Abstract
Calvatia nipponica, a puffball mushroom (Agaricaceae), is thought to be an aphrodisiac, as this mushroom is traditionally known to improve sexual function in males. As part of the systematic study to determine the bioactive secondary metabolites from C. nipponica responsible for aphrodisiac effects, chemical analysis of methanol (MeOH) extracts of the fruiting bodies of C. nipponica resulted in the isolation of two major compounds: N,N-dimethyl-anthranilic acid (1) and (7Z,10Z)-7,10-octadecadienoic acid methyl ester (2). Compounds 1 and 2 were evaluated for cumulative dose-dependent relaxation responses to precontracted penile corpus smooth muscle (PCCSM). Results show that compounds 1 and 2 exhibited a maximum relaxation effect of 20.33 ± 2.18% and 24.63 ± 3.60%, respectively. These findings indicate that compounds 1 and 2, major components of C. nipponica, could potentially be used to treat erectile dysfunction, functioning as natural aphrodisiacs.
Puffball mushrooms in the genus Calvatia are used as food (prior to complete spore maturation) and traditional medicine for hemostasis and wound dressings, as well as to treat pharyngodynia [Citation1–6]. Calvatia mushrooms possess medicinal components such as calvatic acid (an antibiotic) and structurally related components [Citation1,Citation7,Citation8]. Calvatia nipponica is one of the rarest species in the genus Calvatia and was first validated in 2008 [Citation9]. Because of its extreme rarity, C. nipponica is not commonly consumed as food. However, it is used as an aphrodisiac since it is traditionally believed to improve sexual function in males, though this has not been previously examined in scientific studies.
As part of a continuous program to discover novel biological products from natural sources [Citation10–13], we recently explored bioactive compounds in C. nipponica. Anti-angiogenic compounds [Citation14] and anti-inflammatory compounds [Citation15] were previously identified from the chemical investigation of methanol (MeOH) extract of C. nipponica. In the present study, we focused on bioactive metabolites responsible for the aphrodisiac effect of C. nipponica as its folk remedy. Chemical analysis of the MeOH extract of C. nipponica was carried out, which led to the identification of two major compounds (1 and 2), the structures of which were elucidated by using liquid chromatography/mass spectrometry (LC/MS) analysis and comparing nuclear magnetic resonance (NMR) spectroscopic data with those previously reported. The study also examined the relaxant effects of two major compounds (1 and 2) from C. nipponica on penile corpus cavernosum smooth muscle (PCCSM) to evaluate the therapeutic effect of the compounds on penile erection.
Infrared (IR) spectra were measured using a Bruker IFS-66/S FT-IR spectrometer (Bruker, Karlsruhe, Germany). Ultraviolet (UV) spectra were obtained using an Agilent 8453 UV-visible spectrophotometer (Santa Clara, CA, USA). LC/MS analysis was carried out using an Agilent 1200 series high performance liquid chromatography (HPLC) system equipped with a diode array detector and a 6130 series ESI mass spectrometer. NMR spectra were documented using a Bruker AVANCE III 700 NMR spectrometer with a 5 mm TCI CryoProbe (Bruker) operating at 700 MHz (1H) and 175 MHz (13C). Preparative HPLC was performed using a Waters 1525 binary HPLC pump (Milford, MA, USA) with a Waters 996 photodiode array detector and Agilent Eclipse C18 column (21.2 × 250 mm; flow rate: 5 mL/min). Semi-preparative HPLC was performed using a Shimadzu Prominence HPLC System with SPD-20A/20AV UV-visible detector (Kyoto, Japan) and Phenomenex Luna HPLC phenyl-hexyl column (250 × 10 mm; flow rate: 2 mL/min; Torrance, CA, USA). Column chromatography was performed using silica gel 60 (230–400 mesh) (Merck, Kenilworth, NJ, USA). Merck precoated silica gel F254 plates and reversed-phase (RP)–18 F254s plates were used for thin-layer chromatography (TLC). Spots were detected on TLC either by using UV light or heating after spraying with anisaldehyde-sulfuric acid.
Fresh fruiting bodies of C. nipponica were collected in Jeonju, Jeollabuk-do, Korea in August 2014. A voucher specimen (HCCN26287) of the mushroom was authenticated by one of the authors (K. H. Kim) and deposited at the Herbarium Conservation Center of National Institute of Agricultural Sciences, RDA, Korea.
To explore the bioactive metabolites of C. nipponica in relation to the aphrodisiac effect, the MeOH extract was obtained from the fruiting bodies. Dried fruiting bodies of C. nipponica (200 g) were extracted using 80% MeOH (2.0 L) three times at room temperature. The MeOH extracts were then filtered and concentrated under reduced vacuum pressure using a rotavapor, resulting in the crude extract (13.8 g). The crude extract, suspended in distilled water (700 mL) and MeOH (30 mL), was subjected to solvent partition using solvents of increasing polarity: hexane, dichloromethane (CH2Cl2), ethyl acetate (EtOAc), and n-butanol (n-BuOH). Accordingly, four main fractions with increasing polarity were obtained, a hexane-soluble fraction (1.1 g), a CH2Cl2-soluble fraction (0.4 g), an EtOAc-soluble fraction (0.8 g), and an n-BuOH-soluble fraction (3.1 g). TLC and LC/MS analysis showed that CH2Cl2-soluble and EtOAc-soluble fractions had similar chemical profiling, so they were combined to obtain a CH2Cl2/EtOAc-soluble fraction. To find the major components of C. nipponica fruiting bodies, a chemical analysis on the solvent-partitioned fractions were carried out based on TLC and LC/MS analysis. Using repetitive techniques of column chromatography and HPLC purification, two major compounds (1 and 2) were isolated (). Silica column chromatography (CC) was used to fractionate the hexane-soluble fraction (1.1 g), eluted with a gradient solvent system of hexane:EtOAc from 20:1 to 1:1, to obtain 12 subfractions (H1-H12). Subfractions H1 (46.8 mg) and H2 (7.6 mg) were combined according to TLC and LC/MS analysis results, and the combined fraction was purified using semi-preparative HPLC equipped with Phenomenex Luna HPLC phenyl-hexyl column (250 × 10 mm; flow rate: 2 mL/min), with the isocratic solvent system of 93% MeOH/H2O (flow rate: 2 mL/min) to produce compound 2 (9.7 mg, tR 36.0 min). The CH2Cl2/EtOAc-soluble fraction (1.2 g) was also subjected to silica CC, eluting a gradient solvent system of CH2Cl2:MeOH from 30:1 to 0:1, to provide 8 subfractions (C1-C8). Subfraction C4 (295.9 mg), which appeared to possess major constituents based on TLC analysis, was fractionated using preparative HPLC with 65% MeOH/H2O (flow rate: 5 mL/min) to create 8 subfractions (C41-C48). Subfraction C41 (164.3 mg) was isolated by semi-preparative HPLC using the same column, with 17% MeOH/H2O to produce compound 1 (87.3 mg, tR 13.5 min). The isolated compounds were identified as N,N-dimethyl-anthranilic acid (1) [Citation14] and (7Z,10Z)-7,10-octadecadienoic acid methyl ester (2) [Citation16] by the comparison of their spectroscopic data with those previously reported, along with LC/MS analysis.
Experiments were performed to investigate the cumulative dose-dependent relaxation effects of compounds 1 and 2 on PCCSM, pre-contracted with L-phenylephrine (Phe). All animal procedures followed the NIH guidelines for care and were approved by the Institutional Animal Care and Use Committee of Chonbuk National University Laboratory Animal Center (IACUC, chu-IACUC-2016-12). Healthy male New Zealand white rabbits were intravenously anesthetized using ketamine (50 mg/kg) plus xylazine (25 mg/kg) and exsanguinated. The penis was excised rapidly and the PCCSM was carefully dissected from the surrounding tunica albuginea. During preparation, each step was undertaken cautiously to prevent damaging the functional endothelium or overstretching the tissue.
PCCSM strips (1.5 × 1.5 × 7 mm) were placed vertically in 2 mL organ baths and connected by threads to the prong of a force transducer (FT03, Grass Telefactor; West Warwick, RI, USA) on one end and a holder for isometric tension measurement on the other end. The organ bath, which contained a HEPES buffer (36 °C), was constantly aerated with 100% O2. The HEPES buffer contained the following: NaCl (118 mM), KCl (4.7 mM), CaCl2 (2.5 mM), MgCl2 (1.2 mM), NaHCO3 (25 mM), glucose (10.0 mM), and HEPES (10 mM) with NaOH (pH 7.4). After mounting, the strip was equilibrated for 60 min with several adjustments of length until a baseline force stabilized at 1 g. The oxygenated buffer was replaced every 15 min. After stabilization, 10−5 M l-phenylephrine (Phe) was added to adjust the maximal contractile tension and samples were added to the organ bath containing the desired final concentration. The relaxation effects of compounds 1 and 2 isolated from C. nipponica were studied by cumulative addition at final concentrations of 10−7, 10−6, 10−5, and 10−4 M at the plateau of the Phe-induced contraction. Changes in isometric force were measured and recorded using the PowerLab data 400 acquisition system (Software Chart, version 5.2; AD Instruments, Castle Hill, Australia). Relaxation was expressed as the percent decline in contractile tension relative to the 10−5 Phe-induced maximum.
Submaximal penile contractile responses induced by Phe were used as 100% values and all subsequent responses to compounds were expressed as a percentage of this value. Results were expressed as the mean ± SD, and n represents the number of tissues in each group. Statistical significance was calculated using one-way analysis of variance (ANOVA) followed by Bonferroni’s multiple comparison test. p < 0.05 was considered significant.
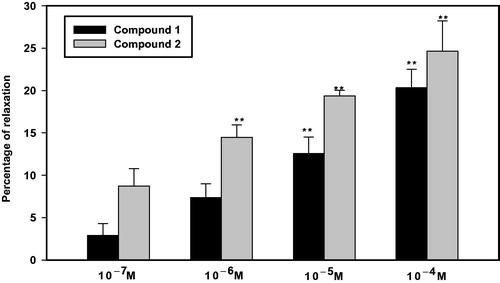
Compounds 1 and 2 exhibited a concentration-dependent relaxant effect (). Relaxations induced by the treatment of 10−7, 10−6, 10−5, and 10−4 M of compound 1 were 2.91 ± 1.39%, 7.36 ± 1.63%, 12.55 ± 1.96% (p < 0.01), and 20.33 ± 2.18% (p < 0.01), respectively. Compound 2 exhibited higher potency in relaxant effect than compound 1, where the relaxant effects induced by the treatment with the same concentrations of compound 2 were 8.73 ± 2.06%, 14.48 ± 1.47%, 19.37 ± 0.65%, and 24.63 ± 3.60%, respectively.
Figure 2. Percentage of relaxation induced by compounds 1 and 2 (n = 4). Precontracted PCCSM with 10−5 M L-phenylephrine (Phe) was treated with four concentrations of 1 and 2. Submaximal penile contractile responses induced by Phe were taken as the 100% values, and all subsequent responses to 1 and 2 were expressed as a percentage of this value. Each point represents the mean ± SD of the percentages. Statistical analysis was carried out by ANOVA followed by Bonferroni’s test (**p < 0.01 vs. 10−7 M).
Several natural products have historically been used as aphrodisiacs, such as yohimbine and mandrake in Africa and Europe, rhinoceros horn in China, and Spanish fly (Lytta vesicatoria) in Europe [Citation17,Citation18]. Even in today’s culture, there are cases where some foods are used as aphrodisiacs, such as strawberries and raw oysters. In addition, chocolate, coffee, and honey are also known to possess aphrodisiac potential. However, these assertions have not been scientifically approved. Recently, many research groups have developed profound interest in examining natural aphrodisiacs. Studies have shown that coumarins and lignans, including scopoletin, esculetin, scoparone, and gomisin A exhibit relaxant effects on PCCSM in vivo [Citation19–22]. In the 1990s, the development of Viagra (sildenafil), the first pharmacologically approved remedy for impotence, has drawn much public attention. However, the drug has limited efficacy, unpleasant side effects, and contraindications in certain disease conditions. As an alternative to this drug, the search for natural supplements from herbal drugs or natural resources is being reinforced anticipating reduced side effects [Citation23]. In this study, we demonstrated that N,N-dimethyl-anthranilic acid (1) and (7Z,10Z)-7,10-octadecadienoic acid methyl ester (2) from C. nipponica showed significant relaxant effect on Phe precontracted PCCSM. Thus, evidence suggests that two major compounds of C. nipponica could be used as aphrodisiacs or supplements for the treatment of erectile dysfunction.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Anke H, Sterner O. Insecticidal and nematicidal metabolites from fungi. In: Osiewacz HD, editor. The Mycota X. Industrial applications. Berlin: Springer-Verlag; 2002. p. 109.
- Coetzee JC, van Wyk AE. The genus Calvatia (‘Gasteromycetes’, Lycoperdaceae): A review of its ethnomycology and biotechnological potential. Afr J Biotechnol. 2009;8:6007–6015.
- Kawahara N, Sekita S, Satake M. A novel dimeric steroid, calvasterone from the fungus Calvatia cyathiformis. Chem Pharm Bull. 1993;41:1318–1320.
- Kawahara N, Sekita S, Satake M. Steroids from Calvatia cyathiformis. Phytochemistry. 1994;37:213–215.
- Morris B. Common mushrooms of Malawi. Oslo (Norway): Fungiflora A/S; 1987.
- Rinaldi A, Tyndalo V. Mushrooms and other fungi, an illustrated guide. London: Hamlyn; 1974.
- Takaishi Y, Adachi R, Murakami Y, et al. A polyoxygenated steroid from Lasiosphaera nipponica. Phytochemistry. 1992;31:243–246.
- Takaishi Y, Murakami Y, Uda M, et al. Hydroxyphenylazoformamide derivatives from Calvatia craniformis. Phytochemistry. 1997;45:997–1001.
- Kasuya T, Katumoto K. Notes on Japanese Lycoperdaceae. 4. Validation of Japanese giant puffball, Calvatia nipponica. Mycoscience. 2008;49:271–275.
- Baek SC, Choi E, Eom HJ, et al. LC/MS-based analysis of bioactive compounds from the bark of Betula platyphylla var. japonica and their effects on regulation of adipocyte and osteoblast differentiation. Nat Prod Sci. 2018;24:235–240.
- So HM, Eom HJ, Lee D, et al. Bioactivity evaluations of betulin identified from the bark of Betula platyphylla var. japonica for cancer therapy. Arch Pharm Res. 2018;41:815–822.
- Trinh TA, Park EJ, Lee D, et al. Estrogenic activity of sanguiin H-6 through activation of estrogen receptor α coactivator-binding site. Nat Prod Sci. 2019;25:28–33.
- Yu JS, Roh HS, Baek KH, et al. Bioactivity-guided isolation of ginsenosides from Korean Red Ginseng with cytotoxic activity against human lung adenocarcinoma cells. J Ginseng Res. 2018;42:562–570.
- Lee S, Park JY, Lee D, et al. Chemical constituents from the rare mushroom Calvatia nipponica inhibit the promotion of angiogenesis in HUVECs. Bioorg Med Chem Lett. 2017;27:4122–4127.
- Lee S, Lee D, Lee JC, et al. Bioactivity-guided isolation of anti-inflammatory constituents of the rare mushroom Calvatia nipponica in LPS-stimulated RAW264.7 macrophages. Chem Biodiversity. 2018;15:e1800203.
- Zhang D, Liang C, Xiulan X, et al. Study on chemical components of blackberry seed oil and its antioxidant activity. Zhongguo Liangyou Xuebao. 2011;26:55–58.
- Ang HH, Chan KL, Gan EK, et al. Enhancement of sexual motivation in sexually naive male mice by Eurycoma longifolia, jack. Int J Pharmacogn. 1997;35:144–146.
- Evans WO. Chemical aphrodisiacs. Psychopharmacol Bull. 1969;5:10–17.
- Choi BR, Kumar SK, Zhao C, et al. Additive effects of Artemisia capillaris extract and scopoletin on the relaxation of penile corpus cavernosum smooth muscle. Int J Impot Res. 2015;27:225–232.
- Choi BR, Kim HK, Park JK. Penile erection induced by scoparone from Artemisia capillaris through NO-cGMP signaling pathway. World J Mens Health. 2017;35:196–204.
- Kim HK. Effect of esculetin from Artemisia capillaris on the relaxation of penile corpus cavernosum smooth muscle. Yakhak Hoeji. 2016;61:48–54.
- Choi BR, Kim HK, Park JK. Effects of Schisandra chinensis fruit extract and gomisin A on the contractility of penile corpus cavernosum smooth muscle: A potential mechanism through the nitric oxide – cyclic guanosine monophosphate pathway. Nutr Res Pract. 2018;12:291–297.
- Kotta S, Ansari SH, Ali J. Exploring scientifically proven herbal aphrodisiacs. Phcog Rev. 2013;7:1–10.