ABSTRACT
Eating habits as well as physical exercise are very important for a healthy lifespan. Ramadan-type fasting, which is food and water avoidance during the daylight period for four weeks, has drawn attention due to its positive impacts on metabolism and health. The aim of this study was to compare the blood and urine catecholamine (CA) levels in fasting and non-fasting rats, in terms of stress response. A total of 20 male rats were randomly divided into a fasting group and a control group. Four weeks later, blood and urine samples were taken after decapitation. Analysis of CAs was done using high-performance liquid chromatography with florescence detection (HPLC-FLD). The dopamine (DA), adrenaline (ADR) and noradrenaline (NA) blood and urine concentrations were found to be higher in the fasting group compared to the control group, but the difference was statistically significant only for the blood DA levels (p < 0.05). In the fasting group, the blood values of ADR and NA correlated with each other but not with the DA levels, whereas there was correlation among the urine levels of DA, ADR and NA. In the control group, the blood and urine values of DA, ADR and NA correlated with each other. The differences observed in the blood and urine CAs indicate a specific regulation of CAs in Ramadan-type fasting, which needs to be investigated thoroughly in future studies.
Introduction
It is a common fact that inadequate eating habits can affect a series of biochemical processes such as coping with stress, excretion of toxic materials, inflammatory response and may result in increased risk of allergic diseases and metabolic disorders.[Citation1] On the other hand, having a regular and balanced diet, avoiding overeating and performing religious fasting can regulate the metabolism, decrease inflammatory processes, improve reproductive functions and prevent chronic diseases.[Citation2,Citation3]
Fasting is abstinence from food and/or water, completely or to some degree, for a defined period of time and mostly implemented as a religious obligation. The fasting styles and rules vary from religion to religion. In this context, Ramadan fasting involves refraining from food and water from dawn till sunset for Muslims throughout the Hijri month, Ramadan.[Citation4] The effects of this fasting style have recently attracted major interest, since its positive impacts on human metabolism and health have become widely known over the past three decades.[Citation5–8]
In the early stages of fasting, hepatic glycogen stores are used for energy. However, if the fasting is prolonged, the fat stored in the adipose tissue begins to be utilized. These processes are regulated by a series of hormonal changes such as reduction in insulin levels and increase in the levels of glucagon, catecholamines (CAs), growth hormone, thyroid-stimulating hormone and corticosteroids.[Citation9] Additionally, the lack of access to food and water when needed might lead to a form of stress in the organism and activation of stress-related mechanisms. One of the two major stress hormones is a CA called adrenaline (ADR), which is rapidly released into the bloodstream together with noradrenaline (NA) and generates the ‘fight-or-flight’ response.[Citation10]
CAs, the catechols with two adjacent hydroxyl groups on a benzene ring, are neurotransmitters and hormones of the adrenomedullary, sympatho-neural and brain catecholaminergic systems.[Citation11] These systems are among the systems that have effective responses and provide a rapid reaction to any stressor. Moreover, they have a critical role in adaptation to acute and chronic stresses as well as in behavioural and physical well-being.[Citation10–12] CAs, ADR, NA and dopamine (DA) are secreted seconds after the stimulation and are rapidly degraded.[Citation12] The release of CAs is a key initial response to stressors, which is affected by the characteristics of the stimuli and is regulated by the hypothalamic–pituitary–adrenal axis. The release is followed by an increase in the expression of genes encoding the CA-synthesizing enzymes via the transcriptional mechanisms that occur in the adrenal medulla and the locus coeruleus.[Citation13]
Stress-triggered responses of CAs are generally studied in the context of immobilization and cold stress; however, fasting has been insufficiently investigated.[Citation14,Citation15] The effect of fasting on stress-related mechanisms including CAs depends on the type of the food and water restriction and the duration of abstinence. In contrast to the general consideration, it has been reported that 48-hour fasting markedly suppresses the activity of the sympathetic nervous system, which might lead to prolongation of survival during famine, but concurrently stimulates the adrenal medulla (reviewed in [Citation16]). Similarly, in a recent study, it has been shown that intermittent fasting increased the hypothalamic NA content in diet-induced obese male mice.[Citation17] Nevertheless, the findings regarding the exact variation in CAs levels in fasting in terms of stress are limited. In this study, we aimed to compare the blood and urine CA levels of fasting and non-fasting rats in order to investigate whether and how the circulating CAlevels and their urinary excretion vary in Ramadan-type fasting.
Materials and methods
Animals
A total of 20 male, 12-week-old Swiss Albino rats weighing 220–261 g were used in the study. All the rats were housed in a temperature-controlled (22 °C —24 °C) facility with daylight allowance. The rats were allowed to adapt to their environment for a period of one week prior to the study and then were randomly divided into two equal groups: a fasting group and a control group. In the fasting group, a Ramadan-type fasting model was employed: the rats were kept on a fasting regimen with total abstinence from food and water from 7.00 am to 5.00 pm and were fed ad libitum during the remaining part of the day. The rats in the control group were fed ad libitum for four weeks. At the beginning of week five, in the morning, the rats in both groups were anesthetized by intraperitoneal injection of ketamine/xylazine (100/7.5 mg/kg) and were decapitated. Blood samples were immediately collected from the left ventricle and were inserted into gel tubes with silica particles, centrifuged at 1500 g for 10 min and placed into 2 mL Eppendorf tubes. Urine samples were collected from the bladder and were also placed into 2 mL Eppendorf tubes. Both the blood and urine samples were stored at −80 °C until analysis.
This work was conducted at the Medical Faculty of Dicle University with the formal approval of the local animal care committees. All the procedures and protocols were approved by the Dicle University Animal Experiments Local Ethics Committee.
Catecholamine analyses
High performance liquid chromatography with florescence detection (HPLC-FLD) was used for quantitative analysis of the three CAs (ADR, NA and DA) in blood (serum) and urine samples. In addition to this method, a validated CA dual kit (Eureka Lab Division, Code Z10550) was utilized for analysis by fluorimetry, following the manufacturer's instructions. Briefly, the analytes were derivatized with two specific reagents and incubated at 70 °C for 15 minutes, and the derivatized solution was injected in HPLC columns. A Shimadzu Nexera HPLC system equipped with an RF-20A XS fluorescence detector was used for the analysis.
The liquid chromatography was equipped with LC-30AD binary pumps, a DGU-20A3R degasser, a CTO-10ASvp column oven and an SIL-30AC autosampler. The chromatographic separation was performed on a C18 reversed-phase Inertsil ODS-4 (150 × 4.6 mm, 3 m) analytical column. The column temperature was fixed at 40 °C. The elution gradient consisted of mobile phase A (water, 5 mmol/L ammonium formate and 0.1% formic acid) and mobile phase B (methanol, 5 mmol/L ammonium formate and 0.1% formic acid). The gradient programme that was applied contained the following proportions of solvent B as (time–minute, B%): (0 min, 40%), (20 min, 90%), (23.99 min, 90%), (24 min, 40%) and (29 min, 40%). The solvent flow rate was maintained at 0.5 mL/min and the injection volume was set at 4 L.[Citation18]
Blood and urine concentrations of ADR, NA and DA were analyzed both in fasting and non-fasting rats for the purpose of comparative analysis. In the control group, the amount of urine in Sample 4 was too small for analysis, which is why the CAs could not be calculated by the device. For this reason, the analyses were only performed for the remaining nine urine samples. The concentrations of ADR, NA and DA in the blood samples were presented in [ng/L] and those in the urine samples, in [µg/L].
Statistical analysis
Data were analyzed by SPSS for Windows (version 18.0, SPSS, Chicago, IL, USA). The data presented in figures and tables are median values with min–max values. Mann–Whitney U Test was used for comparative analysis of the fasting and control groups, and their effect sizes were calculated. To evaluate the correlations between the concentrations of the studied CAs in blood and urine, Spearman's rho-correlation was used. Values of p < 0.05 were considered significant.
Results and discussion
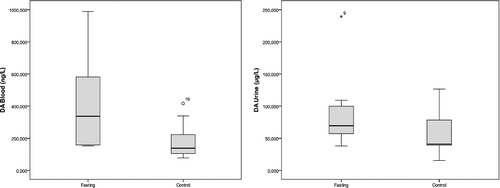
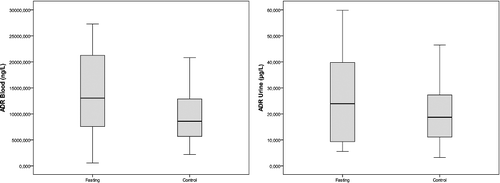
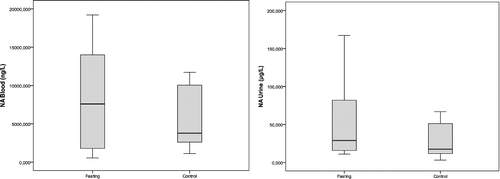
The stress response of an organism is a unique phenomenon, since it can either be lifesaving during a threat, or may cause undesirable consequences if prolonged.[Citation15,Citation19] Moreover, studies have shown that although the stress response is thought to be identified, more aspects of this response have been found that need elucidation. Considering the complexity of the urine and blood serum, it is difficult to detect and quantify the CAs in these matrices. However, after derivatizing the CAs, it is possible to detect them in parts per trillion (pg/mL) levels with HPLC fluorescence detection since fluorescence detectors are probably the most sensitive ones among the existing modern HPLC detectors.[Citation20,Citation21] presents the medians and comparisons of the blood CAs levels in the two studied groups, and presents the medians and comparisons of the urine CAs levels in these groups. Crude HPLC-FLD data are given in the Online Supplementary Appendix. The HPLC-FLD method has been validated in terms of linearity, precision and accuracy, recovery, matrix effect, reproducibility of retention time and stability [Citation22], and the method is known to be simple, accurate and suitable for routine analysis.[Citation23] Moreover, it allows complete purification of CAs by an organic and specific complexing reagent and allows having a complete extraction using a C-18 solid phase column. The results showed that after four weeks of fasting, the DA, ADR and NA blood and urine concentrations were higher in the fasting group compared to the control group but only the differences in the blood DA levels were statistically significant (p < 0.05) with a large effect size (r = 0.56). Despite the non-significant increase in the urine DA and NA levels in the fasting group, both of these values had a medium effect size (r = 0.36 and r = 0.28, respectively).
Table 1. Comparison of blood catecholamines.
Table 2. Comparison of urine catecholamines.
As Kvetnansky et al. [Citation12] emphasized, stressful stimuli evoke complex endocrine as well as autonomic and behavioural responses that are extremely variable and specific, depending on the type and the nature of the stressors. Stress also activates the transcription and gene expression of CA enzymes, particularly tyrosine hydroxylase, which is the rate-limiting enzyme in CA synthesis and has an important impact in terms of stress response. However, the exact mechanism of this activation remains unknown.[Citation10,Citation12,Citation15,Citation24–27]
Activation of stress-dependent systems is likely to allow the improvement of homeostasis adjustment and to enhance the survival; therefore, it is highly important.[Citation28] Exposure to any kind of stress only once or for a short time could cause a three- to seven-fold increase in enzyme activity above basal levels.[Citation12,Citation15,Citation25] Even single, prolonged or repeated stress exposures could generate different responses and activate various pathways.[Citation12,Citation29] Sensitive measurement tools such as HPLC devices allow the detection of small amounts of CAs in blood and urine samples as well as the monitoring of the changes during the stress. However, it is highly difficult to understand the exact mechanism of these changes. In the analysis of CAs, sympatho-neural, sympatho-adrenomedullary, dihydroxyphenylalanine (DOPA)–DA autocrine/paracrine and hypothalamic–pituitary–adrenocortical pathways must be taken into consideration.[Citation12]
Box plot graphics of the blood and urine concentrations of DA, ADR and NA observed in the two studied groups are shown in , and
Of the adrenal medulla CAs, 80% are ADR, 16% are NA and only 4% are DA because phenylethanolamine-N-methyltransferase (PNMT), the enzyme that converts NA to ADR, is mainly localized in the adrenal medulla and is both hormonally and neurally controlled.[Citation25,Citation30] Stress also elicits a rise in PNMT, resulting in increased ADR levels.[Citation10] Plasma ADR levels usually reflect neural outflow to the adrenal medulla and these levels increase during a series of conditions such as hypoglycemia, asphyxia and distress, more than NA.[Citation11] Our findings supported this suggestion, since the ADR levels were greater than the NA levels. This finding suggests that the adrenomedullary activation during fasting is greater than that of the sympathetic noradrenergic system, as approximately 70%–80% of the blood NA comes from sympathetic noradrenergic nerves, particularly around the blood vessels.[Citation11,Citation12] However, in our study, the increase in ADR and NA levels in the fasting group were not as significant as the increase in the DA levels.
Landsberg [Citation16] reported that, in terms of NA, the sympathetic nervous system is suppressed by fasting and stimulated by overfeeding. The author also added that, although the sympathetic nervous system is suppressed, the adrenal medulla is stimulated. Ramadan-type fasting also fits into this model, since the blood pressure remarkably decreases during the fasting period.[Citation16] Accordingly, the increased fasting NA levels in our study are likely to originate from the adrenal medulla and thus, the suppression is likely to occur in the sympathetic noradrenergic system; however, when high DA levels are considered, this model does not appear to be applicable for the dopaminergic nerves. Only a small fraction of CAs that is released from the storage vesicles of the sympathetic nerves enters into the bloodstream due to neuronal uptake.[Citation31] In fasting, the release from the storage vesicles is likely to increase as a result of the overproduction of CAs, especially of DA, or the decreased neuronal uptake; however, the latter is less likely to occur.
It is widely known that the release of ADR and NA into the bloodstream rapidly increases with stressful stimulation; however, in contrast to our findings, it is generally accepted that the DA levels remain unchanged.[Citation12,Citation15,Citation24] The saliently increased activity occurring during the first encounter with a stressor continues if the stimulation is repeated or prolonged but the amplitude of the response decreases. Moreover, it is possible to observe high levels of enzymes days after the cessation of the stress.[Citation24] The non-significant increase in the levels of ADR and NA in the fasting group in our study may result from the decreased but ongoing response. Similarly, Tsigos and Chrousos [Citation28] reported that as the severity of the stressor increased, the specific adaptive response was progressively lost. Another issue suggested by the same authors is that the increased ADR levels reduce the neurovegetative functions such as eating.[Citation28] In fasting, the increased ADR levels might also help to withstand the desire to eat.
In the fasting group, there was correlation between the blood ADR and NA levels (r = 0.842, p < 0.01) but they did not correlate with the DA levels (r = 0.515, p > 0.05; r = 0.624, p > 0.05, respectively). The urine levels of DA, ADR and NA in this group also correlated with each other (r = 0.794, p < 0.01 for DA and ADR; r = 0.891, p < 0.01 for DA and NA; r = 0.891, p < 0.01 for ADR and NA).
In the control group, there was correlation among the blood levels of the three studied CAs (r = 0.830, p < 0.01 for DA and ADR; r = 0.745, p < 0.05 for DA and NA; r = 0.964, p < 0.01 for ADR and NA). Correlations were also observed among the urine levels of DA, ADR and NA in this group (r = 0.883, p < 0.01 for DA and ADR; r = 0.917, p < 0.01 for DA and NA; r = 0.883, p < 0.01 for ADR and NA).
DA concentrations are considered to be similar to ADR concentrations.[Citation11] The plasma concentrations of free DA increase in association with events that increase the sympathetic tone, though to a much lesser extent compared to NA or ADR. [Citation32] Since most of the DA in the blood is synthesized in the brain and is derived from the sympathetic nerves as well as the non-neuronal gut cells and the circulating L-3,4-dihydroxyphenylalnine (L-DOPA), the significantly increased concentrations of DA in the fasting group cannot be explained by the adrenomedullary stimulation induced by fasting. In fasting, the stress response of the sympathetic dopaminergic nerves might be more pronounced.[Citation11] This implies a contribution of non-neuronal gut cells to DA blood concentration. Considering the concept of Ramadan fasting, it is likely that the activity of the gut cells is affected.
On the other hand, Grouzman and Lamine [Citation31] reported that the free concentrations of CAs measured in plasma and urine were not affected by feeding. The higher levels of blood concentrations of DA in the fasting group cannot be explained with the food intake because the ingestion of a standard meal increases the plasma DA sulphate levels, resulting in a slight increase in the DA levels.[Citation11] Additionally, it is reported that the regulatory hormones of the homeostatic system directly affect the DA neurons (e.g. leptin and insulin inhibit the DA neurons and ghrelin activates these neurons).[Citation33] Also, food restriction enhances the magnitude of the DA signals evoked by food, which indicates that the increase in the DA system activity with food intake is more prominent after fasting.[Citation34] Numerous animal studies have reported that lack of DA results in death from starvation and dehydration, since DA is essential for feeding, and that excessive DA signalling may inhibit feeding (reviewed in [Citation33]).
In our study, the urine concentrations of CAs were higher compared to the blood concentrations. Similarly, Grouzmann and Lamine [Citation31] showed that the reported reference intervals for plasma CAs were far below the concentrations reported for urine.
In the kidneys, proximal tubular cells synthesize DA from L-DOPA. Therefore, most of the DA in urine comes from decarboxylation of L-DOPA in circulation but not from the renal excretion of circulating DA.[Citation11,Citation31,Citation35] This could explain why the DA concentrations in the urine were not significantly higher in our fasting group than in the control group despite the statistically higher blood levels of DA in the fasting group. Similarly, Mühlbauer and Osswald [Citation35] showed a remarkable increase in the renal DA excretion in fed compared to fasted animals. Accordingly, the reason for the statistically insignificant urine DA levels in the groups may probably be due to increased renal excretion of DA in our control group. However, no correlations were observed between the blood and urine levels of CAs in both groups.
Consequently, the differences observed in the blood and urine CAs indicate a specific regulation of CA release, synthesis and gene expression of CA biosynthetic enzymes in Ramadan fasting. As Carasco and Van de Kar [Citation26] pointed out, stress, as a response to aversive stimuli, is a concept that is difficult to define fully because its interpretation tends to vary according to individual disciplines. The effect of Ramadan fasting on CA concentrations, particularly in terms of DA levels either in blood or urine samples, should be further investigated thoroughly in future studies.
Conclusions
The results from this study showed statistically significant higher levels of blood DA, but not ADR or NA, in fasting rats, which is indicative of the complexity of the hypothalamic–pituitary–adrenal axis as well as the sympathetic dopaminergic system responses during stress. The sympathetic dopaminergic system response must be more pronounced during fasting. Moreover, the reaction of the dopaminergic system and the non-neuronal gut cells to food intake after fasting may be contributed to the observed higher blood DA levels. Additionally, the higher, but statistically insignificant DA levels in the urine samples of fasting rats may indicate increased renal excretion of DA in satiety and DA synthesized in the kidney. In evaluating the CA levels during fasting, the sympatho-neural, sympatho-adrenomedullary, DOPA–DA autocrine/paracrine and hypothalamic–pituitary–adrenocortical pathways must be taken into consideration.
1172510.pdf
Download PDF (741.2 KB)Acknowledgements
The authors thank the staff of the Animal Laboratory at Dicle University for their help.
Disclosure statement
The authors declare no conflict of interest.
Additional information
Funding
References
- Battaloglu Inanc B. The relation of asthma and allergic diseases diagnosed by doctor with fast foods in school children. Konuralp Med J. 2014;6:19–24.
- Trepanowski JF, Canale RE, Marshall KE, et al. Impact of caloric and dietary restriction regimens on markers of health and longevity in humans and animals: a summary of available findings. Nutr J [Internet]. 2011 [cited 2016 Jan 27];10:107. Available from: http://dx.doi.org/10.1186/1475-2891-10-107.
- Varady KA, Hellerstein MK. Alternate-day fasting and chronic disease prevention: a review of human and animal trials. Am J Clin Nutr. 2007;86:7–13.
- Kul S, Savas E, Ozturk ZA, et al. Does Ramadan fasting alter body weight and blood lipids and fasting blood glucose in a healthy population? A meta-analysis. J Relig Health. 2014;53:929–942. doi: 10.1007/s10943-013-9687-0
- Quarterman J, Morrison E. The effects of short periods of fasting on the absorption of heavy metals. Br J Nutr. 1981;46:277–287.
- Trepanowski JF, Bloomer RJ. The impact of religious fasting on human health. Nutr J [Internet]. 2010 [cited 2016 Jan 27];9:57. Available from: http://dx.doi.org/10.1186/1475-2891-9-57.
- Unalacak M, Kara IH, Baltaci D, et al. Effects of Ramadan fasting on biochemical and hematological parameters and cytokines in healthy and obese individuals. Metab Syndr Relat Disord. 2011;9:157–161. doi: 10.1089/met.2010.0084.
- Alkandari JR, Maughan RJ, Roky R, et al. The implications of Ramadan fasting for human health and well-being. J Sports Sci. 2012;30:9–19. doi: 10.1080/02640414.2012.698298.
- Maughan RJ, Fallah SJ, Coyle EF. The effects of fasting on metabolism and performance. Br J Sports Med. 2010;44:490–494. doi: 10.1136/bjsm.2010.072181.
- Wong DL, Tank AW. Stress-induced catecholaminergic function: transcriptional and post-transcriptional control. Stress. 2007;10:121–130.
- Goldstein DS. Catecholamines 101. Clin Auton Res. 2010;20:331–352. doi:10.1007/s10286-010-0065-7.
- Kvetnansky R, Sabban EL, Palkovitz M. Catecholaminergic systems in stress: structural and molecular genetic approaches. Physiol Rev. 2009;89:535–606. doi:10.1152/physrev.00042.2006.
- Sabban EL, Kvetnansky R. Stress-triggered activation of gene expression in catecholaminergic systems: dynamics of transcriptional events. Trends Neurosci. 2001;24:91–98.
- Sabban EL, Serova IL. Influence of prior experience with homotypic or heterotypic stressor on stress reactivity in catecholaminergic systems. Stress. 2007;10:137–143.
- Sabban EL, Liu X, Serova L, et al. Stress-triggered changes in gene expression in adrenal medulla: transcriptional responses to acute and chronic stress. Cell Mol Neurobiol. 2006;26:843–854.
- Landsberg L. Feast or famine: the sympathetic nervous system response to nutrient intake. Cell Mol Neurobiol. 2006;26:497–508.
- Gotthardt JD, Verpeut JL, Yeomans BL, et al. Intermittent fasting promotes fat loss with lean mass retention, fasting increased hypothalamic norepinephrine content, increased neuropeptide Y gene expression in diet-induced obese male mice. Endocrinology. 2016;157:679–691.
- Gostkowski ML, Wei J, Okerberg E, et al. Attomole electrophoretic analysis of catecholamines using copper-catalyzed intramolecular cyclization. Anal Biochem. 2002;303:199–202.
- Sabban EL. Catecholamines in stress: molecular mechanisms of gene expression. Endocr Regul. 2007;41:61–73.
- Mitsui A, Nohta H, Ohkura Y. High-performance liquid chromatography of plasma catecholamines using 1,2-diphenylethylenediamine as precolumn fluorescence derivatization reagent. J Chromatogr. 1985;344:61–70.
- Alberts G, Boomsma F, Man in 't Veld AJ, et al. Simultaneous determination of catecholamines and dobutamine in human plasma and urine by high-performance liquid chromatography with fluorimetric detection. J Chromatogr. 1992;583:236–240.
- Wang H, Walaszczyk EJ, Li K, et al. High-performance liquid chromatography with fluorescence detection and ultra-performance liquid chromatography with electrospray tandem mass spectrometry method for the determination of indoleamine neurotransmitters and their metabolites in sea lamprey plasma. Anal Chim Acta. 2012;721:147–153.
- Li Y, Tang AG, Mu S. HPLC-FLD determination of serum aromatic amino acids: application in chronic kidney disease patients. Clin Chim Acta. 2011;412:1032–1035.
- Sabban EL, Hebert MA, Liu X, et al. Differential effects of stress on gene transcription factors in catecholaminergic systems. Ann New York Acad Sci. 2004;1032:130–140.
- Wong DL, Tai TC, Wong-Faull DC, et al. Genetic mechanisms for adrenergic control during stress. Ann New York Acad Sci. 2004;1018:387–397.
- Carrasco GA, Van de Kar LD. Neuroendocrine pharmacology of stress. Eur J Pharmacol. 2003;463:235–272.
- Gavrilovic L, Spasojevic N, Tanic N, et al. Chronic isolation of adult rats decreases gene expression of catecholamine biosynthetic enzymes in adrenal medulla. Neuro Endocrinol Lett. 2008;29:1015–1020.
- Tsigos C, Chrousos GP. Hypotalamic–pituitary–adrenal axis, neuroendocrine factors and stress. J Psychosom Res. 2002;53:865–871.
- Kvetnansky R. Stressor specificity and effect of prior experience on catecholamine biosynthetic enzyme phenyletanolamine-N-methyltransferase. Ann New York Acad Sci. 2004;1032:117–129.
- Omer B. [Hormones constitute of proteins and derived from amino acids]. In: Gurdol F, editor. Medical biochemistry. Istanbul: Nobel Medical Editions; 2015. p. 426–429. Turkish.
- Grouzmann E, Lamine F. Determination of catecholamines in plasma and urine. Best Pract Res Clin Endocrinol Metab. 2013;27:713–723. doi:10.1016/j.beem.2013.06.004.
- Van Loon GR. Plasma dopamine: regulation and significance. Fed Proc. 1983;42:3012–3018.
- Palmiter RD. Is dopamine a physiologically relevant mediator of feeding behaviour?. Trends Neurosci. 2007;30:375–381.
- Cone JJ, McCutcheon JE, Roitman MF. Ghrelin acts as an interface between physiological state and phasic dopamine signaling. J Neurosci. 2014;34:4905–4913. doi:10.1523/JNEUROSCI.4404-13.2014.
- Mühlbauer B, Osswald H. Feeding but not salt loading is the dominant factor controlling urinary dopamine excretion in conscious rats. Naunyn Schmiedebergs Arch Pharmacol. 1992;346:469–471.