might be difference between the two corns considering their different genotypes and harvest time. As an increasingly important component of a healthy diet, sweet corn is believed to provide high nutrition value besides a taste of sensory enjoyment. Previous studies determined the content of main nutrients in sweet corn,[Citation11,Citation12] yet little is known about the non-nutrient phytochemicals in sweet corn. To our knowledge, this topic was only involved in few studies. Liu’s research group analyzed the changes in phenolic content of sweet corn during thermal processing.[Citation13] They also reported the free phenolic content and CAA activity of sweet corn as one of 27 determined vegetables commonly consumed in America.[Citation14] Furthermore, Das et al. compared the phytochemical content and antioxidant activity in the skin, germ, and endosperm fractions of four Indian maize varieties including quality protein maize (QPM), baby corn, popcorn, and sweet corn.[Citation15] However, the differences in phenolic content and antioxidant activity among different sweet corn varieties, especially those in the bound phenolic fractions are still unknown.
Therefore, the phenolic content and antioxidant activity, including the CAA activity of eight representative sweet corn varieties commercially planted in South China, were investigated in the present study. The purpose of our research is to determine the content of free and bound phenolics and their predominant phenolic compounds and to compare the antioxidant activity of different sweet corn varieties determined by various methods including the CAA assay.
Materials and methods
Chemicals and reagents
6-Hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox), 2,2-diphenyl-1-picrylhydrazyl (DPPH·), 2,4,6-tripyridyl-s-triazine (TPTZ), 2′,7′-dichlorofluorescin diacetate (DCFH-DA), gallic acid, quercetin dehydrate, (+)-catechin, ferulic acid, p-coumaric acid, fluorescein disodium salt, 2,2′-azobis (2-amidinopropane) dihydrochloride (AAPH), and Folin–Ciocalteu’s phenol reagent were all purchased from Sigma-Aldrich Inc. (St. Louis, MO, USA). Dulbecco’s modified eagle’s medium (DMEM) and fetal bovine serum were from HyClone (Logan, UT, USA). Hank’s Buffered Salt Solution (HBSS), HPLC-grade acetic acid, and acetonitrile were purchased from Fisher Scientific (Waltham, MA, USA). Human hepatoma cell line HepG2 was obtained from the American Type Culture Collection (Rockville, MD, USA).
Sweet corn samples and sample preparation
Eight sweet corn cultivars (Fotian 2, Huabaotian 8, Zhentian 1, Zhutian 3, Jinyinsu 2, Xiantian 5Suiyoutian 1and Zhongtian 2), known as supersweet varieties, were kindly provided by Crop Research Institute of Guangdong Academy of Agricultural Sciences, China. The sweet corns were grown in the Experimental Station of Guangdong Academy of Agricultural Sciences from mid March to early June. Each variety was harvested according to their appropriate time of harvest mainly at the 18th–22nd day after silk emergence. The kernels were removed immediately from the cobs after harvest and stored at –80°C until analysis. All the experiments were repeated three times and data were reported as means ± standard deviation (SD).
Extraction of free phenolics
Free phenolic compounds were extracted according to the modified method reported previously.[Citation16] Briefly, 50 g of corn kernels were homogenized with 80% chilled acetone (1:2, w/v) in a Philips blender for 5 min. The homogenate was then homogenized further using an XHF-D homogenizer (Ningbo Xin-zhi-Bio-Technology Co., Ltd. Ningbo, China) at 5000 rpm for additional 3 min. The homogenate was centrifuged at 4500 rpm for 15 min at 4°C. The residue was extracted again under the same conditions. The supernatants were combined and concentrated under vacuum at 45°C until approximately 90% of the supernatant had been evaporated. The residues were saved for the extraction of bound phenolics. The concentrated supernatant was then recovered with distilled water to a final volume of 25 mL and then aliquoted and stored at –80°C until use.
Extraction of bound phenolics
Extraction of bound phenolics was performed according to a previous method with some modification.[Citation17,Citation18] Briefly, the residues of sweet corn from the free phenolic extraction were hydrolyzed with 25 mL of 4 M NaOH at room temperature for 1 h with continuous shaking under nitrogen gas. The mixture was acidified to pH 2 with 6 M HCl and extracted six times with ethyl acetate. The above ethyl acetate fractions were combined and evaporated at 45°C till dryness. The dried extracts were then redissolved in distilled water to a volume of 10 mL and stored at –80°C until analysis.
Determination of the total phenolic content
The modified Folin Ciocalteu (FC) colorimetric method described previously was applied to analyze the content of the total phenolics of samples.[Citation13] Briefly, a 125 μL of appropriately diluted phenolic extract was mixed with 0.5 mL of distilled water and subsequently 125 μL of the FC reagent. After 6-min reaction, 1.25 mL of 7% aqueous sodium carbonate solution and 1 mL of distilled water were added into the mixture in sequence. The final solution was mixed and then kept for 90 min in the dark to develop color. The absorbance was read at 760 nm using a Shimadzu UV-1800 spectrometer (Shimadzu Inc., Kyoto, Japan). Gallic acid was used as standard and the total phenolic content was expressed as milligrams of gallic acid equivalents (GAE) per 100 g of fresh weight (FW) of the sample.
Determination of the total flavonoid content
The total flavonoid content of the sweet corn kernel extracts was determined using a modified colorimetric method described earlier.[Citation19] A 250 μL of diluted extract was added into a tube containing 1.25 mL of distilled water. Seventy five microliters of 5% NaNO2 solution was subsequently added into the mixture. After 6-min reaction, 150 μL of 10% AlCl3·6H2O solution was added and allowed to stand for 5 min before the addition of 0.5 mL of 1 M NaOH solution. The final volume was adjusted to 2.5 mL with distilled water and the absorbance was read immediately at 510 nm using a Shimadzu UV-1800 spectrometer. Catechin was used as the standard compound, and the total flavonoid content was expressed as milligrams of (+)-catechin equivalents (CE) per 100 g FW of the sample.
Determination of ferulic acid and p-coumaric acid
All the samples were analyzed using an Agilent 1200HPLC system (Waldbronn, Baden-Württemberg, Germany) equipped with an Agilent 1200 series VWD detector and autosampler. An Agilent Zorbox SB-C18 column, 250 mm × 4.6 mm column (Palo Alto, CA, USA), was used for the separation of phenolic compounds. The column temperature was maintained at 30°C. The mobile phase was composed of 0.4% aqueous solution of acetic acid (solution A) and acetonitrile (solution B). Linear gradient elution was used as follows: 0–40 min, solution B 5–25%; 40–45 min, solution B 25–35%; and 45–50 min, solution B 35–50%. The flow rate was 1.0 mL/min and the injection volume was 20 μL. Detection was set at 280 nm. Prior to analysis, all of the samples were filtered through a 0.25 μm membrane filter (Millipore, Billerica, MA, USA). The identification and quantification of ferulic acid and p-coumaric acid was based on the retention time and UV-visible spectral data compared to known standards.[Citation19]
Antioxidant capacity determined by the DPPH assay
The DPPH radical scavenging capacities of sweet corn kernels were performed according to a previous report.[Citation20] Briefly, 200 μL of serially diluted sample or methanol (control) were added to 2.8 mL of 70 μM DPPH methanol solution. After vigorous shaking, the mixture was placed in the dark for 30 min at room temperature, and then absorbance value was determined using a Shimadzu UV-1800 spectrometer at 517 nm. The antioxidant activity was expressed as inhibition percentage (I%) and calculated using the following equation: , where Abscontrol represents the absorbance of control and Abssample represents the absorbance of sample. Fifty percent inhibitory concentration (IC50) of each sample represents the concentration of sample in which 50% DPPH was inhibited. IC50 was calculated by constructing curves of the percentage of DPPH inhibition versus log (extract concentration expressed as the weight of sweet corn kernels). The results were expressed as mg FW of sample/mL.
Antioxidant capacity determined by the FRAP assay
The FRAP assay was carried out according to a modified previous method.[Citation21] The working solution was prepared by mixing 25 mL of 300 mM acetate buffer (3.1 g of CH3COONa·3H2O and 16 mL of CH3COOH pH 3.6), 2.5 mL TPTZ solution (10 mM TPTZ in 40 mM HCl), and 2.5 mL of 20 mM FeCl3·6H2O solution. The mixture was incubated at 37°C before use. A 200 µL of sweet corn kernel extracts or distilled water (blank) were mixed with 2.8 mL of working solution. The mixture was allowed to stand for 30 min in dark at room temperature. Absorbance was measured at 593 nm using a Shimadzu UV-1800 spectrometer. Trolox was used as standard. The antioxidant activity by FRAP was expressed as milligrams of trolox equivalents (TE) per 100 g FW of sample.
Antioxidant activity determined by the CAA assay
The CAA assay was performed according to the procedure of Wolfe et al.[Citation5] Briefly, HepG2 cells were seeded into a black 96-well microplate (Corning) at a density of 6 × 104 cells in 100 μL of DMEM medium containing 10% fetal bovine serum per well. After 24-h culture, the medium was removed, and cells in each well were treated with different concentrations of quercetin or phenolic extracts with DCFH-DA (25 μmol/L) in DMEM for 1 h. Wells where cells treated with 25 μmol/L DMEM alone were set as blank or control wells. Then, 100 μL of 600 μmol/L AAPH dissolved in HBSS was added to each well except blank ones, where 100 μL of HBSS without AAPH was added. Fluorescence in each well was measured using an Infinite® M200 PRO plate reader with 485 nm excitation and 538 nm emission and recorded every 5 min at 37°C for 1 h. The CAA results are expressed as micromoles of quercetin equivalents (QE) per 100 g FW of sweet corn. Each treatment in the same plate was analyzed in triplicates and the CAA values of each sample were from three separate experiments.
Statistical analysis
Values were expressed as means ± SD for triplicate determinations of each sample. The differences among sweet corn varieties were analyzed using one-way ANOVA followed by SNK-q test. A two-tailed unpaired Student’s t-test was used to compare the differences in CAA qualities of the free and bound fractions of the same sweet corn variety. A value of p < 0.05 was considered statistically different. The correlation between variables was analyzed by the Pearson correlation test. All statistical analyses were performed by SPSS statistical package version 13.0 (SPSS Inc. Chicago, IL, USA).
Results
Total phenolic and flavonoid content
The free and bound phenolic and flavonoid content in kernels of 8 sweet corn varieties is presented in . The range of free phenolic content was from 30.35 (Suiyoutian 1) to 47.76 (Fotian) mg GAE/100 g, and the range of bound phenolic content was from 4.45 (Zhutian 3) to 14.47 (Jinyinsu 2) mg GAE/100 g. The percentage contribution of free phenolic content to the total ranged from 74.6% (Jinyinsu 2) to 88.3% (Zhutian 3). The total phenolic content of eight sweet corn varieties varied from 38.00 to 57.04 mg GAE/100 g. The coefficient of variation (CV) of the total phenolic content in sweet corn varieties was 15.9%. Jinyinsu 2 and Fotian 2 had the highest total phenolic content and Zhutian 3 and Suiyoutian 1 had the lowest total phenolic content. The free flavonoid content of eight sweet corn kernel samples ranged from 3.03 (Suiyoutian 1) to 5.08 (Fotian 2) mg CE/100g, and the bound flavonoid content ranged from 1.56 (Zhutian 3) to 2.60 (Jinyinsu 2) mg CE/100g. The percentage contribution of free flavonoids to the total was from 61.7 (Zhongtian 2) to 73.7% (Fotian 2). The total flavonoid content was from 4.89 (Suiyoutian 1) to 7.34 (Jinyinsu 2) mg CE/100g. The CV of the total flavonoid content of different sweet corn varieties was 15.2%.
Table 1. Content of phenolics and flavonoids in different sweet corn varieties and percentage contribution of free and bound fractions to the total (means ± SD, n = 3).
Ferulic acid and p-coumaric acid content of sweet corn
The content of free and bound ferulic acid and p-coumaric acid of sweet corn kernels and the percentage contribution of each fraction to the total in different varieties are presented in . The free ferulic acid content ranged from 270.22 (Huabaotian 8) to 814.53 (Jinyinsu 2) μg/100 g. The bound ferulic acid content ranged from 2357.44 (Zhutian 3) to 11492.27 (Suiyoutian 1) μg/100 g. Ferulic acid in sweet corn kernels occurred mainly in bound form. The CV of the total ferulic acid content in different sweet corn varieties was 42.8%, which indicated that significant genotypic differences existed in the tested sweet corn varieties. Jinyinsu 2 had the highest while Zhutian 3 had the lowest total ferulic acid content. The free and bound p-coumaric acid content ranged from 192.26 (Huabaotian 8) to 398.68 (Jinyinsu 2) μg/100 g, and from 143.58 (Zhutian 3) to 492.31 (Jinyinsu 2) μg/100 g, respectively. The percentage contribution of free p-coumaric acid to the total in the eight sweet corn varieties was from 37.4 (Huabaotian 8) to 71.7% (Zhutian 3). The total content of p-coumaric acid in sweet corn kernels ranged from 483.34 (Zhentian 1) to 890.99 (Jinyinsu 2) μg/100g. The CV of the total p-coumaric acid in different sweet corn varieties was 22.6%. The total content of p-coumaric acid in Jinyinsu 2 was higher than all the other seven sweet corn varieties.
Table 2. The content of ferulic acid and p-coumaric acid in different sweet corn varieties tested (μg/100g FW) (means ± SD, n = 3).
DPPH radical scavenging activity of sweet corn
The results of the DPPH radical scavenging activity of eight sweet corn varieties, expressed as the concentration of fresh sweet corn where 50% DPPH radicals is inhibited (IC50) are shown in . Both the extracts of free and bound fractions showed significant differences in DPPH scavenging activity among tested sweet corn varieties. The IC50 values of free fraction varied from 85.01 to 281.97 mg/mL. Fotian 2 had the lowest IC50 value of DPPH scavenging, indicating the highest antioxidant activity. The bound fraction of Zhutian 3 (314.61 mg/mL) and Jinyinsu 2 (117.48 mg/mL) showed the lowest and the highest DPPH scavenging activity, respectively.
Table 3. IC50 values of DPPH scavenging activity of different sweet corn varieties determined (mg FW of sample /mL) (means ± SD, n = 3).1
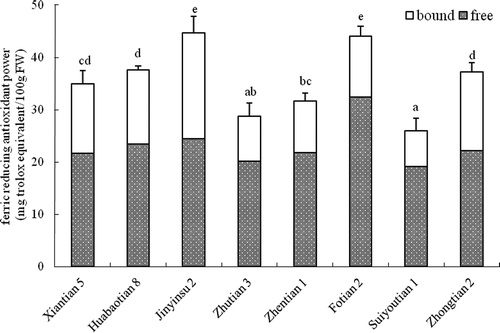
Antioxidant capacity of sweet corn determined by FRAP
The antioxidant capacity of the free and bound fraction of 8 sweet corn varieties determined by the FRAP assay are presented in . The FRAP values of free fraction in eight sweet corn varieties ranged from 19.16 to 32.42 mg TE/100 g. The percentage contribution of free fraction to the total antioxidant activity was from 54.8% in Jinyinsu 2 to 73.6% in Suiyoutian 1. The total antioxidant activity ranged from 26.02 to 44.67 mg TE/100 g. The CV of the total antioxidant activity in different sweet corn varieties was 18.8%. Jinyinsu 2 and Fotian 2 had the highest total antioxidant activity (p > 0.05), and Suiyoutian 1 and Zhutian 3 showed the lowest total antioxidant activity by FRAP (p > 0.05).
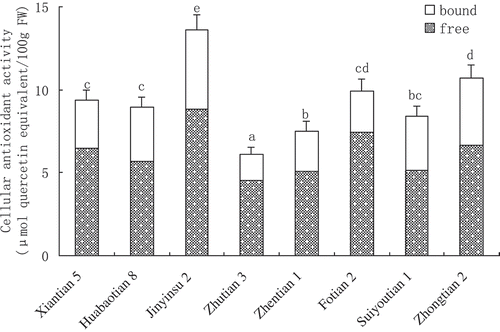
Antioxidant activity of sweet corn determined by CAA
CAA values of the free and bound fraction of eight sweet corn varieties are presented in . The CAA values of free fraction in 8 sweet corn varieties ranged from 4.55 to 8.83 μmol QE/100 g. The percentage contribution of free fraction to the total CAA value was from 60.7% in Suiyoutian to 74.8% in Fotian 2. Jinyinsu 2 had the highest total CAA value (13.59 μmol QE/100 g) followed by Zhongtian 2 and Fotian 2 (10.73 and 9.92 μmol QE/100 g, respectively). There were no significant differences between Xiantian 5 (9.36 μmol QE/100 g), Huabaotian 8 (8.96 μmol QE/100 g) and Suiyoutian (8.43 μmol QE/100 g). The last one had a similar total CAA value to that of Zhentian 1 (7.49 μmol QE/100 g). Zhutian 3 showed the lowest total CAA value of 6.09 μmol QE/100 g.
Discussion
Phenolics are synthesized in plants as secondary metabolites during normal growth and development. However, more phenolics can be produced when the plants suffer from stress conditions such as infection, wounding and UV radiation.[Citation22] Besides the environmental factors, the phenolic profiles of fruits, vegetables, and grains also show significant genetic variance. The results of the current study indicate that there are statistical differences in phenolic content of the most sweet corn varieties. Dewanto et al. reported the total phenolic content of sweet corn was 72.0 mg GAE/100 g (25 and 47 mg GAE/100 g in free and bound fraction, respectively), which is higher than that determined in the eight sweet corn varieties in the present study (38.00–57.04 mg GAE/100g).[Citation13] However, in another study, the free phenolic content in sweet corn was as low as 26.7 mg GAE/100 g,[Citation14] which is less than that determined in the 8 varieties in the present study (30.35–47.76 mg GAE/100g). It can be found that the higher total phenolic content in Dewanto’s study is mainly due to the much higher bound phenolic percentage contribution in the sweet corn variety they used than that in the eight sweet corn varieties analyzed in the current study.
As stated earlier, phenolics exist in significant quantities as insoluble bound form, especially in grain. Although consumed as vegetables, sweet corn belongs to subspecies of maize like common corn. Corns and other grains were reported to have much more bound than free phenolics.[Citation9,Citation23] The bound phenolics were reported to account for 65% of the total in sweet corn according to Dewanto’s report.[Citation13] On the other hand, the current results showed that the phenolics in sweet corn kernels exist mainly in free form (74.6–88.3%), which is similar to those found in vegetables. Bound phenolics in maize mainly distribute in the bran fraction.[Citation24] A thin bran is an important superiority of sweet corn over common corn cultivars to maintain its tasting quality. Breeders try to improve the mouthfeel of sweet corn by decreasing bran thickness. The sweet corn varieties analyzed in the present study are the widely grown cultivars in South China with relatively thin bran. The variations in bran thickness along with the different agro-climatic condition might partly account for the discrepancy of bound phenolic percentage contribution in the current study and that reported by Dewanto et al.
As a vegetable, the total phenolic content of sweet corn is equivalent to that of cabbage, radish, and mushroom, but higher than that of celery, lettuce, and cucumber.[Citation14] The phenolic content of sweet corn in the present study is close to that of common corn (15.55 μmol GAE/g =264 mg GAE/100 g dry weight) considering nearly 80% water content in fresh sweet corn, and higher than other grains, including wheat (135.8 mg GAE/100 g), oats (111.0 mg GAE/100 g), and rice (94.5 mg GAE/100 g).[Citation9]
Ferulic acid and p-coumaric acid, which are hydroxycinnamic acid derivatives, are reported to be the predominant phenolic acids detected in maize and other grains.[Citation9] Due to its diverse health benefits, including anti-oxidant, anti-inflammatory, and anti-cancer activities, ferulic acid is regarded as one of the most important phenolic acids and receiving increasingly more attention.[Citation25] The antioxidant and cardiovascular protective properties of p-coumaric acid makes it to be of great interest.[Citation26] Therefore, HPLC method was employed in the present study to determine the content of these two important phenolic acids. Dewanto et al. previously reported that ferulic acid content of sweet corn was 42677 μg/100 g (calculated according to the reported data by adding free, soluble conjugated and bound ferulic acid content together), which is much higher than that of 8 determined varieties in the present study (2890.01–13084.40 μg/100 g). Ferulic acid mainly exists in bound form in grain. A much lower percentage contribution of bound phenolics to the total in our study (11.7–25.4%) than that in Dewanto’s (65.3%) may account for the great variation in ferulic acid content between the two studies.
Many in vitro methods have been developed to estimate the antioxidant activity of plant-extracts and pure compounds by measuring the radical scavenging capacity, the reducing power and the chelation of the transition metal ions, etc. DPPH assay, which is based on hydrogen atom transfer reaction, has been widely used for assessing radical scavenging capacity.[Citation27] The FRAP assay measures the reducing capacity of antioxidants based on the electron transfer (ET) mechanism. CAA method might give more indicative information due to the fact that a physiological reaction system and cells are involved in the method. Therefore, in the present study, 3 different methods DPPH, FRAP, and CAA assay were used to estimate the antioxidant activity of 8 sweet corn varieties. For the free fraction, Fotian 2 showed the highest antioxidant activity by DPPH and FRAP assays but the second highest activity following Jinyinsu 2 by the CAA method. It was notable that the free fraction of Jinyinsu 2 showed the second lowest and the second highest antioxidant activity by DPPH and FRAP methods, respectively. For the bound fraction, Jinyinsu 2 shows the highest and Zhutian 3 shows the lowest antioxidant activity by the 3 assay methods, while Suiyoutian 1 has equivalent FRAP value to Zhutian 3. It seems that DPPH scavenging activity can not provide the same information as the other 2 methods when they are used to evaluate the antioxidant activity of the free phenolic fractions of sweet corn.
Correlation analysis showed that the FRAP antioxidant capacity of the investigated sweet corn varieties had significantly positive correlation with their total phenolic content (r = 0.966, p < 0.001). IC50 values of DPPH scavenging capacity of the bound fractions showed statistically negative correlation with phenolic content (p < 0.01) with r = –0.872, suggesting that DPPH scavenging capacity was positively correlated with the bound phenolics. However, free phenolic content in determined sweet corn varieties did not show significant correlation with DPPH scavenging capacity. The CAA values expressed as per 100 g sweet corn fresh weight were significantly positive correlated with the phenolic contents with r = 0.806 (p < 0.05) in free fraction and r = 0.934 (p < 0.01) in bound fraction. Therefore, phenolics might be the main contributor for the antioxidant activity in sweet corn kernels.
Since DPPH can only be dissolved in organic solvents not in water, the DPPH assay is usually performed in a methanol or ethanol reaction system where lipophilic antioxidants can react with DPPH radical easily.[Citation28] Besides phenolics, corn also contains some lipophilic antioxidants like lutein and zeaxanthin, which might be extracted partly by an acetone aqueous solution.[Citation29] Although the content of carotenoids was not determined in the present study, they might exhibit antioxidant activity in the free phenolic fraction of different sweet corn varieties. The interference from these nonphenolic antioxidants may at least partly account for the inconsistency in antioxidant activity between the DPPH assay and the other two methods.
To our knowledge, it is the first study to report the antioxidant activity of different sweet corn varieties, especially with CAA assay. Song et. al.[Citation14] reported the CAA value of sweet corn (4.62 μmol QE/100 g) as one of selected 27 commonly consumed vegetables in the United States, which is lower than CAA values of all the sweet corn varieties except Zhutian 3 in our study. When compared to other vegetables, the CAA activity of free fraction of eight sweet corn varieties (4.55–8.83 μmol QE/100 g) is equivalent to that of chilli pepper (8.80 μmol QE/100 g), sweet potato (8.56 μmol QE/100 g), radish (7.35 μmol QE/100 g), yellow onion (6.40 μmol QE/100 g), lettuce (5.07 μmol QE/100 g), and potato (4.76 μmol QE/100 g), but significantly lower than that of eggplant, broccoli, cabbage, etc.[Citation14] It can be concluded that the determined sweet corn varieties can provide equivalent CAA activity to many common vegetables.
In order to compare the antioxidant potential of phytochemicals in various sweet corn varieties, the CAA values per 100 μmol of phenolic compounds present in eight varieties were calculated as cellular antioxidant qualities in . The free fraction of Jinyinsu 2 had much higher cellular antioxidant quality than other seven sweet corn varieties. It is noticeable that the bound phenolics had much higher cellular antioxidant qualities than the free in all the varieties. The bound fraction of Suiyoutian 1 had the highest cellular antioxidant quality followed by Huabaotian 8. All the other varieties showed lower antioxidant quality and had no statistical difference among tested varieties. The variation in cellular antioxidant quality among different varieties is partly due to their difference in phenolic profiles, especially the percentage contribution of ferulic acid to the total phenlics. Jinyinsu 2, which has much higher percentage of ferulic acid to the total phenolics in free fraction (19.13 μg/mg) as compared to all the other varieties (7.00–15.87 μg/mg). Similarly, the bound fraction of Suiyoutian 2 and Huabaotian 8 had higher cellular antioxidant qualities than that of other varieties, also have much higher percentage of ferulic acid to the total phenolics than the other six varieties (1502.26 μg/mg and 981.37 μg/mg, respectively). It can be concluded that ferulic acid is an important component contributing to the CAA activity in sweet corn. Besides, other phenolics including vanillic acid, vanillin, and protocatecuic acid were detected in corn previously.[Citation30] These may also contribute to the CAA quality of different sweet corn.
Table 4. Comparison of antioxidant quality of sweet corn extracts with the cellular antioxidant activity (CAA) assay (μmol quercetin equivalent/100 μmol phenolics).1
In conclusion, eight Chinese sweet corn varieties showed significant varietal difference in terms of phenolics, flavonoids and antioxidant activity. Conversely to common corn, the phenolics and flavonoids determined in sweet corn mainly existed in free form. Ferulic acid was the predominant phenolic acid in sweet corn, present mainly in bound form. Sweet corn could provide equivalent phenolics and antioxidant potential to many common vegetables like lettuce and potato, etc.
Acknowledgments
We sincerely appreciate Dr. Sher Ali Khan in our lab for his kind help in language editing and Dr. Jianguang Hu from Crop Research Institute of Guangdong Academy of Agricultural Sciences for providing helpful information about sweet corn breeding.
Funding
This work was supported by the Group Program of Natural Science Foundation of Guangdong Province (2016A030312001) and Guangdong Provincial Science and Technology Projects (2016B070701012, 2016A040403081).
Additional information
Funding
References
- Lertrat, K.; Pulam, T. Breeding for Increased Sweetness in Sweet Corn. International Journal of Plant Breeding 2007, 1, 27–30.
- Turati, F.; Rossi, M.; Pelucchi, C.; Levi, F.; La Vecchia, C. Fruit and Vegetables and Cancer Risk: A Review of Southern European Studies. British Journal of Nutrition 2015, 113, S102–S110.
- Alissa, E.M.; Ferns, G.A. Dietary Fruits and Vegetables and Cardiovascular Diseases Risk. Critical Reviews in Food Science & Nutrition 2015. DOI:10.1080/10408398.2015.1040487.
- Wu, Y.; Zhang, D.; Jiang, X.; Jiang, W. Fruit and Vegetable Consumption and Risk of type 2 Diabetes Mellitus: A Dose-Response Meta-analysis of Prospective Cohort Studies. Nutrition, Metabolism and Cardiovascular Diseases 2015, 25, 140–147.
- Wolfe, K.L.; Liu, R.H. Cellular Antioxidant Activity (CAA) Assay for Assessing Antioxidants, Foods, and Dietary Supplements. Journal of Agricultural and Food Chemistry 2007, 55, 8896–8907.
- Bender, C.; Graziano, S.; Zimmermann, B.F. Study of Stevia rebaudiana Bertoni Antioxidant Activities and Cellular Properties. International Journal of Food Sciences & Nutrition 2015, 66, 1–6.
- Faller, A.L.K.; Fialho, E.; Liu, R.H. Cellular Antioxidant Activity of Feijoada Whole Meal Coupled with an in Vitro Digestion. Journal of Agricultural and Food Chemistry 2012, 60, 4826–4832.
- Acosta-Estrada, B.A.; Gutierrez-Uribe, J.A.; Serna-Saldivar, S.O. Bound Phenolics in foods, a Review. Food Chemistry 2014, 152, 46–55.
- Adom, K.K.; Liu, R.H. Antioxidant Activity of Grains. Journal of Agricultural and Food Chemistry 2002, 50, 6182–6187.
- Guo, J.; Zhang, J.; Wang, W.; Liu, T.; Xin, Z. Isolation and Identification of Bound Compounds from Corn Bran and their Antioxidant and Angiotensin I-converting Enzyme Inhibitory Activities. European Food Research and Technology 2015, 241, 37–47.
- Warman, P.R.; Havard, K.A. Yield, Vitamin and Mineral Contents of Organically and Conventionally Grown Potatoes and Sweet Corn. Agriculture, Ecosystems & Environment 1998, 68, 207–216.
- Makhlouf, J.; Zee, J.; Tremblay, N.; Bélanger, A.; Michaud, M.H.; Gosselin, A. Some Nutritional Characteristics of Beans, Sweet Corn and Peas (raw, canned and frozen) produced in the Province of Quebec. Food Research International 1995, 28, 253–259.
- Dewanto, V.; Wu, X.; Liu, R.H. Processed Sweet Corn Has Higher Antioxidant Activity. Journal of Agricultural and Food Chemistry 2002, 50, 4959–4964.
- Song, W.; Derito, C.M.; Liu, M.K.; He, X.J.; Dong, M.; Liu, R.H. Cellular Antioxidant Activity of Common Vegetables. Journal of Agricultural and Food Chemistry 2010, 58, 6621–6629.
- Das, A.K.; Singh, V. Antioxidative Free and Bound Phenolic Constituents in Botanical Fractions of Indian Specialty Maize (Zea mays L.) Genotypes. Food chemistry 2016, 201, 298–306.
- Pedreschi, R.; Cisneros-Zevallos, L. Phenolic Profiles of Andean Purple Corn (Zea mays L.). Food chemistry 2007, 100, 956–963.
- Krygier, K.; Sosulski, F.; Hogge, L. Free, Esterified, and Insoluble-bound Phenolic Acids. 1. Extraction and Purification Procedure. Journal of Agricultural and Food Chemistry 1982, 30, 330–334.
- Madhujith, T.; Shahidi, F. Antioxidant Potential of Barley as Affected by Alkaline Hydrolysis and release of Insoluble-bound Phenolics. Food chemistry. 2009, 117, 615–620.
- Zhang, R.; Zeng, Q.; Deng, Y.; Zhang, M.; Wei, Z.; Zhang, Y.; Tang, X. Phenolic Profiles and Antioxidant Activity of Litchi Pulp of Different Cultivars Cultivated in Southern China. Food Chemistry 2013, 136, 1169–1176.
- Materska, M. Flavone C-glycosides from Capsicum annuum L.: relationships between Antioxidant Activity and Lipophilicity. European Food Research and Technology 2015, 240, 549–557.
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Analytical Biochemistry 1996, 239, 70–76.
- Beckman, C.H. Phenolic-storing cells: Keys to Programmed Cell Death and Periderm Formation in Wilt Disease Resistance and in General Defence Responses in plants? Physiological & Molecular Plant Pathology 2000, 57, 101–110.
- Liyana-Pathirana, C.M.; Shahidi, F. Importance of Insoluble-bound Phenolics to Antioxidant Properties of Wheat. Journal of Agricultural and Food Chemistry 2006, 54, 1256–1264.
- Lapierre, C.; Pollet, B.; Ralet, M.C.; Saulnier, L. The Phenolic Fraction of Maize Bran: Evidence for Lignin-Heteroxylan Association. Phytochemistry 2001, 57, 765–772.
- Kroon, P.A.; Williamson, G. Hydroxycinnamates in Plants and Food: Current and Future Perspectives. Journal of the Science of Food and Agriculture 1999, 79, 355–361.
- Jyoti, R.A.; Stanely, M.P.P. Preventive Effects of p-coumaric acid on Lysosomal Dysfunction and Myocardial Infarct Size in Experimentally Induced Myocardial Infarction. European Journal of Pharmacology 2013, 699, 33–39.
- Magalhães, L.M.; Segundo, M.A.; Reis, S.; Lima, J.L. Methodological Aspects about in Vitro Evaluation of Antioxidant Properties. Analytica Chimica Acta 2008, 613, 1–19.
- Locatelli, M.; Gindro, R.; Travaglia, F.; Coïsson, J.D.; Rinaldi, M.; Arlorio, M. Study of the DPPH -scavenging activity: Development of a Free Software for the Correct Interpretation of Data. Food Chemistry 2009, 114, 889–897.
- Perry, A.; Rasmussen, H.; Johnson, E.J. Xanthophyll (lutein, zeaxanthin) content in Fruits, Vegetables and Corn and Egg Products. Journal of Food Composition & Analysis 2009, 22, 9–15.
- Luthria, D.L.; Memon, A.A.; Liu, K. Changes in Phenolic Acid Content during Dry-grind Processing of Corn into Ethanol and DDGS. Journal of the Science of Food and Agriculture 2014, 94, 1723–1728.