ABSTRACT
A unique internal mould of the patelliform mollusc Eesticonus aariensis n. gen. n. sp. is described from the Middle Ordovician (Darriwilian Series, Kunda Stage) of northern Estonia. Well-preserved muscle attachment scars are compared to those of Floripatella from strata of Middle Ordovician (Dapingian Series) age in Utah, originally considered to be the oldest known patelloidean gastropod but possibly an untorted mollusc. Comparison with the muscle scar pattern in Archinacellina from the Ordovician of Bohemia suggests that Eesticonus is an archinacelloidean gastropod, but not a patellogastropod.
Bilaterally symmetrical, limpet-like mollusc shells are known from the Cambrian to the present. Vermeij (Citation2017) noted that the morphology has arisen more than 50 times in gastropod history, indicating that the acquisition of this shell form is not a unifying phylogenetic feature. It has long been suggested that the earliest gastropods had limpet-like shells, based on numerous claims of Palaeozoic patellids in historical and more recent literature (Knight Citation1952; Golikov & Starobogatov Citation1975; Haszprunar Citation1988; Yochelson & Webers Citation2006; Frýda Citation2012; Parkhaev Citation2017). However, the Palaeozoic fossil record of gastropods is overwhelmingly dominated by conispiral morphologies (Koken Citation1897; Ulrich & Scofield Citation1897; Koken & Perner Citation1925; Wenz Citation1938-Citation44; Knight et al. Citation1960; Frýda Citation2012).
Lindberg (Citation1986, Citation1988) introduced the name Patellogastropoda, corresponding to the Docoglossa of traditional usage, for a concept based on the familiar present-day limpet Patella Linnaeus, Citation1758 and its relatives. Patellogastropoda was later placed within the Subclass Eogastropoda of Ponder & Lindberg (Citation1995) that was recognised as the sister group of essentially all other gastropods, the latter being referred to Orthogastropoda. However, based on studies of shell structure, Lindberg (Citation2008, Citation2009), Ponder & Lindberg (Citation1997) and Frýda (Citation2012) noted that the oldest confirmed patellogastropod is Triassic in age (Hedegaard et al. Citation1997).
Lindberg (Citation1988) sensibly proposed that the ancestor of patellogastropod limpets was probably a coiled gastropod (see also discussion by Frýda Citation2012; Frýda et al. Citation2008). This viewpoint was formalised by Ponder et al. (Citation2020) in their presentation of two infraclasses within Eogastropoda. The Infraclass Euomphaliformii included characteristically coiled, mainly conispiral Palaeozoic superfamilies such as Euomphaloidea, Macluritoidea, Palaeotrochoidea, Platyceratoidea, Orthonychoidea and the patelliform Archinacelloidea. Limpets placed within the Infraclass Patellogastropoda included the late Palaeozoic to Recent orders Patellida and Nacellida.
Discussion of the Eogastropoda–Orthogastropoda model has developed through phylogenomic analyses of living taxa, although the methodology obviously excludes members of extinct fossil groups such as the Euomphaliformii of Ponder et al. (Citation2020). Zapata et al. (Citation2014) reviewed models for the internal relationships of the five principal extant gastropod clades (Patellogastropoda, Vetigastropoda, Neritomorpha, Caenogastropoda, Heterobranchia) and rejected the Orthogastropoda hypothesis. Cunha & Giribet (Citation2019) placed Patellogastropoda together with Vetigastropoda, an orthogastropod group (sensu Ponder & Lindberg Citation1995) with a prominent Palaeozoic record, in a clade Psilogastropoda (new). Neritomorpha, Caenogastropoda and Heterobranchia were referred to the clade Angiogastropoda (new). A close relationship between Patellogastropoda and Vetigastropoda was also one of the possibilities considered by Zapata et al. (Citation2014).
The lack of proven patellid patellogastropods in the Palaeozoic should not imply that patellogastropods or patelliform shells were absent. Rather, the issue of patellogastropod origins is complicated by the occurrence of numerous conical (cap-shaped) or slightly coiled bilaterally symmetrical shells assigned to other molluscan groups, or left unassigned due to a lack of preserved diagnostic features. Yochelson & Webers (Citation2006) summarised the development of scientific thought concerning many of these groups. Foremost amongst these are the untorted, monoplacophorous, molluscs placed within Class Tergomya (alternative names include Monoplacophora and Tryblidia) that are well represented in Late Cambrian–Devonian strata (Horný Citation1961, Citation1963, Citation1965a, Citation1965b, Citation2002; Peel Citation1990, Citation1991a, Citation1991b; Peel & Horný Citation1999; Yochelson & Webers Citation2006) but readily compared to extant members of the class (Lemche & Wingstrand Citation1959; Wingstrand Citation1985; Lindberg Citation2009). Classic descriptions of the shells of Tryblidium Lindström in Angelin & Lindstrom, Citation1880 and Pilina Koken & Perner, Citation1925 from the Ordovician and Silurian of the Baltic region, with a series of paired internal muscle scars along the dorsum, were given by Lindström (Citation1884) and Koken & Perner (Citation1925).
Cambrian and earliest Ordovician strata have yielded numerous univalves referred to the Class Helcionelloida by Peel (Citation1991a, Citation1991b), which is approximately equivalent in composition to the Order Helcionelliformes (within gastropod Subclass Archaeobranchia) of Parkhaev (Citation2019) and earlier papers cited therein (see discussion by Geyer Citation1994 and Peel & Kouchinsky Citation2022). However, the resemblance of their bilaterally symmetrical shells, which are generally coiled through a quarter of a whorl or more, to patellids is superficial and a variety of structures on the shell interior in Helcionelloida are seemingly unique to the group (Peel Citation1991a, Citation1991b; Kouchinsky Citation2000; Parkhaev Citation2000, Citation2002, Citation2006; Vendrasco et al. Citation2010, Citation2011; Peel & Kouchinsky Citation2022). Late Cambrian to early Ordovician Hypseloconida were placed within Class Tergomya by Peel (Citation1991a) and Yochelson & Webers (Citation2006), but a provocative submission by Dzik (Citation2010) argued that similarities in muscle scars patterns with the bivalved Angarella Asatkin, Citation1932 suggested brachiopod affinities.
The patelliform Floripatella Yochelson, Citation1988, originally described from the Middle Ordovician (Dapingian) of Utah, USA has aroused particular interest on account of the claim by Yochelson (Citation1988) that it was the earliest patelloidean gastropod. As such, Floripatella would represent an extension of the known geological range of patelloids back to the Ordovician. While the oldest known patellogastropod based on preserved shell structure is of Triassic age (Hedegaard et al. Citation1997), claims of patellid affinity have been made on the basis of muscle scar patterns on rare internal moulds from the Silurian. Thus, Damilina Horný, Citation1961 from the Silurian of Bohemia has been interpreted as a patellogastropod limpet (Horný Citation1961, Citation1963; Starobogatov & Mazaev Citation1999; Mazaev Citation2015; see also Ponder et al. Citation2020). Bouchet et al. (Citation2017) placed Family Damilinidae Horny, 1961 in Superfamily Lottioidea Gray, Citation1840 of Subclass Patellogastropoda, Order Patellida. The holotype of Floripatella rousseaui Yochelson, Citation1988, the type species of Floripatella, displays well preserved muscle scars on the internal mould (A,B,G,J). However, Lindberg (Citation2009) and Vermeij (Citation2017) suggested that Floripatella was a tergomyan monoplacophoran and not a gastropod.
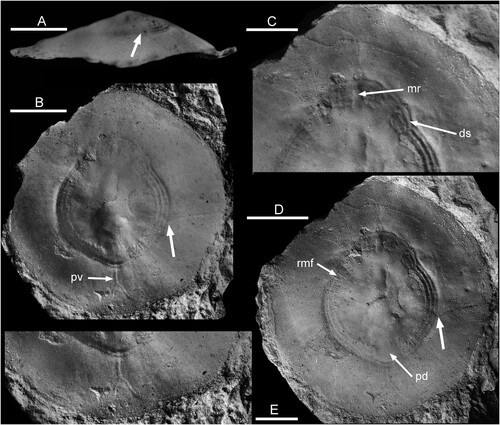
Figure 1. Floripatella rousseaui Yochelson, Citation1988, USNM PAL 410165, holotype, internal mould, Middle Ordovician, Darriwilian, Kanosh Shale, Millard County, Utah. A. lateral view oblique to the plane of symmetry B where large arrows locates intersection of a radial crack with the muscle scar in B and D. B. dorsal view with arrow pv locating the putative pallial vessel impression discussed by Lindberg (Citation2009, fig. 6). C. detail of muscle scar showing median ridge (mr) and one discrete scar (ds). D. dorsal view, as B but with alternative lighting, showing radial muscle fibres (rmf) and pericardium depression (pd). E. detail of margin with pallial vessel. Scale bars: 3 mm, C,E; 5 mm, A,B,D. Photographs prepared from negatives supplied by E.L. Yochelson.
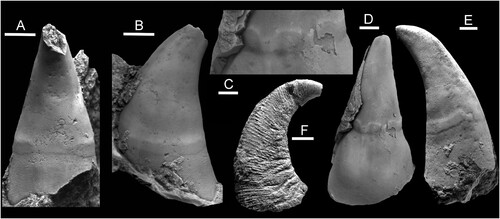
In the context of Ordovician strata in the Baltic region, cap-shaped shells of the genera Archinacella Ulrich & Scofield, Citation1897 and Pollicina Koken in Holzapfel, Citation1895 () are conspicuous (Koken Citation1897; Koken & Perner Citation1925; Peel Citation2020a, Citation2020b); Archinacella is widely distributed in the Ordovician (Ulrich & Scofield Citation1897; Wahlman Citation1992; Yochelson & Webers Citation2006). Archinacella has been interpreted variously as a gastropod or a tergomyan, as summarised by Peel (Citation1990, Citation2020a) and Peel & Horný (Citation1999) who considered it to be a gastropod, but not a patellogastropod, an opinion maintained by Ponder et al. (Citation2020). Pollicina was interpreted as a gastropod related to Archinacella by Peel (Citation2020a, Citation2020b), largely on the basis of the muscle scars on the internal moulds in both taxa. However, the distinctive tall and shallowly curved shell form of Pollicina () is not seen in patellids and Evans & Cope (Citation2003) suggested it was a tergomyan.
Figure 2. Pollicina Koken in Holzapfel, Citation1895, Ordovician, Darriwilian, Kunda Stage. A–E. Pollicina crassitesta Koken, Citation1897, internal moulds. A,B. ELM G1:2919, sub-apical and lateral views with muscle attachment scar, Tallinn. C,D. ELM g1:2323, oblique views of supra-apical surface with muscle attachment scar, Tallinn. E. CNIGRM 15600, lateral view with muscle attachment scar, Laaksberg ( = Lasnamägi), Estonia. F. Pollicina corniculum (Eichwald, Citation1860), CNIGRM 15702, lateral view showing comarginal ornamentation on shell exterior, Pulkowa (=Pulkovo), St. Petersburg, Russia. Scale bars: 2 mm, C; 4 mm, A,B,D,E; 5 mm, F.
This paper describes a unique internal mould of a patelliform mollusc from the Middle Ordovician, Darriwilian Series, Kunda Stage of Aari, Haljala, in northern Estonia, as Eesticonus aariensis n. gen. n. sp. (). Eesticonus aariensis displays a well preserved comarginal muscle attachment scar developed around the shell apex that is similar in general disposition to the muscle scar seen in Floripatella rouseaui () but close comparison is made also with Pollicina crassitesta Koken (Citation1897) from eastern Baltic Darriwilian strata (), and with archinacellid species, as a prelude to evaluating its systematic position.
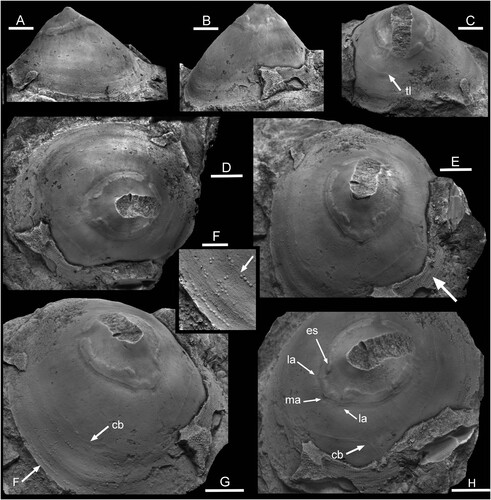
Figure 3. Eesticonus aariensis n. gen. n. sp., TUG 1787-21, holotype, internal mould with broken apex showing muscle attachment scars. Ordovician, Darriwilian Series, Kunda Stage, Aari Quarry Noonu Parish, Haljala, northern Estonia. A. lateral view. B. supra-apical surface. C. oblique view of sub-apical surface showing trace of shell laminae (tl). D. dorsal view, supra-apical surface to left. E. oblique view of supra-apical surface, with radially ornamented shell fragment (arrow). F. tubercles on the internal mould representing pits in the dissolved shell, located by arrow F in G. G. oblique view of supra-apical surface with raised comarginal band (arrow cb) that may represent an endolith burrow; position of Fig. F indicated by arrow. H. oblique view of supra-apical surface showing medial (ma) and lateral (la) angulations; es indicates the extension of the muscle scar band towards the median plane. Scale bars: 2 mm, F; 6 mm, A–E,G,H.
Systematic palaeontology
Abbreviations and repositories. – Repositories of figured specimen are indicated by the following prefixes: CNIGRM, F.N. Chernyshev Central Geological Survey Research Museum, St. Petersburg; ELM, Estonian Museum of Natural History, Tallinn; NM L, National Museum, Prague; TUG, geological collections of the University of Tartu, Natural History Museum; USNM PAL, paleobiological collections of the National Museum of Natural History (Smithsonian Institution), Washington D.C.
PHYLUM MOLLUSCA CUVIER (Citation1797)Genus Floripatella Yochelson (Citation1988)1988 Floripatella Yochelson, p. 196.2009 [Floripatella] Lindberg, p. 198.
Type species. – Floripatella rousseaui Yochelson (Citation1988), from the Kanosh Shale of Utah, USA; Middle Ordovician, Dapingian Series.
Discussion. – Yochelson (Citation1988, p. 197) commented that initial examination of available material of the type species Floripatella rousseaui from Utah prompted him to recognise several genera but he subsequently united the specimens into a single variable species. His illustrations, some of which are reproduced here on the basis of new digital scans of the original negatives (), show substantial variation in the shape of the aperture in dorsal perspective and the lateral profile. The holotype is almost circular in plan view, with a diameter of about 20 mm (B,D). In the lateral profile presented by Yochelson (Citation1988; A), its height is slightly more than one-quarter of its length. The apparent sub-apical surface (left in A) is flat in this view, whereas the apparent supra-apical surface is shallowly convex (right in A); the apex is located slightly closer to the supra-apical margin. According to Yochelson (Citation1988), this illustration represents the right side of a latex cast from the external mould, with the convex supra-apical surface thereby interpreted as anterior. Comparison of the poor photograph (A) with dorsal views of the internal mould (B,D) is difficult, but a faint radial crack (large arrows in A,B,D) seems to provide a linkage. If so, the illustrated lateral profile (A) lies at about 45 degrees (clockwise rotation relative to B) oblique to the plane of symmetry, and represents a view from the lower right in B,D. This would place the shallowly convex supra-apical surface (right in A) on the upper right in B,D, antero-lateral in the interpretation of Yochelson (Citation1988). The specimen is composite, with impression of the muscle scar from the internal mould onto the mould of the exterior.
Yochelson (Citation1988) figured two paratypes with an oval dorsal profile that he interpreted as a laterally compressed specimen, although no evidence of distortion was seen. The apex is located much closer to the margin (Yochelson Citation1988, fig. 2.1, 2.5, 2.8) than in the holotype (A) and their lateral profiles are therefore dissimilar. Based on the published illustrations, the assignment of these two specimens to Floripatella rousseaui is considered to be tenuous.
Genus Eesticonus new genus
Type species. – Eesticonus aariensis n. gen. n. sp. from the Middle Ordovician, Darriwilian Series, Kunda Stage of northern Estonia.
Derivation of name. – From Eesti Vabariik, the offical name of the Republic of Estonia.
Diagnosis. – Univalve, with oval aperture and height about two-thirds of length; early growth stage laterally compressed. Apex located at about one-quarter of length measured from sub-apical margin. Sub-apical surface shallowly concave, supra-apical surface very shallowly convex. Shell thin, with fine comarginal growth lines crossed by closely spaced, sharp radial ridges. Semi-continuous muscle attachment scar located on internal mould at about one-third of distance from apex to aperture.
Discussion. – The holotype of Floripatella rousseaui differs from Eesticonus in being almost circular in plan view (B,D) compared to the oval form of Eesticonus. Floripatella is much lower in lateral perspective than Eesticonus (A), with height only about one-quarter of the length (A). Additionally, the apex in Eesticonus lies closer to the sub-apical margin (A). The muscle attachment scar in Eesticonus (A,D) is located at less than one-third of the distance from the apex to the apertural margin but at half of the distance from the apex to the apertural margin in Floripatella (B,D).
When viewed in lateral perspective, the shell in Pollicina is much more slowly expanding than that in Eesticonus, such that its height is more than twice the length of the aperture (F) compared to two-thirds in Eesticonus (A). Additionally, in contrast to Eesticonus, the apex in Pollicina overhangs the sub-apical margin.
The well-preserved muscle attachment scar of Eesticonus aariensis invites comparison with Damilina Horný (Citation1961) from the Silurian of Bohemia, but the shell of Damilina is distinguished from Eesticonus by its low conical form and sub-central apex (Horný Citation1963). Calloconus Perner (Citation1903) from the lower Devonian of Bohemia, as re-described by Horný (Citation1963), has a similar shell form to Eesticonus but is ornamented by prominent comarginal lamellae in contrast to the fine radial ridges of Eesticonus (E, arrow). Its musculature is not known.
Numerous limpet-like shells from the Ordovician have been referred to Archinacella Ulrich & Scofield, Citation1897, which is widely distributed and varied in form, but often poorly known (Koken Citation1897; Koken & Perner Citation1925; Wahlman Citation1992; Peel & Horný Citation1999; Yochelson & Webers Citation2006). The holotype of the type species, Archinacella powersi Ulrich & Scofield (Citation1897), was re-described by Peel & Horný (Citation1999) who noted that the apex in the low, oval shell was located above the short, concave sub-apical surface almost above the apertural margin. The apex in the morphologically similar Archinacellina Horný, Citation1961 overhangs the sub-apical apertural margin. Eesticonus is readily distinguished from both by its taller shell and proportionately longer sub-apical surface.
Eesticonus aariensis new species
.
Holotype. – TUG 1787-21 from Aari Quarry, Noonu Parish, Haljala, northern Estonia (59.514111°N; 26.205069°E); Ordovician, Darriwilian Series, Kunda Stage.
Derivation of name. – From its occurrence in Aari Quarry.
Diagnosis. – As for genus, by monotypy.
Description. – In this unique internal mould, the width of the oval aperture is about three-quarters of its length. Its height is about two-thirds of length. The sub-apical surface is shallowly concave. The supra-apical surface is flattened, initially shallowly concave but becoming very shallowly convex in the main portion of the shell (A). The convexity is retained until the apertural margins in lateral areas (B,C) but the shell becomes slightly concave due to minor flaring of the aperture medially (A,H). The apex is located at about one-quarter of the length measured from the sub-apical margin (A). The early growth stage, although largely broken away in the available specimen, is narrower than the mature shell, with shallowly concave lateral areas evident on the adapical side of the muscle scar (D,G); it is slightly raised on the internal mould but the protoconch not known. The shell is thin (E, arrow), slightly reflexed at the apertural margin (A,G), with fine comarginal growth lines crossed by closely spaced, sharp radial ridges spaced at four ridges per mm. A semi–continuous muscle attachment scar on internal mould is located at about one-third of the distance from the apex to the aperture. The scar is raised slightly above the internal mould surface, although a narrow, shallow, depression corresponding to a ridge on the shell interior delimits its abapical margin.
Discussion. – The description is based on a single internal mould about 42 mm long, the holotype (), with small adherent small patches of thin shell (E, arrow); the apex is broken away.
The holotype of Floripatella rousseaui, the type species of Floripatella, differs in having an almost circular aperture (B,D) when compared to the oval aperture of Eesticonus aariensis, and is about half its overall length. Its height is about one quarter of the length (A), much lower than Eesticonus aariensis (A). The apex in Floripatella rousseaui lies more centrally than in Eesticonus aariensis where it is noticeably closer to the sub-apical margin (A). The muscle attachment scar is located at about half of the distance from the apex to the apertural margin in the holotype of Floripatella rousseaui (B) but at less than one-third of the distance from the apex to the apertural margin in Eesticonus aariensis (A,D). On account of the greater curvature of the shell, the muscle band is inclined to the apertural plane in Eesticonus aariensis (A) whereas it is parallel to the aperture in Floripatella rousseaui (A).
The supposed laterally compressed paratype internal mould of Floripatella rousseaui (Yochelson Citation1988, fig. 2.1, 2.5) is about 50% longer than the holotype of that species, narrower in plan view and apparently taller. It also shows a different lateral profile, with both the supra-apical and sub-apical slopes being shallowly convex. Additionally, the muscle scar band is located closer to the apex than in the holotype, in a position similar to that seen in Eesticonus ().
In lateral profile,?Micropileus ordovicinus (Horný, Citation1963) from the Middle Ordovician of Bohemia shows similar inclination of the sub-apical surface, but the supra-apical surface is more strongly convex, parallel to the aperture near the apex (Horný Citation1963, Citation2002). Its muscle scar is poorly known.
Muscle scar in Eesticonus
Description. – The muscle attachment scar in the holotype of Eesticonus aariensis is raised above the surface of the internal mould and is thus equivalent to a depression formed by muscle insertion on the shell interior. The relief of the scar in the following description refers to the internal mould. The muscle scar is dominated by a raised U-shaped band that extends around the supra-apical surface across one lateral area, over the dorsum, and across the other lateral area. Narrow gaps at the two prongs of the U-shape separate a crescentic scar beneath the apex from the principal scar. This crescentic scar is damaged on its apical margin as a result of breakage of the apex of the internal mould, and may be divided medially, However, a fine trace on its adapertural side is continuous. This trace also continues in weakened form across the gaps between the crescentic scar and the principal U-shaped scar. The two narrow gaps and the crescentic scar are not perfectly symmetrical about the median dorsal plane (C,D), although the apparent asymmetry is exaggerated by the breakage of the apex of the internal mould.
The adapertural margin of the U-shaped scar on each lateral surface is a relatively smooth curve marked by two narrow ridges and, outside of these, a broad shallow depression. As this adapertural margin approaches the mid-dorsal line of the supra-apical surface, slight changes in curvature produce a pair of slightly protruding angulations (H, arrows la) lying one on each side of a pointed median angulation (H, arrow ma). The adapical margin of the U-shaped scar is irregular but symmetrical from one lateral surface to the other (D,E). Each lateral branch of the scar is approximately uniform in width but the band doubles its width (along a radial line) as the median dorsal plane of the supra-apical surface is approached to produce a small, but prominent, elongate, extension to the scar on each dorso-lateral shoulder (H, arrow es). Indentations in the adapical margin of the U-shaped scar on the mid-dorsum correspond to the angulations (H, la, ma) on the adapertural scar margin. Thus, the median dorsal area of the U-shaped attachment scar appears to be composed of two pairs of smaller scars (E), although these are conjoined and with their adapertural margin delimited by the unbroken pair of marginal ridges. The lateral areas of the internal mould are shallowly convex, steepening towards the apertural margin (B). Fine radial striations are developed on their surface close to the muscle scar, while weak comarginal undulations develop near to the margin (A).
Remarks. – The slight asymmetry of the sub-apical scar may suggest that the bilateral symmetry of the single specimen of Eesticonus aariensis is derived from a trochiform gastropod ancestor, but slight asymmetry is not uncommon in the muscle scar patterns of other Palaeozoic univalve specimens, such as the tryblidiid tergomyan Pilina cheyennica Peel, Citation1977.
The muscle attachment scar in the tall, slowly expanding Pollicina () is located much closer to the aperture than in the more rapidly expanding shell of Eesticonus aariensis (A,B). However, the adapertural deflection of the muscle scar in the median dorsal area of both taxa is similar, but more strongly expressed than that developed in Eesticonus aariensis (compare C–E and E). The adapertural margin of the scar on the lateral areas is also clearly marked in both, but the muscle band in Pollicina displays abrupt terminations to the attachment area below the apex, with a continuous adapertural margin trace but without the crescentic scar crossing the median plane of the sub-apical surface (A).
The muscle scar in the low, cone-shaped shell of Damilina Horný (Citation1961), from the Silurian of Bohemia, is developed as a prominent U-shaped band in which the slightly wider prongs are joined by a thin but distinct pallial line (Horný Citation1963). The scar is crossed by radial grooves on the internal mould, equivalent to ridges on the shell interior, which caused Horný (Citation1963) to suggest that it was composed of numerous juxtaposed scars, rather than a continuous single muscle scar. Apparent segmentation of the comarginal scar is often seen in patellids due to the passage of blood vessels to the circum-apertural gills (Ponder et al. Citation2020). Damilina was interpreted as a typical patellid (Horný Citation1963, Starobogatov & Mazaev Citation1999), see also Mazaev (Citation2015), Bouchet et al. (Citation2017) and Ponder et al. (Citation2020), although information concerning its shell structure is lacking. Damilina lacks the extension of the U-shaped scar on the mid-dorsum of Eesticonus and the small scars placed between the prongs of the principal scar.
The narrow muscle scar on the internal mould of the holotype of Archinacella powersi was re-evaluated by Peel & Horný (Citation1999). It is continuous across the median line of the sub–apical surface, in contrast to Eesticonus, but it fades as it passes along the lateral areas towards the supra-apical apertural margin. In Eesticonus the scar is continuous across the median dorsal area of the supra-apical surface (G,H). Archinacellina from the Upper Ordovician of Bohemia (Horný Citation1961) is similar in shape to Archinacella powersi but with a slightly more prominent overhanging apex. A paralectotype illustrated by Peel & Horný (Citation1999, fig.10) shows the thin muscle scar crossing the median dorsal in similar fashion to the taller Eesticonus, although the scar in the latter is located much closer to the apex (A,D). The scars in the two taxa are similar, however, in developing angulations where the muscle scar crosses the dorsum of the supra-apical surface, although this feature is much more strongly developed in Archinacellina (B).
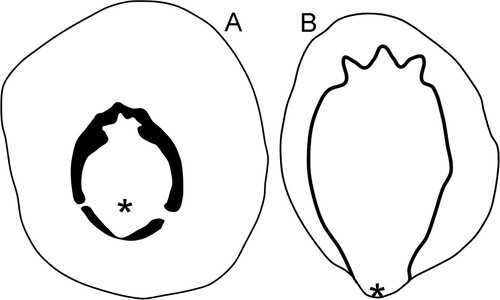
Figure 4. Drawings of the muscle attachment scars on internal moulds of the holotype of Eesticonus aariensis (A) and a paratype (NM L 5903) of Archinacellina modesta (B), the latter based on Peel & Horný (Citation1999, fig. 10A). Specimens are oriented as gastropods, with the anterior uppermost. Asteriscs locate position of shell apex.
While generally similar, with an oval, circum-apical pattern, the muscle attachment scar of Floripatella differs in detail from that of Eesticonus. It should be recalled, however, that comparison is made on the basis of just a single specimen of each taxon. The surface of the internal mould in Floripatella shows several swellings that may reflect the position of internal organs, a feature in patellids discussed by Lindberg (Citation2009). The absence of similar structures in Eesticonus probably reflects the greater height of the shell (). While the median extension of the U-shaped scar across the dorsum on the supra-apical surface of Eesticonus preserves three small angulations, the extension in Floripatella is diffuse and apparently made up of two pairs of discrete scars (C, D, arrow ds), the pairs being separated medially by a ridge on the internal mould (C, arrow mr). However, the angulations in Eesticonus may represent incipient separation of the paired muscle seen in Floripatella.
In Floripatella, a distinct radially striated, comarginal scar on each lateral surface terminates at a smooth, raised area located on the mid-dorsum, termed the pericardium depression of the inner shell surface by Lindberg (Citation2009, fig. 6; Fig. 1D, arrow pd). This depression is not recognised in Eesticonus where narrow gaps at the two prongs of the U-shape scar separate a crescentic scar beneath the apex from the principal scar that dominates the lateral areas. As in Eesticonus, a continuous, distinct, pallial line delimiting the adapertural side of the muscle scars, crosses the dorsum from one lateral area to the other in Floripatella. Comarginal ridges are prominent in the U-shaped scar of Floripatella (B–D) but restricted to the adapertural margin of the scar in Eesticonus (G,H). The radial striations seen within the lateral scars in Floripatella (D, arrow rmf) are not observed in Eesticonus.
Other structures. – The internal mould of Eesticonus aariensis shows a fine comarginal trace on the sub-apical surface reflecting the edge of an inner shell laminae (C, arrow tl). The division of an irregular comarginal band on the supra-apical surface (G,H, arrow cb) may represent an endolith burrow on the shell interior. Repeated comarginal series of small tubercles (F, arrow F in 3G) represent small oval pits associated with the growing edge of the innermost shell layer, in some cases seemingly joined by a faint trace (F, arrow). In their shape, they resemble cell strands of the present-day cyanobacterium Hyella Bornet & Flahault (Citation1888), as illustrated by Radtke & Golubic (Citation2011).
Discussion
Floripatella rousseaui was interpreted as a patelloidean gastropod by Yochelson (Citation1988) but Lindberg (Citation2009) suggested that it was as an untorted monoplacophorous mollusc. His opinion was based on the presence of an inverted Y-shaped ridge on the internal mould of the holotype that he interpreted as the trace of the efferent pallial vessel located at the posterior margin of the shell (B,E, arrow pv). Circum-pallial vessels also occur in various gastropods, including patellogastropods where they are located antero-laterally, by the side of the head (Ponder et al. Citation2020, fig. 18.12). The presence of such pallial vessels is not established in Eesticonus, nor described in other similar Lower Palaeozoic patelliform taxa. While Lindberg (Citation2009) considered Floripatella to be untorted, he recognised that it was not a member of the lineage with multiple dorsal muscle scars leading through Tryblidium and Pilina to the present-day tergomyans.
Bandel (Citation1982), Harper & Rollins (Citation1982, Citation2000), Peel (Citation1991a, Citation1991b; Citation2020a, Citation2020b), Peel & Horný (Citation1999) and others have noted that the distribution of muscle scars in cap-shaped molluscs reflects mechanical function as well as systematic position. The series of paired muscle scars located on the supra-apical surface of fossil Pilina, Tryblidium and numerous other genera convincingly support their interpretation as Tergomya, by comparison to living forms. However, even this conservative pattern (Cambrian–Recent) is modified in the Silurian Archaeopraga pinnaeformis (Perner, Citation1903), where a large elongate muscle is developed on each lateral area between the dorsal series of paired muscles and the apertural margin (Horný Citation2005).
In Tergomya, the shell apex lies outside (anterior) of the muscle scar ring, but in numerous other cap-shaped shells the apex lies within the muscle scar ring, the cyclomyan condition of Horný (Citation1965a, Citation1965b). Peel (Citation1991b) sought to explain the formation of many muscle scar patterns in terms of changes in shell morphology associated with diversifying modes of life. He abandoned the Subclass Cyclomya Horný, Citation1965b in a formal sense, assigning the constituent orders recognised by Horný (Citation1965b) to the gastropods (archinacellids) and Tergomya (cyrtonellids). Patellids are also cyclomyan in a morphological sense, but it does not follow that all cyclomyan muscle patterns indicate patellid, or even gastropod affinity.
The orientation of the protoconch relative to the adult shell suggests that patellogastropods were derived from a sinistral or hyperstrophic coiled ancestor (Ponder et al. Citation2020). Peel (Citation2019) described a pair of muscle scars on internal moulds of the open coiled, sinistral/hyperstrophic euomphaline gastropod Asgardaspira evolvens (Koken, Citation1897) from the Middle Ordovician of eastern Baltica, a member of the Infraclass Euomphaliformii, the sister group of Patellogastropoda within Eogastropoda (Ponder et al. Citation2020) and a possible indicator of the form of the ancestors of patellogastropods. The muscle scars are located one on each side of the angular junction between the umbilical wall and basal surfaces of the whorl, in a position appropriate to their function in retracting soft parts into the narrow shell. Similar paired muscle scars occur in many gastropod groups and, disposed symmetrically, fulfil the same function in Ordovician–Carboniferous isometric bellerophontoideans as in Asgardospira (Peel Citation1976, Citation1982, Citation1993). They are also described in secondarily symmetrical Carboniferous pleurotomarioidean vetigastropods (Peel Citation1986, Citation2001, Citation2004).
Development of a rapidly expanding patelliform shell, adapted to clamping against the substratum, from a slowly expanding trochiform ancestor such as Asgardospira evolvens would promote relocation of the pair of muscles to the lateral areas of the shell, with eventual comarginal extension or coalescence to form a muscle attachment band around the apertural margin. Muscle scar patterns in Pollicina (Peel Citation2020a, Citation2020b) may represent an intermediate stage. The dominance of the lateral muscle attachment areas is seen in both Eesticonus and Floripatella, but the acquisition of large muscles in Archaeopraga indicates that adoption of this location is an adaptive feature not just in gastropods, but also in some tergomyans. Thus, considered in isolation, the overall similarity in the muscle scar patterns described herein provides little reason for suggesting that Floripatella and Eesticonus were representatives of two different molluscan classes, or overwhelming evidence that they were not.
Peel (Citation2020a) acknowledged the suggestion by Evans & Cope (Citation2003) that the tall, slowly expanding Pollicina was a cyrtonellid tergomyan, but chose to interpret this genus typical of the Baltic Middle Ordovician as an archinacelloidean gastropod on the basis of comparison of muscle scars. Archinacella has been interpreted as both a monoplacophorous mollusc and a gastropod, but together with the closely related Archinacellina it was referred to the gastropods by Peel & Horný (Citation1999). Ponder et al. (Citation2020) considered Archinacella to be a patelliform euomphaliformean eogastropod but not a patellogastropod. Close similarity between the muscle scars of Eesticonus and Archinacellina with respect to the three angulations developed in the scar astride the dorsal area of the supra-apical surface () motivates assignment of Eesticonus to the Archinacelloidea Knight (Citation1956). The status of Floripatella is not satisfactorily resolved, but the interpretation of Lindberg (Citation2009) that it represents a monoplacophorous mollusc unrelated to the tergomyan lineage of present-day seas is tentatively accepted. The status of many other Palaeozoic cap-shaped or patelliform molluscs remains arbitrary.
Data archiving statement
This published work and the nomenclatural acts it contains have been registered in Zoobank: https://zoobank.org./References/75FB857E-D015-4FBC-8662-A4CE85236AE5
Acknowledgements
Mare Isakar (Tartu Museum of Natural History) kindly arranged the loan of Estonian material. Nina K. Kadlets (St Petersburg) provided access to the collections of the F. N. Chernyshev Central Geological Survey Research Museum (CNIGRM), St Petersburg, Russia. Many years ago, Ellis L. Yochelson (1929–2006) generously donated photographic negatives of Floripatella rousseaui that form the basis for . Michael J. Vendrasco (Pasadena City College) and three anonymous reviewers are thanked for comments concerning the original manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Angelin, N.V. & Lindstrom, G., 1880: Fragmenta Silurica e dono Caroli Henrici Wegelin. Samson & Wallin, Stockholm. 60 pp.
- Asatkin, B.P., 1932: Ecardines from the lower Silurian of the Siberian platform. Izvestiya Vsesoyuznogo Geologo-Razvednochnogo Obedineniya 51, 483–495, In Russian.
- Bandel, K., 1982: Morphologie und Bildung der frühontogenetischen Gehäuse bei conchiferan Mollusken. Facies 7, 1–198.
- Bornet, E. & Flahault, C., 1888: Note sur deux nouveaux genres d’algues perforantes. Journals de Botanique 2, 161–165.
- Bouchet, P., Rocroi, J.-P., Hausdorf, B., Kaim, A., Kano, Y., Nützel, A., Parkhaev, P., Schrödl, M. & Strong, E.E., 2017: Revised classification, nomenclator and typification of gastropod and monoplacophoran families. Malacologia 61, 1–526.
- Cunha, T.J. & Giribet, G., 2019: A congruent topology for deep gastropod relationships. Proceedings of the Royal Society B 286, 20182776. https://doi.org/10.1098/rspb.2018.2776.
- Cuvier, G., 1797: Tableau élémentaire de l’histoire naturelle des animaux. Baudouin, Paris. 710 pp.
- Dzik, J., 2010: Brachiopod identity of the alleged monoplacophoran ancestors of cephalopods. Malacologia 52, 97–113.
- Eichwald, E., 1860: Lethaea Rossica ou paléontologie de la Russié, décrite et figurée. Vol. 1, seconde sectione de l'ancienne période, 681–1657. E. Schweizbart, Stuttgart.
- Evans, D.H. & Cope, J.C.W., 2003: Systematic position of Pollicina corniculum (Eichwald, 1860; Mollusca, Tergomya) from the Middle Ordovician of the United Kingdom. Palaeontology 46, 139–149.
- Frýda, J., 2012: Phylogeny of Palaeozoic gastropods inferred from their ontogeny. In Talent J.A. (ed.): Earth and Life, 395–435. Springer Science and Business Media B.V., Heidelberg.
- Frýda, J., Racheboeuf, P.R. & Frýdová, B., 2008: Mode of life of Early Devonian Orthonychia protei (Neritimorpha, Gastropoda) inferred from its post-larval shell ontogeny and muscle scars. Bulletin of Geosciences 83, 491–502.
- Geyer, G., 1994: Middle Cambrian mollusks from Idaho and early conchiferan history. New York State Museum Bulletin 481, 69–86.
- Golikov, A.N. & Starobogatov, Y.I., 1975: Systematics of prosobranch gastropods. Malacologia 15, 185–232.
- Gray, J.E., 1840: A Manual of the Land and Freshwater Shells of the British Islands by W. Turton. Longman, Rees, Orme, Brown & Green, London. 324 pp.
- Harper, J.A. & Rollins, H.B., 1982: Recognition of Gastropoda and Monoplacophora in the fossil record. Proceedings of the Third North American Paleontological Convention 1, 227–232.
- Harper, J.A. & Rollins, H.B., 2000: The bellerophont controversy revisited. American Malacological Union 15, 147–156.
- Haszprunar, G., 1988: On the origin and evolution of major gastropod groups, with special reference to the Streptoneura. Journal of Molluscan Studies 54, 367–441.
- Hedegaard, C., Lindberg, D.R. & Bandel, K., 1997: Shell microstructure of a Triassic patellogastropod limpet. Lethaia 30, 331–335.
- Holzapfel, E., 1895: Das Obere Mitteldevon (Schichten mit Stringocephalus Burtini und Maeneceras terebratum) im rheinischen Gebirge. Abhandlungen der Königlich Preußischen Geologischen Landesanstalt 16, 1–460.
- Horný, R.J., 1961: New genera of Bohemian Monoplacophora and patellid Gastropoda. Vestník Ústredniho Ústavu Geologického 36, 299–302.
- Horný, R.J., 1963: Lower Palaeozoic Monoplacophora and patellid Gastropoda (Mollusca) of Bohemia. Sborník Ústředniho Ústavu Geologickeho 28, 7–83.
- Horný, R.J., 1965a: On the sytematical position of Cyrtolites Conrad, 1838 (Mollusca). Časopis Národního muzea 134, 8–10.
- Horný, R.J., 1965b: Cyrtolites Conrad, 1838 and its position among the Monoplacophora (Mollusca). Sborník Národního Muzea v Praze 21, 57–70.
- Horný, R.J., 2002: Ordovician Tergomya and isostrophic Gastropoda (Mollusca) of Bohemia: Types and referred specimens in the collections of the National Museum, Prague, Czech Republic. Acta Musei Nationalis Pragae, Series B 57(2001), 69–102.
- Horný, R.J., 2005: Muscle scars, systematics and mode of life of the Silurian Family Drahomiridae (Mollusca, Tergomya). Acta Musei Nationalis Pragae, Series B 61, 53–76.
- Knight, J.B., 1952: Primitive fossil gastropods and their bearing on gastropod classification. Smithsonian Miscellaneous Collections 114(13), 1–55.
- Knight, J.B., 1956: New families of Gastropoda. Journal of the Washington Academy of Sciences 46, 41–42.
- Knight, J.B., Cox, L.R., Keen, A.M., Batten, R.L., Yochelson, E.L. & Robertson, R., 1960: Systematic descriptions [Archaeogastropoda]. In Moore R.C. (ed.): Treatise on Invertebrate Paleontology. Part I, Mollusca 1, I169–I310. Geological Society of America and University of Kansas Press, Boulder, CO.
- Koken, E., 1897: Die Gastropoden des baltischen Untersilurs. Bulletin de l’Académie Impériale des Sciences de St Petersburg 7, 97–214.
- Koken, E. & Perner, J., 1925: Die Gastropoden des baltischen Untersilurs. Mémoires de l’Académie des Sciences de Russie, Série. 8, Classe Physico-mathématique 37, 326.
- Kouchinsky, A.V., 2000: Shell microstructures in Early Cambrian molluscs. Acta Palaeontologica Polonica 45, 119–150.
- Lemche, H. & Wingstrand, K.G., 1959: The anatomy of Neopilina galatheae Lemche, 1957. Galathea Report 3, 9–71.
- Lindberg, D.R., 1986: Radular evolution in the Patellogastropoda. American Malacological Bulletin 4, 115.
- Lindberg, D.R., 1988: The patellogastropoda. Malacological Review 4(Supplement), 35–63.
- Lindberg, D.R., 2008: Patellogastropoda, Neritomorpha, and Cocculinoidea. In Ponder W.F. & Lindberg D.R. (eds.): Phylogeny and Evolution of the Mollusca, 271–296. University of California Press, Berkeley.
- Lindberg, D.R., 2009: Monoplacophorans and the origin and relationships of mollusks. Evolution: Education and Outreach 2, 191–203.
- Lindström, G., 1884: On the Silurian Gastropoda and Pteropoda of Gotland. Kongliga svenska Vetenskaps-Akademiens Handlingar 19(6), 250.
- Linnaeus, C., 1758: Systema naturae per regna tria naturae, 10th Edition. Laurentii Salvii, Stockholm. 824 pp.
- Mazaev, A.V., 2015: Upper Kazanian (Middle Permian) Gastropods of the Volga–Urals Region. Palaeontological Journal 49, 869–986.
- Parkhaev, P.Y., 2000: The functional Morphology of the Cambrian univalved mollusks – Helcionellids, 1. Paleontological Journal 34, 392–399.
- Parkhaev, P.Y., 2002: Phylogenesis and the system of the Cambrian univalved mollusks. Paleontological Journal 36, 25–36.
- Parkhaev, P.Y., 2006: On the genus Auricullina Vassiljeva, 1998 and shell pores of the Cambrian helcionelloid mollusks. Paleontological Journal 40, 20–33.
- Parkhaev, P.Y., 2017: On the position of Cambrian archaeobranchians in the system of the Class Gastropoda. Paleontological Journal 51, 453–463.
- Parkhaev, P.Y., 2019: Cambrian molluscs of Australia: overview of taxonomy, biostratigraphy and paleobiogeography. Stratigrafiya, Geologicheskaya Korrelatsiya 27, 52–79.
- Peel, J.S., 1976: Musculature and systematic position of Megalomphala taenia (Bellerophontacea, Gastropoda) from the Silurian of Gotland. Bulletin of the Geological Society of Denmark 25, 49–55.
- Peel, J.S., 1977: Relationship and internal structure of a new Pilina (Monoplacophora) from the late Ordovician of Oklahoma. Journal of Paleontology 51, 116–122.
- Peel, J.S., 1982: Muscle scars in Bellerophon recticostatus (Mollusca) from the Carboniferous of Ireland. Journal of Paleontology 56, 1307–1310.
- Peel, J.S., 1986: Muscle scars in Porcellia (Gastropoda; Pleurotomariacea) from the Carboniferous of England. Bulletin of the Geological Society of Denmark 35, 53–58.
- Peel, J.S., 1990: Morphology and systematic position of Tryblidium canadense Whiteaves, 1884 (Mollusca) from the Silurian of North America. Bulletin of the Geological Society of Denmark 38, 43–51.
- Peel, J.S., 1991a: Functional morphology of the Class Helcionelloida nov., and the early evolution of the Mollusca. In Simonetta A.M. & Conway Morris S. (eds.): The Early Evolution of Metazoa and the Significance of Problematic Taxa, 157–177. Cambridge University Press, Cambridge.
- Peel, J.S., 1991b: The Classes Tergomya and Helcionelloida, and early molluscan evolution. Bulletin Grønlands Geologiske Undersøgelse 161, 11–65.
- Peel, J.S., 1993: Muscle scars and mode of life of Carinaropsis (Bellerophontoidea, Gastropoda) from the Ordovician of Tennessee. Journal of Paleontology 67, 528–534.
- Peel, J.S., 2001: Musculature and asymmetry in a Carboniferous pseudo-bellerophontoidean gastropod (Mollusca). Palaeontology 44, 157–166.
- Peel, J.S., 2004: Asymmetry and musculature in some Carboniferous bellerophontiform gastropods (Mollusca). GFF 126, 213–220.
- Peel, J.S., 2019: Muscle scars in euomphaline gastropods from the Ordovician of Baltica. Estonian Journal of Earth Sciences 68, 88–100.
- Peel, J.S., 2020a: Muscle scars, mode of life and systematics of Pollicina (Mollusca) from the Ordovician of Baltica. Estonian Journal of Earth Sciences 69, 20–36.
- Peel, J.S., 2020b: The Dala thumb: shell morphology and failed predation in Pollicina cyathina (Gastropoda) from the Ordovician of Dalarna, Sweden. GFF 142, 139–146.
- Peel, J.S. & Horný, R.J., 1999: Muscle scars and systematic position of the Ordovician limpets Archinacella and Barrandicella gen. nov. (Mollusca). Journal of the Czech Geological Society 44, 97–115.
- Peel, J.S. & Kouchinsky, A., 2022: Middle Cambrian (Miaolingian Series, Wuliuan Stage) molluscs and mollusc-like microfossils from North Greenland (Laurentia). Bulletin of the Geological Society of Denmark 70, 69–104.
- Perner, J., 1903: Systême Silurien du centre de la Bohême par Joachim Barrande. Vol. IV Gastéropodes 1, Texte (Patellidae et Bellerophontidae) et Planches 1 à 89. C. Bellman, Prague. 164 pp.
- Ponder, W.F. & Lindberg, D.R., 1995: Gastropod phylogeny, Challenges for the 90s. In Taylor J.D. (ed.): Origin and Evolutionary Radiation of the Mollusca, 135–154. Oxford University Press, Oxford, dated 1996, published 10 December 1995.
- Ponder, W.F. & Lindberg, D.R., 1997: Towards a phylogeny of gastropod molluscs: an analysis using morphological characters. Zoological Journal of the Linnean Society 119, 83–265.
- Ponder, W.F., Lindberg, D.R. & Ponder, J.M., 2020: Biology and Evolution of the Mollusca, Volume 2. CRC Press, Boca Raton, Florida. 870 pp.
- Radtke, G. & Golubic, S., 2011: Microbial euendolithic assemblages and microborings in intertidal and shallow marine habitats: insight in cyanobaterial speciation. In Reitner J., Quéric N. & Arp G. (eds.): Advances in Stromatolite Geobiology, Lecture Notes in Earth Sciences, 131, 233–263. Springer-Verlag, Berlin & Heidelberg.
- Starobogatov, Y.I. & Mazaev, A.V., 1999: A new genus Novlepatella (Gastropoda), and some problems of taxonomy of Paleozoic limpets. Ruthenica 9, 87–90.
- Ulrich, E.O. & Scofield, W.H., 1897: The Lower Silurian Gastropoda of Minnesota. Minnesota Geological Survey 3(2), 813–1081.
- Vendrasco, M.J., Kouchinsky, A.V., Porter, S.M. & Fernandez, C.Z., 2011: Phylogeny and escalation in Mellopegma and other Cambrian molluscs. Palaeontologia Electronica 14(2), 11A, palaeo-electronica.org/2011_2/221/index.html
- Vendrasco, M.J., Porter, S.M., Kouchinsky, A., Li, G. & Fernandez, C.Z., 2010: New data on molluscs and their shell microstructures from the Middle Cambrian Gowers Formation, Australia. Palaeontology 53, 97–135.
- Vermeij, G.J., 2017: The limpet form in gastropods: evolution, distribution, and implications for the comparative study of history. Biological Journal of the Linnean Society 120, 22–37.
- Wahlman, G.P., 1992: Middle and Upper Ordovician symmetrical univalved molluscs (Monoplacophora and Bellerophontina) of the Cincinnati arch region. U. S. Geological Survey Professional Paper 1066–O, 1–213.
- Wenz, W., 1938–1944: teil 1: Allgemeiner Teil und Prosobranchia. In Schindewolf O.H. (ed.): Handbüch der Paläzoologie, Band 6, Gastropoda, 1–1639. Gebrüder Bornträger, Berlin.
- Wingstrand, K.G., 1985: On the anatomy and relationships of recent Monoplacophora. Galathaea Report 16, 7–94.
- Yochelson, E.L., 1988: A new genus of Patellacea (Gastropoda) from the Middle Ordovician of Utah: the oldest known example of the superfamily. New Mexico Bureau of Mines and Mineral Resources Memoir 44, 195–200.
- Yochelson, E.L. & Webers, G.F., 2006: A restudy of the Late Cambrian molluscan fauna of Berkey (1898) from Taylor Falls, Minnesota. Minnesota Geological Survey Report of Investigations 64, 1–66.
- Zapata, F., Wilson, N.G., Howison, M., Andrade, S.C.S., Jörger, K.M., Schrödl, M., Goetz, F.E., Giribet, G. & Dunn, C.W., 2014: Phylogenomic analyses of deep gastropod relationships reject Orthogastropoda. Proceedings of the Royal Society B 281, 20141739. https://doi.org/10.1098/rspb.2014.1739.

