 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Many of the β-glucans are known to have antihypertensive activities, but, except for angiotensin-converting enzyme II inhibition, the underlying mechanisms remain unclear. Corin is an atrial natriuretic peptide (ANP)-converting enzyme. Activated corin cleaves pro-ANP to ANP, which regulates water–sodium balance and lowers blood pressure. Here, we reported a novel antihypertensive mechanism of β-glucans, involved with corin and ANP in mice. We showed that multiple oral administrations of β-glucan induced the expression of corin and ANP, and also increased natriuresis in mice. Microarray analysis showed that corin gene expression was only upregulated in mice liver by multiple, not single, oral administrations of the β-glucan fraction of Phellinus baumii (BGF). Corin was induced in liver and kidney tissues by the β-glucans from zymosan and barley, as well as by BGF. In addition to P. baumii, β-glucans from two other mushrooms, Phellinus linteus and Ganoderma lucidum, also induced corin mRNA expression in mouse liver. ELISA immunoassays showed that ANP production was increased in liver tissue by all the β-glucans tested, but not in the heart and kidney. Urinary sodium excretion was significantly increased by treatment with β-glucans in the order of BGF, zymosan, and barley, both in 1% normal and 10% high-sodium diets. In conclusion, we found that the oral administration of β-glucans could induce corin expression, ANP production, and sodium excretion in mice. Our findings will be helpful for investigations of β-glucans in corin and ANP-related fields, including blood pressure, salt–water balance, and circulation.
Keywords:
1. Introduction
Hypertension is a leading cause of morbidity and mortality from stroke, myocardial infarction, heart failure, and ischemic heart diseases. Crisis symptoms associated with hypertension are cerebral infarction or hemorrhage, acute pulmonary edema and hypertensive encephalopathy [Citation1]. Many of the conventional medicines were developed to treat hypertension by acting as diuretics, adrenergic receptor agonist/antagonists, calcium channel blockers, angiotensin-converting enzyme (ACE) inhibitors, aldosterone antagonists, and vasodilators [Citation2]. Genes responsible for inducing hypertension include renin (REN), ACE, Na-Cl cotransporter (SLC12A3), and endothelin receptor type A (EDNRA). In contrast, blood pressure-lowering genes were reported by the Framingham Heart Study (FHS) [Citation3]. These include solute carrier family 12 member 3 (SLC12A3), SLC12A1, and KCNJ1. Different from genes, vasoconstricting peptides, such as angiotensin II, endothelins, and neuropeptide Y, and vasodilating peptides, such as bradykinin, ANP, BNP, CGRP, and VIP, have been reported [Citation4].
Corin is an important enzyme in the natriuretic peptide system and its gene was first identified as an unusual mosaic serine protease from human heart. As a transmembrane protein, corin has various extracellular domains containing two frizzled-like cysteine-rich motifs, seven LDL receptor repeats, a macrophage scavenger receptor-like domain, and a trypsin-like protease domain [Citation5]. Among the extracellular domains of corin, the trypsin-like protease domain is responsible for the processing of pro-ANP to active ANP, which is responsible for various biological functions, such as natriuresis, vasodilation, and anticancer activities. Diseases associated with corin include preeclampsia and eclampsia. Pregnant corin- or ANP-deficient mice were shown to develop high blood pressure and proteinuria, characteristics of pre-eclampsia [Citation6].
ANP peptide can be produced in the heart by the enzymatic action of corin. ANP is a 28-amino acid peptide that belongs to a family of cardiac hormones, performing a crucial role in the cardiovascular homeostasis of blood volume and pressure regulation. In the heart, ANP is originally synthesized as a 151-amino acid pre-propeptide. Through signal peptide processing in the endoplasmic reticulum, pro-ANP is produced in atrial cardiomyocyte granules by the removal of a signal peptide with the aid of signal peptidase. Pro-ANP is further proteolytically cleaved by the convertase corin, producing mature ANP (COOH-terminal) and an NH2-terminal propeptide. The latter is further cleaved to three natriuretic heart hormones, long-acting natriuretic peptide (LANP), vessel dilator (VSDL), and kaliuretic peptide (KP) [Citation7]. All the corin-mediated heart hormones of ANP, LANP, VSDL, and KP have natriuresis functions, as well as very strong anticancer activities [Citation8].
β-Glucan is one of the most abundant forms of polysaccharides found on the cell wall of fungi, mushrooms, and cereals. Glucans can be synthesized either as an α-glucan or β-glucan, depending on the type of linkage between the constituting subunits. As mammalians do not have β-glucanase, orally administered β-glucans reach the intestine in an intact state and can be absorbed into the intestinal membrane, finally reaching the circulating systems [Citation9]. Therefore, orally administered β-glucans show many biological activities including antitumor, immunomodulating, bone injury healing, anti-diabetic, cholesterol-lowering, and antigenotoxic activities [Citation10]. β-Glucans have been reported to have antihypertensive efficacy in hypertensive and hyperlipidemic persons, patients with type 2 diabetes, hypertensive patients, and obese persons [Citation11]. However, most of the previous research did not elucidate the antihypertensive mechanism of β-glucans. Antihypertensive polysaccharides have also been obtained from various sources, including microbes, Gastrodia elata Blume, oysters, and brown alga, with the ability to block the ANP receptor, increase nitric oxide (NO), and inhibit ACE II [Citation12,Citation13].
We previously demonstrated that polysaccharide of P. linteus [Citation14] prevented tumor metastasis by inducing tissue inhibitor of metalloproteinase-1 (TIMP-1) and IL-23 in mice and RAW264.7 cells. We also reported that β-glucan from P. baumii induced flavin-containing monooxygenase (FMO) and activated NADPH oxidase, suggesting that β-glucan could enhance the detoxification of xenobiotics and macrophage-mediated phagocytosis, respectively [Citation15,Citation16]. In this study, we reported the induction of corin gene expression by oral β-glucan treatment, which led to an increase in natriuresis by augmenting ANP production in mice. In addition to β-glucan from mushrooms, we also showed that β-glucans from Saccharomyces cereviceae and Hordeum vulgare had ANP-augmenting properties. Although β-glucan has been reported to have antihypertensive properties, the mechanism of action has not yet been reported. As far as we know, this report on corin expression and ANP production is the first one elucidating the antihypertensive mechanism of β-glucan. As ANP has powerful antihypertensive activity, ANP-inducing β-glucans from natural products can be applied to hypertensive patients without any side effects.
2. Materials and methods
2.1. Materials and chemicals
The β-glucan fraction (BGF) was obtained from the carpophores of P. baumii as described previously [Citation16]. Briefly, dried and powdered carpophores were extracted with distilled water at 100 °C for 10 h. The filtered extract was concentrated in vacuo, and three volumes of ethanol were added at 4 °C for 4 h to precipitate the crude BGF. The crude BGF was purified by passing through a Sephacryl S-400 HR column (Sigma-Aldrich, St. Louis, MO, USA) and BGF was obtained as a strong single band. The β-glucan content in the BGF was 84% by analysis with a mushroom and yeast assay kit (Megazyme, Bray, Ireland). β-Glucans of yeast zymosan A from Saccharomyces cerevisiae and barley were obtained from Sigma-Aldrich. Mouse diets containing 1% normal and 10% high-salt were obtained from Samtako (Osan, Korea).
The polymerase chain reaction (PCR) primers for corin and GAPDH were synthesized by Cosmogenetech (Seoul, Korea). The forward and reverse primer sequences (5′→3′) of corin were 5′GTC CGC ATT ATT CCT CTG GA3′ and 5′ CAA ACC AGA GGA CCA CCA CT3′, respectively. Those of GAPDH were 5′ ACC GCA GCT AGG AAT AAT GGA ATA 3′ and 5′ CTT TCG CTC TGG TCC GTC TT 3′, respectively. Oligo deoxythymine, 5X reaction buffer, Moloney murine leukemia virus (M-MLV) reverse transcriptase, and SYBR green qPCR PreMIX were obtained from Enzynomics (Daejeon, Korea).
2.2. Animal treatment for microarray
To perform microarray analysis for the induction of some genes in the liver, heart, and kidney, crude BGF was orally administered to ICR mice at a concentration of 100 mg/kg (n = 3 mice/group) in a volume of 0.25 mL once per day for one or seven days. Total RNA extracts were obtained from the liver, heart, and kidney as described previously [Citation15]. Briefly, after a single (SOA) or multiple (MOA) oral administrations of crude BGF in mice over seven days, total mRNA was obtained from the liver for microarray. To obtain total RNA extract from the liver, about 200 mg of liver tissue was treated with 1.5 mL of an RNA-stabilizing reagent (Sigma-Aldrich) to preserve the total RNAs. After a quality analysis of the RNA, only the well-stabilized RNA samples were analyzed using a mouse gene 1.0 ST array (Affymetrix Inc., Santa Clara, CA, USA).
2.3. Real-time PCR
BGF or zymosan was administered to ICR male mice (8 weeks old, n = 4, Samtako) at a concentration of 100 mg/kg for seven days. Total mRNA was obtained as previously reported [Citation15]. Tissues (0.2 g) from the liver, heart, and kidney of the mice were washed with phosphate-buffered saline (PBS) and lysed with 1 mL Trizol reagent (Favorgen, Ping-Tung, Taiwan) for 5 min. The RNA-containing aqueous phase was obtained by adding 250 μL chloroform (Sigma-Aldrich) and the RNA was precipitated with 550 μL of isopropyl alcohol (Sigma-Aldrich). The obtained RNA pellet was washed with 1 mL of 75% ethanol/DEPC and dissolved in 50 μL of RNase-free DEPC water. First-strand complementary DNA (cDNA) was synthesized using Moloney murine leukemia virus (M-MLV) reverse transcriptase (Promega, Madison, WI, USA), which is an RNA-dependent DNA polymerase. Mixtures of 2 μg mRNA, 1 μL 0.5 μg/μL oligo dT (Enzynomics, Daejeon, Korea), and 4 μL DEPC-treated water were incubated at 70 °C for 10 min, then placed on ice for at least 5 min. The final reaction mixture was prepared by adding 4 μL of 5X reaction buffer, 2 μL of 10 mmol/L DNTP mixture (Promega), 2.4 μL double-distilled water, and 0.1 μL RNase inhibitor. The reaction was incubated at 42 °C for 3 min and 1 μL M-MLV reverse transcriptase was added, followed by further incubation for 60 min at 42 °C. cDNA synthesis was terminated by heating at 70 °C for five min. The products were stored at −20 °C until PCR analysis. To determine corin mRNA expression in the mouse liver, lung, and kidney, real-time PCR (RT-PCR) amplification was performed by adding Taq polymerase to RT-PCR plates (multi-well PCR plates, 96-well clear; Bio-Rad, Hercules, CA, USA) containing 2 X Ampigene qPCR Green Mix Lo-Rox (Enzo Life Science, Farmingdale, NY, USA), cDNA, and primers specific for corin. The PCR plates were covered with optical adhesive covers (Applied Biosystems, Foster City, CA, USA) and centrifuged for 5 min at 1,500 rpm. Amplification was performed by heating for 2 min at 95 °C (polymerase activation), followed by repeated 40 cycles of 30 s at 95 °C (denaturation), 30 s at 65 °C (annealing/extension), and 1 min at 72 °C using an RT-PCR machine (CFX-Connect; Bio-Rad). The PCR products were analyzed by 1% agarose gel electrophoresis.
2.4. ELISA assay of ANP peptide
ANP peptides were extracted from the heart, liver, and kidney of mice that were orally administered 100 mg/kg/day of β-glucans from yeast, barley, and P. baumii for seven days. Briefly, tissues (0.5 g) from the organs were washed with PBS, followed by adding 1 mL PRO-PREP protein extraction solution (Intron Biotechnology, Sungnamsi, Korea) and lysed by adding bead-beater (TACO-prep; PhileKorea, Seoul, Korea) at 4 °C using a homogenizer (Wheaton, Millville, NJ, USA). The homogenized tissues were centrifuged at 600 g for 10 min and the supernatants were again centrifuged at 15,000 g for 5 min and the supernatants were finally centrifuged at 15,000 g for 30 min at 4 °C. The protein concentrations of the obtained tissue supernatants were determined by the Bradford assay, which measures the optical density of 2 μL tissue supernatant and 200 μL of 1X solution of protein assay dye reagent concentrate (Bio-Rad) diluted with ultra-pure distilled water at 595 nm. The ANP concentrations in the tissue supernatants were measured by the RayBio Mouse ANP Enzyme Immunoassay Kit (RayBiotech, Peachtree Corners, GA, USA). Briefly, the microplate wells were treated with 100 μL of anti-ANP antibody solution for 1.5 h at room temperature with 1 cycle/sec shaking. The solution in the well was discarded and washed four times with 200 μL of 1X wash buffer solution. Samples of 100 μL were added to each well and incubated for 2.5 h at room temperature with 1 cycle/sec shaking and the solution was discarded and washed four times with wash buffer. The plate was incubated for 45 min at room temperature with shaking after adding 100 μL of HRP-streptavidin solution to each well. The solution in the well was discarded and washed four times with wash buffer. Finally, the plate was incubated at room temperature in the dark with 1 cycle/sec shaking after adding 100 μL of TMB One-Step substrate reagent. After 30 min incubation, 50 μL of stop solution was added to each well and the OD450 was measured immediately with a spectrophotometer.
2.5. Urinary excreted sodium assay
Mouse urine was collected in a specially designed metabolic cage (4 mice/group). Before the experiment, the ICR mice were acclimated to the laboratory environment for five days. Urine was collected every day from day 1 to day 21 with the daily oral administration of 100 mg/kg/day BGF or zymosan in mice fed normal diet (1% sodium) or high-sodium diet (10% sodium). The body weights and amount of diet and water consumed were measured every day. The sodium contents of the urine were measured in 96-well clear flat-bottomed plates using a BioVision Sodium Assay Kit (K391-100; Biovision, Milpitas, CA, USA), which employs sodium ions as a cofactor for the enzymatic activity of β-galactosidase. Briefly, 10 μL of the collected urine, 20 μL of β-galactosidase, and 10 μL of 10 mM Na assay buffer were added to the microplate wells and the plates were incubated for 10 min in the dark at 37 °C. After incubation, 40 μL of the substrate solution was added to each well and the plate was incubated for 30 min at 37 °C in the dark. Then, 100 μL of Na developer was added to each well, mixed well, and the optical density was measured at 405 nm. Urinary sodium concentrations were calculated by the following equation:
where, B was the amount of sodium in the sample well from the standard curve (nmol), V was the sample volume added to the reaction well (μL), and D was the sample dilution factor.
2.6. Statistical analysis
All values are expressed as mean ± standard deviation. One-way analysis of variance (ANOVA) was used to detect statistical significance. A value of p < 0.05 was considered to be significant.
3. Results
3.1. Microarray analysis of mouse liver gene
To find novel functions of β-glucan from Phellinus baumii, BGF was orally administered to ICR mice for one or seven days at a concentration of 100 mg/kg/day. Among the modulated gene expression in the liver by microarray analysis, we were interested in proteolysis-associated gene expression and found that genes for five proteins, phospholipid transfer protein (PLTP), solute carrier family 3 member 1 (SLC3A1), cystatin 3 (CST3), corin, and serpin family A member 7 (Serpin A7), were increased or decreased in the liver. The average fold-changes in PLTP, SLC3A1, and CST3 were 2.2, 0.5, and 0.5, respectively, from a SOA of BGF. Those of corin and Serpin A7 were 5.8 and 0.4, respectively, by MOA (). As the corin expression was the most strongly increased among the five proteolysis-associated genes, we further investigated the effect of corin on the biological activities in mice.
Table 1. Expression of proteolysis-associated genes in mice liver by single (SOA) or multiple oral (MOA) BGF administrations.
3.2. Validation of corin expression by RT-PCR analysis
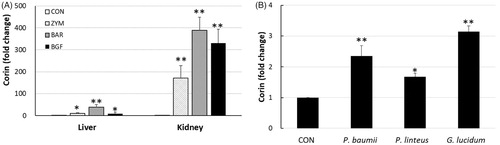
Microarray analysis of liver genes showed that BGF induced corin 5.8-fold compared to the untreated control group. We further validated the mRNA expression of corin in mice liver and kidney tissues by RT-PCR analysis. In addition to the BGF of mushroom, β-glucans of yeast zymosan A from Saccharomyces cerevisiae and barley were also analyzed by PCR. For the RT-PCR analysis of corin, cDNA was constructed from the mRNAs obtained from the liver and kidney tissues of ICR mice orally administered BGF or the β-glucan of zymosan or barley at 100 mg/kg/day for seven consecutive days. The PCR analysis of corin showed that the fold-increases in corin expression in liver tissue by the zymosan, barley, and BGF treatments were 10.7, 38.3, and 8.3, respectively, compared to the PBS-treated control group. The expression of corin in kidney tissue following treatment with zymosan, barley, and BGF was increased 171.7-, 388.3-, and 330.0-fold, respectively, compared to the control group (). Not only did mushroom β-glucan and BGF induce corin expression in mouse liver and kidney tissues, but yeast and barley β-glucans increased corin expression in kidney tissue much more than in liver tissue. We also investigated whether β-glucans from other mushrooms could induce corin in mice. The RT-PCR analysis showed that the β-glucans from P. baumii, P. linteus, and Ganoderma lucidum also induced the expression of corin in mouse liver tissue 2.4-, 1.7-, and 3.2-fold, respectively, compared to the control group ().
Figure 1. Real-time PCR of corin mRNA expression in mice tissues. (A) ICR mice (n = 4) were orally administered zymosan, barley β-glucan, and BGF at 100 mg/kg for seven consecutive days. RT-PCR analysis of corin was performed on the mRNAs of mice liver and kidney tissues. (B) RT-PCR analysis of corin was performed on the kidney tissue of ICR mice treated with β-glucans from Phellinus baumii, Phellinus linteus, and Ganoderma lucidum at 100 mg/kg for seven consecutive days. CON: control; ZYM: β-glucan of yeast zymosan A from Saccharomyces cerevisiae; BAR: β-glucan of barley; BGF: β-glucan fraction of carpophores of Phellinus baumii. The values are expressed as the average ± SD. *p < 0.01, **p < 0.005.
3.3. Production of ANP by corin in mice
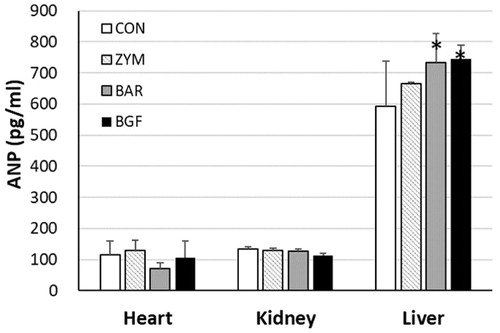
Corin or pro-ANP convertase is a membrane-bound protein enzyme with a trypsin-like protease domain. The enzyme domain breaks down pro-ANP (126 amino acids) into ANP (28 amino acids) and an N-terminal peptide (98 amino acids). Since corin expression was induced by the treatment of mice with β-glucans of P. baumii, yeast, and barley, ANP production should be increased in tissues such as liver, heart, and kidney. The amount of ANP produced was measured using an anti-ANP polyclonal antibody in an ELISA immunoassay. The amount of ANP from the heart tissue of mice treated with zymosan, barley, and BGF for seven days was 129.8 ± 31.9 (112.5% of the control value of 115.3 ± 44.2), 71.6 ± 17.1 (62.1% of the control), and 106.8 ± 52.0 pg/mL (92.6% of the control), respectively. The amount of ANP produced by the kidney following zymosan, barley, and BGF treatment was 128.5 ± 7.2 (96.6% of the control value of 133.0 ± 8.1), 127.9 ± 6.4 (96.2% of the control), and 112.9 ± 6.7 pg/mL (84.9% of the control), respectively. The liver ANP produced from zymosan, barley, and BGF treatment was 664.7 ± 5.4 (111.9% of the control value of 593.7 ± 144.2), 733.0 ± 92.8 (123.5% of the control), and 746.1 ± 43.1 pg/mL (125.7% of the control), respectively (). The ELISA immunoassay showed that ANP production was significantly increased in the liver tissue of mice administered barley β-glucans (123.5%) or BGF (125.7%) for seven days.
Figure 2. Effects of β-glucans on the production of ANP in various mice tissues. ICR mice (n = 6) were orally administered zymosan, barley β-glucan, and BGF at 100 mg/kg/day for seven consecutive days. ANP peptides were extracted from the heart, kidney, and liver of mice with protein extraction kit solution by lysing with a bead-beater homogenizer. The ANP concentrations were determined by an ELISA kit for mouse ANP by measuring the OD450 with a spectrophotometer. CON: control; ZYM: β-glucan of yeast zymosan A from Saccharomyces cerevisiae; BAR: β-glucan of barley; BGF: β-glucan fraction of carpophores of Phellinus baumii. The values are expressed as the average ± SD. *p < 0.01.
3.4. Urinary excretion of sodium by ANP in mice
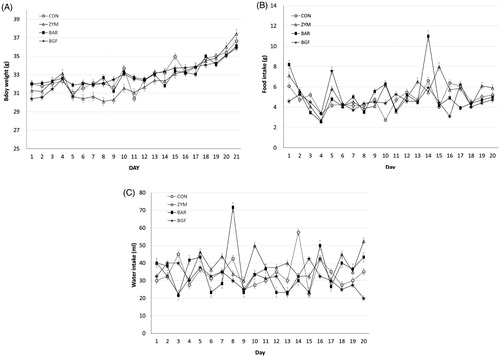
ANP has various biological activities, such as natriuresis, diuresis, vasodilation, smooth muscle relaxation, and anticancer activities. Among the various activities of ANP, we were interested in natriuresis, which is related to the antihypertensive mechanism of β-glucan. The mice were orally administered zymosan or barley β-glucans or BGF for 21 days at 100 mg/kg/day along with a 10% sodium diet (high-salt diet). Body weight, food intake, and water intake were measured daily during the 21 days of oral treatment with the β-glucans. Body weight changes of the control, zymosan, barley, and BGF groups over 21 days were 4.65, 6.17, 4.10, and 5.48 g, respectively. The total food intake amounts in the control, zymosan, barley, and BGF groups were 96.4, 104.3, 100.3, and 94.0 g, respectively, during the 21 days of oral β-glucan administration to the mice. Water intake in the control, zymosan, barley, and BGF groups was 677.5, 751.2, 698.3, and 632.5 g, respectively (). When the mice were fed 10% sodium diet instead of normal 1% sodium diet, neither food and water intake nor body weight was affected during the 21 days of administration of zymosan and barley β-glucans and BGF.
Figure 3. Effects of orally administered β-glucans on changes in body weight and food and water intakes in high-salt diet-fed mice. ICR mice (n = 4) kept in metabolic cages and were orally administered zymosan, barley β-glucan, and BGF at 100 mg/kg/day for seven consecutive days. Mouse body weight (A), food (B) and water (C) intakes were measured daily. CON: control; ZYM: β-glucan of yeast zymosan A from Saccharomyces cerevisiae; BAR: β-glucan of barley; BGF: β-glucan fraction of carpophores of Phellinus baumii. The values are expressed as the average ± SD.
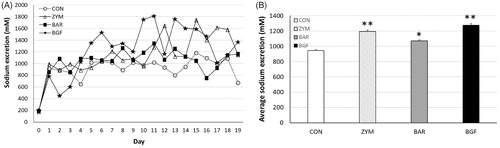
We next measured the amount of urinary sodium excreted by mice fed 1% and 10% sodium diets. The mice fed 1% normal diet and BGF or zymosan and barley β-glucans showed higher urinary sodium excretion during the first four days compared to the control group. From the 5th day, the barley group showed a natriuresis pattern similar to the control group. In contrast, the BGF and zymosan groups showed higher natriuresis than the control group. When we calculated the average urinary sodium excretion of the treated groups fed 1% sodium diet for eight days, the average sodium values of the zymosan, barley, and BGF groups were 567.8 ± 42.8 (191.2% of the control value of 296.9 ± 23.5), 391.3 ± 21.1 (131.8% of the control), and 639.4 ± 26.7 (215.3% of the control) mM sodium, respectively (). During the first four days mice fed the 10% sodium diet, the zymosan and barley groups showed similar natriuresis patterns as the control. The BGF group showed a lower natriuresis pattern than the control group during this period. However, after the ninth day, the BGF and zymosan groups showed much higher excretion of sodium than the control group until the 19th day of treatment. In mice fed the 10% sodium diet, the average sodium values of the zymosan, barley, and BGF groups were 1,195.0 ± 17.5 (126.5% of the control value of 944.9 ± 10.6), 1,072.4 ± 12.2 (113.5% of the control) and 1,276.7 ± 22.1 (135.1% of the control) mM sodium, respectively (). Overall, both zymosan β-glucan and BGF increased urinary sodium excretion in both the normal and high-sodium diet-fed mice.
Figure 4. Effect of orally administered β-glucan on sodium excretion in normal-salt-fed mice. β-Glucans of zymosan, barley, and BGF were orally administered at 100 mg/kg/day to ICR mouse (n = 4) for eight days by feeding with normal-salt diet. (A) Urinary sodium excretion in mice fed a normal-salt diet for eight days. (B) Average urinary sodium excretion in mice fed a normal-salt diet for eight days. The values are expressed as the average ± SE. *p < 0.01, **p < 0.005.
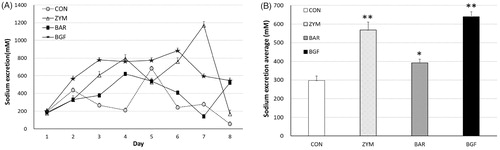
Figure 5. Effect of orally administered β-glucan on sodium excretion in high-salt-fed mice. β-Glucans of zymosan, barley, and BGF were orally administered at a concentration of 100 mg/kg/day to ICR mouse (n = 4) for 21 days by feeding with high-salt diet. (A) Sodium excretion in urine of high-salt fed mice for 21 days; (B) Average sodium excretion in urine of high-salt fed mice for 21 days. Values are average ± SE. *p < 0.01, **p < 0.005.
4. Discussion
In this report, we showed that corin gene expression was upregulated by multiple oral administrations of β-glucans obtained from the carpophores of several mushrooms, including P. baumii, P. linteus, and G. lucidum. We also found increased production of ANP in the liver of mice treated orally with β-glucans from yeast, barley, and mushrooms. To demonstrate the antihypertensive action of those β-glucans, we determined the amount of sodium excreted in mouse urine and showed that the urinary excretion of sodium was increased both in normal and high-salt diet-fed mice by treatments with various β-glucans, presumably through the production of ANP by the increased expression of corin. In our microarray assay, an SOA of BGF did not induce corin in the mouse liver (0.4-fold change), but MOA for seven days induced a 5.8-fold change compared to the untreated control group. The major function of corin is to cleave pro-ANP to ANP, which has natriuretic activity, thereby lowering blood pressure. Our finding that corin was induced by β-glucans led us to investigate the antihypertensive mechanism of β-glucans. Some mushroom β-glucans have been reported to have antihypertensive activities. The β-glucan of G. lucidum reduced systolic pressure in hypertensive patients [Citation11]. Mushroom powders from Lentinus edodes and Grifola frondosa decreased blood pressure in spontaneously hypertensive rats [Citation17]. No studies have yet reported the antihypertensive effect of β-glucan in relation to corin expression or natriuresis in animals or humans.
Corin is synthesized as a zymogen that is activated by proprotein convertase subtilisin/kexin-6 (PCSK6) [Citation18]. Although most abundantly expressed in the atria of the heart, corin can also be expressed in non-cardiac tissues, such as kidneys, bones, and pregnant uteri [Citation5,Citation6]. In our experiment, corin induction was detected in the liver of mice by an mRNA microarray assay. When we compared the expression of corin induced by β-glucans in mice tissues, the expression was much stronger in the kidneys than in the liver. Renal corin expression is important because decreased renal corin expression contributes to sodium retention in proteinuric kidney diseases [Citation19]. Although the corin gene is expressed in the human heart and kidney, corin protein was reported to be expressed in the normal human heart, kidney, and circulating blood. Interestingly, circulating corin protein was significantly higher in men than women and was weakly positively correlated with age [Citation20]. It has been reported that high levels of circulating corin were associated with a risk of cardiovascular diseases, such as hypertension, heart failure, myocardial infarction, preeclampsia, and stroke, suggesting that circulating corin might serve as a biomarker for cardiovascular diseases [Citation21]. In our experiment, the induction of corin mRNA gene expression by β-glucan was confirmed in mice liver. However, we do not have data on the levels of circulating corin protein induced by β-glucan treatment.
The proper regulation of corin expression in the membrane is important. Transcription factor GATA-4 is a major factor in the control of corin expression in cardiomyocytes [Citation22]. Expressed corin and PCSK6 in the nucleus move separately to the cell surface, where PCSK6 activates zymogen corin to active corin. To downregulate corin activity in the membrane, ectodomain shedding of corin occurs in the cardiomyocytes. After shedding, circulating corin loses its activity due to cleavage of the active site, which can be a protective mechanism of the proteolytic enzyme of corin. Thus, for the regulation of corin activity, protease-mediated shedding from the cell surface is essential for reducing corin activity in the cardiomyocytes. However, under pathophysiological conditions, corin expression is downregulated in diabetic cardiomyopathy [Citation23], heart failure [Citation24], and radiation-induced heart injury [Citation25]. Therefore, high corin gene and protein expression are important for the prevention of hypertension and heart failure.
In our experiment, ANP levels, the corin-mediated cleavage product, were increased only in the liver, not in the heart and kidney (). The liver consistently expressed both corin and ANP by treatment with the β-glucans. As the major tissue producing ANP is the atria of the heart, the ANP increased by β-glucan in the liver might come from the heart. Atrial ANP could be secreted in response to an increase in blood pressure or blood volume. To regulate blood pressure, increased ANP can target kidneys and cause a decrease in sodium reabsorption, resulting in decreases in blood fluid and blood pressure by sodium excretion. In addition, ANP also affects blood vessels by promoting vasodilation. In our 1% normal sodium diet study, β-glucans increased sodium excretion after three days of the treatment (). This means that the induction of corin and the production of ANP in mice organs may require two or three days. Since urinary sodium excretion was increased in mice fed 1% sodium diet, we wanted to investigate the effect of β-glucan in mice fed a 10% sodium diet. In the high-sodium diet condition, β-glucans did not increase sodium excretion until day 9 of the treatment. Treatment with β-glucans clearly increased urinary sodium excretion from day 10, with increased excretion following treatment with zymosan or BGF. Although we did not measure blood pressure, increased urinary sodium excretion by treatment with β-glucans might contribute to lowering blood pressure. Among the β-glucans tested, yeast and mushroom β-glucans showed the highest urinary sodium excretions. Zymosan was reported to induce hypotension instantly in a rat model. However, it was caused by acute inflammation due to the activation of the Syk/IĸB‐α/NF‐ĸB signaling pathway by zymosan treatment [Citation26]. In our data, barley β-glucan showed the lowest excretion of urinary sodium both in mice fed the 1% and 10% sodium diets. In addition to the ANP, other peptides from the N-terminal peptide of pro-ANP, including LANP and VSDL, might have contributed to increasing the urinary sodium excretion in our mice experiment. Among the natriuretic hormones of the N-terminal peptide of pro-ANP, ANP was reported to have the strongest natriuresis properties and the other hormones (LANP and VSDL) showed slightly weaker natriuresis than ANP [Citation27].
The underlying antihypertensive mechanisms of β-glucan have not been well elucidated. However, insulin sensitivity improved by β-glucan may contribute to the reduction in systolic blood pressure (SBP) and diastolic blood pressure (DBP) [Citation28]. Blood pressure regulation by β-glucan also could be associated with the excretion of vascular endothelial growth factor (VEGF), which induces endothelium-dependent vasodilation [Citation29]. As the ANP receptor signal is associated with vasodilation, blood pressure could be regulated by the specific binding of polysaccharide with the guanyl cyclase (GC)-linked ANP receptor in glomeruli [Citation12]. The induction of NO and inhibition of ACE II could be a mechanism by which blood pressure is regulated by polysaccharides [Citation30]. Finally, β-glucan-induced gut microbiota alteration could be a potential mechanism of reduced cardiovascular disease risk [Citation31].
Our data showed that corin and ANP, as well as urinary sodium excretion, were increased in mice by β-glucan treatment. The orally administered β-glucans of zymosan, barley, and P. baumii induced the corin gene in mouse liver. β-Glucans of other mushrooms, including P. linteus and G. lucidum, also induced corin gene expression in the kidney (). We also observed the increased production of ANP in mouse liver by β-glucans from zymosan, barley, and P. baumii by ELISA assays (). All the β-glucans tested increased the urinary excretion of sodium, both in mice fed 1% normal and 10% high-sodium diets ( and ). Therefore, we propose that corin, ANP, and natriuresis are the major antihypertensive mechanisms of β-glucan in mice (). Corin induced by β-glucan acts at the beginning of ANP cascade signaling to regulate salt-water balance and blood pressure. The increased ANP binds to the ANP receptor, and the intensified receptor’s signal lowers blood pressure by promoting the excretion of sodium from the kidney into the urine (natriuresis), diuresis and vasodilation, which lower blood volume and pressure. Vasodilation due to cGMP by ANP could be an additional antihypertensive function, as reported by Chen et al. [Citation32]. They reported that ANP induced a substantial, dose-dependent, rapid, and sustained increase in cGMP, whereas NO yielded a weak and transient increase in cGMP.
Figure 6. Proposed corin enzyme induced antihypertension mechanism of β-glucan. Oral administration of β-glucan induced corin in the liver or kidney, which cleaves pro-ANP into biologically active atrial natriuretic peptide (ANP) and N-terminal peptide (NTP). Upon the binding of ANP to its receptor, signals of the receptor promote natriuresis, diuresis, and vasodilation, which lower blood volume and pressure. Corin, a trypsin-like serine protease, induced by β-glucan, acts at the beginning of the ANP pathway to regulate the salt-water balance and blood pressure. The finding of ANP-initiated cGMP vasodilation was reported elsewhere [Citation32]. AAs: amino acids; ANP: atrial natriuretic peptide; NTP: N-terminal peptide.
![Figure 6. Proposed corin enzyme induced antihypertension mechanism of β-glucan. Oral administration of β-glucan induced corin in the liver or kidney, which cleaves pro-ANP into biologically active atrial natriuretic peptide (ANP) and N-terminal peptide (NTP). Upon the binding of ANP to its receptor, signals of the receptor promote natriuresis, diuresis, and vasodilation, which lower blood volume and pressure. Corin, a trypsin-like serine protease, induced by β-glucan, acts at the beginning of the ANP pathway to regulate the salt-water balance and blood pressure. The finding of ANP-initiated cGMP vasodilation was reported elsewhere [Citation32]. AAs: amino acids; ANP: atrial natriuretic peptide; NTP: N-terminal peptide.](/cms/asset/9457cd38-a42d-468e-87de-65b78d9c8122/tmyb_a_1812150_f0006_c.jpg)
The intestinal absorption mechanism of high molecular weight β-glucan has long been reported. Rice et al. [Citation33] demonstrated that orally administered fluorescent glucans could be absorbed either by intestinal epithelial cells (IECs) or M cells of gut-associated lymphoid tissue (GALT). The internalization of glucan by M cells within Payer’s Patches was dectin-1 and toll-like receptor (TLR)2-dependent, but internalization by IEC was not dectin-1-dependent. Orally administered β-glucans were translocated from the gastrointestinal tract into the systemic circulation without any transporters. In another report, β-glucans from barley and yeast whole glucan particles (WGP) were used in mice. Orally administered β-glucans were taken up by macrophages that transported them to the spleen, lymph nodes, and bone marrow. Sandvik et al. [Citation34] reported that significant levels of β-glucans were absorbed into the circulation and detected in serum in experimental rats. When the serum concentration resulting from oral administration was compared to those from subcutaneous injections, the subcutaneous injection group had levels approximately 40-fold higher, despite a 10-fold lower dose. Since humans lack cleavage enzymes, intact β-glucans arriving in the intestine could be absorbed by sampling through M cells of the Peyer’s patches without any digestion. This could be the reason why orally administered β-glucans show biological activities in concentration-independent patterns [Citation9]. Although dectin-1 and complement receptor 3 (CR3) are known as β-glucan receptors [Citation35], we did not reveal how glucans induced corin gene expression in this report. Further research is necessary to elucidate the exact mechanism by which glucans induce corin gene expression.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Papadopoulos DP, Mourouzis I, Thomopoulos C, et al. Hypertension crisis. Blood Press. 2010;19:328–336.
- Padmanabhan S, Aman A, Dominiczak AF. Genomics of hypertension. Pharmacol Res. 2017;121:219–229.
- Ji W, Foo JN, O'Roak BJ, et al. Rare independent mutations in renal salt handling genes contribute to blood pressure variation. Nat Genet. 2008;40:592–599.
- Min YS, Yoon HJ, Je HD, et al. Endothelium independent effect of pelargonidin on vasoconstriction in rat aorta. Biomol Ther (Seoul). 2018;26:374–379.
- Yan W, Sheng N, Seto M, et al. Corin, a mosaic transmembrane serine protease encoded by a novel cDNA from human heart. J Biol Chem. 1999;274:14926–14935.
- Cui Y, Wang W, Dong N, et al. Role of corin in trophoblast invasion and uterine spiral artery remodelling in pregnancy. Nature. 2012;484:246–250.
- Serafino A, Pierimarchi P. Atrial natriuretic peptide: a magic bullet for cancer therapy targeting Wnt signaling and cellular pH regulators. Curr Med Chem. 2014;21:2401–2409.
- Vesely DL. New anticancer agents: hormones made within the heart. Anticancer Res. 2012;32:2515–2521.
- Batbayar S, Lee DH, Kim HW. Immunomodulation of fungal β-glucan in host defense signaling by dectin-1. Biomol Ther (Seoul). 2012;20:433–445.
- Bashir KMI, Choi JS. Clinical and physiological perspectives of β-glucans: the past, present, and future. Int J Mol Sci. 2017;18:1906.
- Sugita P, Fadlan MR, Sargowo D, et al. β-1,3/1,6-d-Glucan of Indonesian Ganoderma lucidum mycelium extract reduces systolic blood pressure & inflammation in hypertensive patients. Hypertension. 2019;74:AP2071.
- Sano T, Imura R, Morishita Y, et al. HS-142-1, a novel polysaccharide of microbial origin, specifically recognizes guanylyl cyclase-linked ANP receptor in rat glomeruli. Life Sci. 1992;51:1445–1451.
- Zhao C, Yang C, Liu B, et al. Bioactive compounds from marine macroalgae and their hypoglycemic benefits. Trends Food Science Technol. 2018;72:1–12.
- Yoon SK, Sung SK, Lee DH, et al. Tissue inhibitor of metalloproteinase-1 (TIMP-1) and IL-23 induced by polysaccharide of the black hoof medicinal mushroom, Phellinus linteus (Agaricomycetes). Int J Med Mushrooms. 2017;19:213–223.
- Sainkhuu B, Park BS, Kim HW. Induction of flavin-containing monooxygenase in mice by oral administration of Phellinus baumii (Agaricomycetes) extract. Int J Med Mushrooms. 2016;18:793–806.
- Sung SK, Batbayar S, Lee DH, et al. Activation of NADPH oxidase by β-glucan from Phellinus baumii (Agaricomycetes) in RAW 264.7 cells. Int J Med Mushrooms. 2017;19:957–965.
- Kabir Y, Yamaguchi M, Kimura S. Effect of shiitake (Lentinus edodes) and maitake (Grifola frondosa) mushrooms on blood pressure and plasma lipids of spontaneously hypertensive rats. J Nutr Sci Vitaminol. 1987;33:341–346.
- Chen S, Cao P, Dong N, et al. PCSK6-mediated corin activation is essential for normal blood pressure. Nat Med. 2015;21:1048–1053.
- Polzin D, Kaminski HJ, Kastner C, et al. Decreased renal corin expression contributes to sodium retention in proteinuric kidney diseases. Kidney Int. 2010;78:650–659.
- Ichiki T, Huntley BK, Heublein DM, et al. Corin is present in the normal human heart, kidney, and blood, with pro-Β-type natriuretic peptide processing in the circulation. Clin Chem. 2011;57:40–47.
- Yu R, Han X, Zhang X, et al. Circulating soluble corin as a potential biomarker for cardiovascular diseases: a translational review. Clin Chim Acta. 2018;485:106–112.
- Pan J, Hinzmann B, Yan W, et al. Genomic structures of the human and murine corin genes and functional GATA elements in their promoters. J Biol Chem. 2002;277:38390–38398.
- Pang A, Hu Y, Zhou P, et al. Corin is down-regulated and exerts cardioprotective action via activating pro-atrial natriuretic peptide pathway in diabetic cardiomyopathy. Cardiovasc Diabetol. 2015;14:134.
- Chen S, Sen S, Young D, et al. Protease corin expression and activity in failing hearts. Am J Physiol Heart Circ Physiol. 2010;299:H1687–H1692.
- Kim EJ, Lee J, Jung YR, et al. Involvement of corin downregulation in ionizing radiation-induced senescence of myocardial cells. Int J Mol Med. 2015;35:731–738.
- Unsal D, Kacan M, Temiz-Resitoglu M, et al. The role of Syk/IĸB-α/NF-ĸB pathway activation in the reversal effect of BAY 61-3606, a selective Syk inhibitor, on hypotension and inflammation in a rat model of zymosan-induced non-septic shock. Clin Exp Pharmacol Physiol. 2018;45:155–165.
- Vesely DL. Which of the cardiac natriuretic peptides is most effective for the treatment of congestive heart failure, renal failure and cancer? Clin Exp Pharmacol Physiol. 2006;33:169–176.
- Keenan JM, Pins JJ, Frazel C, et al. Oat ingestion reduces systolic and diastolic blood pressure in patients with mild or borderline hypertension: a pilot trial. J Fam Pract. 2002;51:369.
- Zhong J, Urch B, Speck M, et al. Endotoxin and β-1,3-d-glucan in concentrated ambient particles induce rapid increase in blood pressure in controlled human exposures. Hypertension. 2015;66:509–516.
- Zhu HB, Geng MY, Guan HS, et al. Antihypertensive effects of D-polymannuronic sulfate and its related mechanisms in renovascular hypertensive rats. Acta Pharmacol Sin. 2000;21:727–732.
- Wang Y, Ames NP, Tun HM, et al. High molecular weight barley β-glucan alters gut microbiota toward reduced cardiovascular disease risk. Front Microbiol. 2016;7:129.
- Chen H, Levine YC, Golan DE, et al. Atrial natriuretic peptide-initiated cGMP pathways regulate vasodilator-stimulated phosphoprotein phosphorylation and angiogenesis in vascular endothelium. J Biol Chem. 2008;283:4439–4447.
- Rice PJ, Adams EL, Ozment-Skelton T, et al. Oral delivery and gastrointestinal absorption of soluble glucans stimulate increased resistance to infectious challenge. J Pharmacol Exp Ther. 2005;314:1079–1086.
- Sandvik A, Wang YY, Morton HC, et al. Oral and systemic administration of β-glucan protects against lipopolysaccharide-induced shock and organ injury in rats [published correction appears in Clin Exp Immunol. 2007 Aug;149(2):399]. Clin Exp Immunol. 2007;148:168–177.
- Brown GD, Gordon S. Immune recognition. A new receptor for beta-glucans. Nature. 2001;413:36–37.

