ABSTRACT
Background/aim: The aim of this study was to determine whether Extremely low frequency electric and magnetic field (ELF-EMF) had any harmful effects on dental pulp tissue and examine histologically whether melatonin (MLT) and Ganoderma lucidum (GL) play a protective role against these harmful effects.
Material and method: A total of 56 adult, male Wistar Albino rats were used in the study. The rats were separated into 8 equal groups and were exposed to ELF-EMF, generated by high voltage source, for 8 hours/day for 26 days – Groups I, II and III and for 52 days-Groups V, VI and VII. For sham-control groups (Groups IV and VIII for 26 and 52 days), rats were placed into same experimental set-up as exposure groups and same procedure was applied except ELF-EMF exposure. GL (20 mg/kg/day) and MLT (10 mg/kg/day) were administered by oral gavage and the intraperitoneal route respectively. At the end of the study, the rats anterior teeth were examined immunohistochemicaly using vimentin and vascular endothelial growth factor (VEGF).
Results: Degeneration in the odontoblast cells, inflammatory cell infiltration, dilatation of the blood vessels and haemorrhagic changes were determined in the pulp of the rats in Groups I and V. A statistically significant difference was determined in the groups treated with MLT and GL (p < 0.05).
Conclusion: It was determined that exposure to ELF-EMF induced histological changes in the dental pulp of rats, the using of MLT and GL could have a protective effect against these effects.
Introduction
In recent years, public concerns and scientific interest increased in relation to the effects of ELF-EMF emitted from many devices used in daily life [Citation1]. Some in vivo and in vitro studies suggest that exposure to EMF can affect the nervous system, body weight, tissue morphology and histology, blood biochemical parameters, hormones, the immune system and the repair system [Citation2]. Different types of studies, included animal and human, reported contradictory results, which indicated that ELF-EMFcan or cannot affect biological systems [Citation1,Citation3]. Some of the studies show that there is a relationship between exposure to power frequency ELF-EMF and cellular changes [Citation4,Citation5]. However, others failed to demonstrate such a link [Citation6]. The main cause of contradictory results may arise from the cellular and molecular changes made by EMF, which vary with the duration of exposure of the tissue, penetration, the healing regeneration of the tissue and some exposure parameters [Citation7].
Free radicals and reactive oxygen species damage macromolecules such as tissue fat, proteins and nucleic acids. Genotoxic, cytotoxic and cancerogenous effects have been reported [Citation8,Citation9]. One of the possible interaction mechanisms of ELF-EMF with biological systems is that ELF-EMF can induce oxidative stress and apoptosis by altered reactive oxygen species (ROS) formation [Citation1,Citation10]. Some authors have also reported that ROS may be active in the mechanisms of ELF-EMF effects [Citation11–13]. If cellular or plasma ROS levels are changed by exposure to ELF electromagnetic fields and radiofrequency radiation, the levels of oxidative DNA damage, cell death and apoptotic processes in some tissues would be altered as well [Citation10,Citation14–16].
The teeth, which are the most important tissues of the internal structures of the mouth, are formed of enamel, dentin, cement and pulp. Dental pulp, which develops from dental papilla, is formed of odontoblasts, fibroblasts, protein, blood and nerve vessels [Citation17]. Odontoblast cells are the most important cells of the pulp and are the cells responsible for the construction of dentin and pre-dentin. In pathological conditions they are responsible for the construction of reparative dentin [Citation18,Citation19].When the integrity of the pulp is threatened, pulp cells, especially fibroblasts, produce various inflammatory mediators such as IL-6, IL-8 and VEGF [Citation20,Citation21]. Dental pulp undertakes a series of biological activities such as nourishment, sensitivity, structure and protection [Citation22]. Changes in the blood pressure and flow in the vessels coming from the apical region significantly affect the health of the pulp [Citation23].When dental pulp damage occurs through mechanical, chemical, thermal or microbial irritants, there are local tissue reactions and various lymphatic and vascular inflammatory responses [Citation24]. The primary cause of inflammation in the pulp is the presence of dentin affected by intra-oral bacteria causing caries [Citation25]. As a result of the restricted environment, changes which occur in the vascular tissue or in the blood flow to the pulp have serious effects on the pulp health [Citation26].
Animal, laboratory and epidemiological studies have reported exposure to ELF-EMF to be related with many diseases [Citation27]. Tissues in the dental and mouth, which is an important structure of the body, are also affected by EMF. The enamel hardness, the element content of the tooth, periodontal tissues and the jawbone may be affected [Citation28–30].There are studies in literature related to dental hard tissue and ELF-EMF [Citation25,Citation30–32]. However, the obtained data is not enough to explain the interaction mechanisms between ELF-MF and oral tissues, and this led us to carry out the present study. The aim of the current study was to determine whether ELF-EMF had any harmful effects on dental pulp tissue and examine histologically whether or not melatonin (MLT) and Ganoderma lucidum (GL) had a protective role against these harmful effects.
Materials and methods
This study was conducted in the Research and Applications Centre of Dicle University Prof. Dr Sabahattin Payzin Healthcare Units. The experimental design and study protocol were approved by the Animal Ethics Committee at the University of Dicle (Diyarbakir, Turkey). A total of 64 adult, male Wistar Albino rats, each weighing 250–300 g, were used in the study. After a one-week adaptation period, the rats were randomly separated into 8 equal groups.
The groups were formed as Group I: (n = 8), ELF-EMF exposure for 26 days, Group II: (n = 8), ELF-EMF exposure for 26 days + GL, Group III: (n = 8), ELF-EMF exposure for 26 days + MLT, Group IV: (n = 8), control, Group V: (n = 8), ELF-EMF exposure for 52 days, Group VI: (n = 8), ELF-EMF exposure for 52 days + GL, Group VII: (n = 8), ELF-EMF exposure for 52 days + MLT, Group VIII: (n = 8), control.
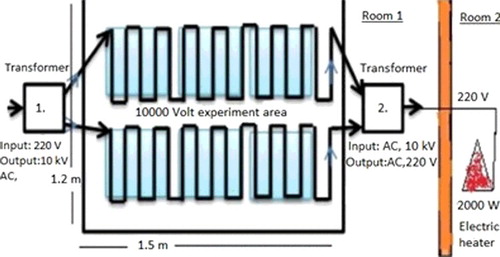
To create ELF-MF, two transformers that produced 10 kV (10,000 V) of high voltage were used. For transformer 1, the input was 220 V, and the output was 10 kV. For transformer 2, the input was 10 kV, and the output was 220 V and 5,000 VA. The rats in the 26-day and 52-day experimental groups (Groups I, II, III, V, VI and VII) were exposed to ELF-EMF for 8 hours each day (). The electric and magnetic field intensity were measured with the aid of a Spectran device NF5035 (AARONIA AG, Strickscheid, Germany), with reference to the method of 6-minute measurement (ICNIRP). The electric and magnetic field intensity measurements in experimental setup were determined as 80.3 V/m and 2.48 µT, respectively.
Throughout the period of exposure to ELF-EMF, GL was administered by oral gavage to Groups II and VI, taking into account the weight of the animals and standard doses (20 mg/kg/day). MLT at a dose of 10 mg/kg/day was administered to Groups III and VII intraperitoneally throughout the period of exposure to ELF-EMF.
At the end of the study, the rats were euthanized by draining intracardiac blood under anaesthesia. The anterior teeth were then removed from the rats and fixed in a 10% formalin solution until histopathological and immunohistochemical examinations.
Histological examination
Tissue preparation for light microscopy
At the end of the study, rat teeth were fixed with neutral buffered 10% formalin solution and decalcified with 5% EDTA (Ethylene-diamine-tetraacetic acid). After preservation, samples were directly dehydrated in a graded series of ethanol and then embedded in paraffin wax. Sections of 4–6 µm thickness were cut with a microtome (Rotatory Microtome, Leica, RM 2265, Germany) and mounted on coated slides. The sections were stained with Haematoxylin and Eosin (H-E),.
Immunohistochemical staining
The antigen retrieval process was performed in a citrate buffer solution (pH:6.0) two times, firstly for 7 minutes, then for 5 minutes in a microwave oven at 700 W. The samples were allowed to cool to room temperature for 20 minutes and then were washed in distilled water for 5 minutes twice. Endogenous peroxidase activity was blocked in 0.1% Hydrogen peroxide for 10 minutes. Ultra V block (Histostain-Plus Kit, Invitrogen, Carlsbad, CA) was applied for 10 minutes prior to the application of primary antibodies Vascular Endothelial Growth Factor (VEGF), (mouse monoclonal, 1/100, Santa Cruz, CA),Vimentin (mouse monoclonal, 1/200, Santa Cruz, CA), and then were stored overnight. A secondary antibody (Histostain-Plus Kit, Invitrogen, Carlsbad, CA) was applied for 20 minutes. After then the slides were exposed to streptavidin-peroxidase for 20 minutes. Diaminobenzidine (DAB, Invitrogen, Carlsbad, CA) was used as a chromogen. Control slides were prepared as stated above but with the omission of the primary antibodies. After counterstaining with Hematoxylin, the slides were washed in tap water for 5 minutes and in distilled water for 2 × 5 minutes, then the slides were mounted.
Data analyses
Conformity to normal distribution of the data was tested with the Shapiro Wilk test and in the comparison of groups not showing normal distribution of data, Kruskal Wallis test variance analysis was applied as a non-parametric test. Differences between the groups according to the results of the Kruskal Wallis variance analysis were deemed to be statistically significant at p<0.05. In the paired comparisons between groups, the Bonferroni corrected Mann Whitney U-test was applied as a multiple comparison test.
Results and discussion
In the histological and immunohistochemical examinations applied at the end of the study period, it was determined that exposure to ELF-EMF had caused degeneration in the odontoblast cells, increased inflammatory cell infiltration, dilatation in the blood vessels and haemorrhage. Compared to the control group, the least damage was seen in the groups where MLT was administered. As a result of the histopathological and immunohistochemical examinations applied at the end of the study period, the following data were obtained:
Histopathological results
As a result of the H&E staining procedure, the following results were obtained:
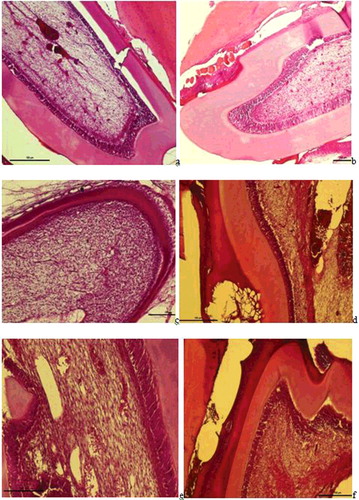
In Group I, dilatation and haemorrhage were observed in the vessels of the pulp, as well as an increase in collagen fibres. Degeneration was observed in the odontoblast cells ((a)). In Group II, haemorrhage was observed in the small vessels of the pulp, and hyperplasia and degeneration in the odontoblastic cells ((b)). In Group III, proliferation of the connective tissue cells in the pulp, regular alignment of the collagen fibres and increased odontoblastic activity were determined ((c)). In Group V, increased dilatation and haemorrhage in the vessels of the pulp, an increase in fibrous tissue and leukocyte infiltrations were observed ((d)). In Group VI, dilatation was observed in the pulp vessels, inflammatory cell infiltrations in the surrounding vessel and degenerative changes in odontoblastic cells ((e)). In Group VII, regular alignment was observed in the odontoblast cells at the border of the dentin and pulp and no change was observed in the fibre and cellular distribution or in the vessel structure ((f)).
Figure 2. a– Group I: Dilatation and haemorrhage in the vessels of the pulp, an increase in collagen fibres, degeneration in odontoblast cells, and a widening of the periodontal membrane and alveolar spaces were observed (H-E staining Bar 100 µm); b – Group II: Haemorrhage in the small vessels of the pulp, hyperplasia and degeneration in odontoblastic cells and dilatation and haemorrhage in the vessels of the periodontal membrane were observed (H-E staining Bar 100 µm); c – Group III: Proliferation in the connective tissue cells in the pulp, regular alignment in collagen fibres and increased odontoblastic activity were observed (H-E staining Bar 100 µm); d -- Group V: Increased dilatation and haemorrhage in the vessls of the pulp, an increase in fibrous tissue and leukocyte infiltrations and loss of tissue with necrotic tissue and hyalinisation in the periodontal membrane were observed (H-E staining Bar 100 µm); e – Group VI: Dilatation in the pulp vessels and inflammatory cell infiltrations surrounding the vessel and degenerative changes in odontoblastic cells were observed (H-E staining Bar 50 µm); f -- Regular alignment was observed in the odontoblast cells at the border of the dentin and the pulp (arrow) and no change in the fibrous, vascular structure or cellular alignment in the pulp (H-E staining Bar 50 µm).
Immunohistochemical results
As a result of the VEGF immune-staining, the following results were obtained: In Group I, VEGF positive expression was observed in dilated vessels in the pulp and in inflammatory cell infiltrations ((a)). In Group II, VEGF positive reaction was observed in the vessel wall in the pulp and a reduced VEGF expression in inflammatory cells was seen to be weak ((b)).
Figure 3. a– Group I: VEGF positive expression observed in dlated vessels and inflammatory cell infiltrations in the pulp (VEGF immune staining 100 µm); b – Group II: VEGF positive reaction in the vessel wall in the pulp (red arrow), reduced and weak VEGF expression in inflammatory cells (VEGF immune staining 100 µm); c – Group III: VEGF positive expression in the vessels close to odontoblastic cells and in vessels in the cement-periodontal space (red arrow) and neo-angiogenesis was seen to have been induced (VEGF immune staining 100 µm); d – Group V: VEGF positive expression in the endothelial cells of the expanded vessel wall in the pulp, in the small vessels between degenerative odontoblast cells and in inflammatory cells (VEGF immune staining 50 µm); e – Group VI: VEGF positive expression in the vessels of the periodontal ligaments and pulp (VEGF immune staining 100 µm), f – Group VII: VEGF positive reaction in the small blood vessels of the pulp and in some inflammatory cells (VEGF immune staining 50 µm).
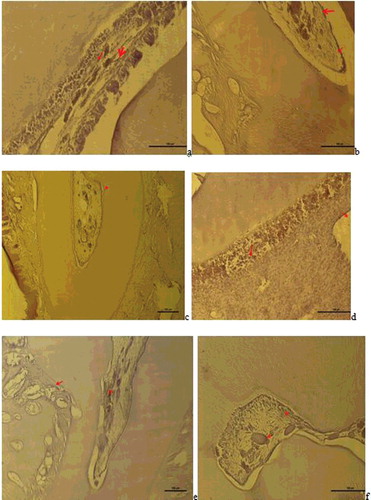
In Group III, VEGF positive expression was seen in the vessels close to odontoblastic cells and neo-angiogenesis was observed to be induced ((c)). In Group V, VEGF positive expression in endothelial cells in the expanded vessel wall in the pulp and VEGF positive expression (50 µm) was observed in inflammatory cells and in the small vessels between degenerative odontoblastic cells ((d)). In Group VI, VEGF positive expression in the vessels in the pulp was observed ((e)). In Group VII, VEGF positive reaction (50 µm) observed in the small blood vessels of the pulp and in some inflammatory cells was observed ((f)).
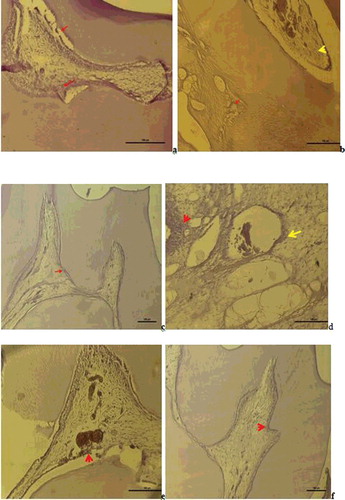
As a result of the Vimentin immune-staining, the following results were obtained: In Group I, Vimentin expression was observed in degenerative odontoblast cells in the pulp, in fibroblast cells and in impaired fibrous structures ((a)). In Group II, weak Vimentin expression was observed in scattered fibrous alignment in the pulp ((b)). In Group III, positive Vimentin expression was observed in odontoblast cells and fibroblast cells and in the regular fibrous alignment in the pulp ((c)). In Group V, degenerative changes were seen in odontoblast cells in the pulp and Vimentin positive reaction in the impaired fibre structure around the dilated vessels and in areas of hyalinisation ((d)). In Group VI, Vimentin positive reaction was observed in odontoblasts and in the fibrous structure around the vessels ((e)). In Group VII, odontoblastic alignment was regular between the dentin and pulp and Vimentin positive reaction was observed in diffuse fibroblasts within the pulp ((f)).
Figure 4. a – Group I: Vimentin expression was observed in degenerative odntoblast cells, fibroblast cells and impaired fibrous structures in the pulp (Vimentin immune staining 50 µm); b – Group II: Vimentin was positive in the degenerative change in the fibres of the periodontal membrane and weak Vimentin expression was observed in scattered fibre distribution in the pulp (Vimentin immune staining100 µm); c – Group III: Vimentin positive expression was observed in odontoblast cells and fibroblast cells and a positive Vimentin reaction was observed in the regular fibre organisation in the pulp (Vimentin immune staining50 µm); d – Group V: Degenerative change was observed in the odontoblast cells of the pulp and positive Vimentin reaction in the impaired fibrous structure around the dilated vessel and in areas of hyalinisation (Vimentin immune staining50 µm); e – Group VI: A positive Vimentin reaction was observed in odontoblasts and in the fibrous structure around the vessel (Vimentin immune staining50 µm); f – Group VII: A positive Vimentin reaction was observed in a diffuse form within the pulp fibroblasts with regular odontoblastic alignment between the dentin and pulp (Vimentin immune staining 50 µm).
Statistical results
The statistical results of the scores in relation to histopathological examinations are shown in –. As a result of the statistical evaluation, no statistically significant difference was determined between the control group and the groups where MLT was administered (Groups III and VII) (–). A statistically significant difference was determined between the control group and Groups I, II, V and VI in respect of odontoblast degeneration and inflammatory cell infiltration created as a result of ELF-EMF ( and ). While no statistically significant difference was determined between the control group and Group II in respect of vasodilation in the pulp blood vessels and haemorrhage as a result of ELF-EMF (), a statistically significant difference was determined between the control group and Groups I, V and VI (p < 0.05) ().
Table 1. The statistical results of odontoblast cell degeneration scores in groups. The values represent mean ± SD. A statistically significant difference was determined between the control group and Groups I, II, V and VI in respect of odontoblast degeneration as a result of ELF-EMF exposure.
Table 2. The statistical results of Inflammatory cell infiltration scores in groups. The values represent mean ± SD. A statistically significant difference was determined between the control group and groups I, II, V and VI in respect of inflammatory cell infiltration as a result of ELF-EMF exposure.
Table 3. The statistical results of dilatation and hemorrhage in blood vessels scores in groups. The values represent mean ± SD. While no statistically significant difference was determined between the control group and Group II in respect of vasodilation in the pulp blood vessels and haemorrhage as a result of ELF-EMF exposure. However, a statistically significant difference was determined between the control group and Groups I, V and VI.
As a result of technological developments, there is increasing concern about the effects of ELF-EMF on human health. It is also important as to whether or not the effects are harmful to biological systems [Citation33]. Previous studies have suggested that EMF has an impact on cancer, reproduction and neurobehavioural reactions [Citation3,Citation34].
There have been reports in previous studies that ELF-EMF has harmful effects on the hardness of teeth, the mineral content and periodontal tissues [Citation28–30,Citation35]. It was also reported that long-term RF exposure can cause some histopathologic significant differences between exposure and sham groups such as vasodilatation in periodontal ligament and alveolar bone and can be a factor to alter the teeth trace elements’ densities [Citation36,Citation37]. In the current study, the harmful effects of high voltage generated ELF-EMF on rat dental pulp were examined and whether antioxidants (MLT and GL) could reduce these harmful effects. From the results of the study, it was determined that rat teeth were affected by ELF-EMF. In the histological and immunohistochemical examinations of this study, it was determined that the electromagnetic field created by high voltage caused degeneration in the odontoblast cells, increased inflammatory cell infiltration, dilatation in the blood vessels and haemorrhage in the dental pulp. As the duration of exposure to ELF-EMF increased, so the damage was seen to increase. Compared to the control group, the least damage was seen to be in the groups where MLT was administered.
In a previous study conducted on rat dental pulp, it was reported that osteogenic differentiation and mineralisation in the pulp cells were accelerated by a static magnetic field [Citation38]. In contrast to that study, Kaya et al reported that ELF-EMF created changes in odontoblasts, fibroblasts, collagen fibres and blood vessels in the dental pulp [Citation35]. Similar to the results of that study, it was determined as a result of the immunohistochemical staining in the current study that ELF-EMF created degeneration in odontoblasts and dilatation in the blood vessels.
In a study related to the effect of ELF-EMF on oral tissues, histological changes in mineral density and in the tissue structure of the head and jaw bones were reported to be created by a magnetic field [Citation25]. Further research is required to determine whether ELF-MF could lead to stimulation on the odontoblast cells activities, cells number, growth and repair ability of the pulp tissue [Citation31]. In another study, as a result of exposure to 900 MHz radio frequency, abnormal histological changes such as focal areas of bleeding and vasodilatation were reported to have formed in rat dental pulp [Citation36]. Similar to the findings of that study, in the current study, vasodilatation and haemorrhage were determined in the pulp vessels as a result of ELF-EMF at high voltage of 10 kV. It was also determined that MLT has a protective effect against this damage.
Significant changes in the element values of rat teeth by EMF created from 50 Hz have been reported [Citation32]. In another study, changes were reported in the values of elements related to oxidative stress (e.g. iron, zinc, silver) in teeth from electromagnetic radiation created from 2.45 GHz. In the Caspase-3 immunohistochemical staining of the same study, a mild effect was determined in the oral mucosa epithelium, odontoblasts and ameloblasts [Citation28]. In the current study, VEGF and Vimentin immunochemical staining methods were used to examine the vessels and cells in the pulp. In the groups exposed to ELF-EMF for 26 days, VEGF positive expression was determined in dilated vessels in the pulp and in inflammatory cell infiltrations and in the groups exposed to ELF-EMF for 52 days, VEGF positive expression was observed in endothelial cells in the expanded vessel walls in the pulp, in small vessels between degenerative odontoblast cells and in inflammatory cells.
Koç et al reported that melatonin and Omega 3 protected cells against neural damage in the hippocampus created by EMF of 900MHz [Citation39]. Melatonin has been reported to have a preventative and protective effect against acute pulpitis and has been stated to have a therapeutic effect for oral diseases [Citation40]. It has also been reported that melatonin could be beneficial due to anti-oxidant and anti-inflammatory effects [Citation41]. In accordance with the findings of these studies, MLT and GL were used in the current study against the harmful effects of ELF-EMF which could have been created in the dental pulp tissues. It was concluded from the results of the current study that MLT protected rat dental pulp against ELF-EMF. In addition, no statistically significant difference was determined between the control group and the groups where MLT was administered (Groups III and VII).
It has been reported that exposure to EMF formed from 50 Hz has a stimulating effect on cells and tissues and can therefore provide recovery from periodontitis [Citation42]. In another study, it was determined that extremely low frequency pulsed electromagnetic fields accelerated osteogenesis and provided early cell proliferation in alveolar bone cells [Citation43]. However, in the current study, ELF-EMF was determined to have caused abnormal changes in the dental pulp structure.
It has been reported that EMF causes oxidative damage and learning and memory damage and that this damage has been prevented by Lotus seedpod procyanidins through stimulation of anti-oxidant enzymes and cleaning of free oxygen radicals [Citation44]. With a similar aim in the current study, Ganoderma lucidum fungus was used. Although GL was observed to have a protective effect against the damage created in the dental pulp by ELF-EMF, it was not as effective as MLT.
Conclusion
From the results in the current study it was concluded that exposure to ELF-EMF causes degeneration and damage to important structures in the dental pulp. Changes were made in odontoblast and fibroblast cells, which are important for pulp and dental health. In addition structural changes occurred in the vessels which are important for the defence, nourishment and construction processes of the pulp. The use of MLT and GL reduces the damage which can occur in the dental pulp as a result of exposure to ELF-EMF. Therefore, for people working in areas where there is ELF-EMF, the use of toothpastes or other products containing MLT could be considered beneficial. There is a need for further studies on this subject.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Cakir DU, Yokus B, Akdag M.Z, et al. Alterations of haematological variations in rats exposed to extremely low frequency magnetic fields (50 HZ). Arch Med Res. 2009;40(5):352–356.
- Oksay T, Naziroglu M, Dogan S, et al. Protective effects of melatonin against oxidative injury in rat testis induced by wireless (2.45 GHz) devices. Andrologia. 2014;46(1):65–72.
- Fiorani M, Biagiarelli B, Vetrano F, et al. In vitro effects of 50 Hz magnetic fields on oxidatively damaged rabbit red blood cells. Bioelectromagnetics. 1997;18:125–131.
- Margonato V, Veicsteinas A, Conti R, et al. Biological effects of prolonged exposure to ELF electromagnetic fields in rats. I. 50-Hz electric field. Bioelectromagnetics. 1993;14:479–493.
- Seto YJ, Majoau-Chargeois D, Lymangrover JR, et al. Chronic 60 Hz electric field exposure induced subtle bioeffects on hematology. Environ Res. 1986;39:143–152.
- Svedenstal BM, Johanson KJ, Mattsson MO, et al. DNA damage, cell kinetics and ODC activities studied in CBA mice exposed to electromagnetic fields generated by transmission lines. In Vivo. 1999;13:507–513.
- Franzellitti S, Valbonesi P, Ciancaglini N, et al. Transient DNA damage induced by high-frequency electromagnetic fields (GSM 1.8 GHz) in the human trophoblast HTR-8/SV neo cell line evaluated with the alkaline comet assay. Mutat Res Fundam Mol Mech Mutagen. 2010;683(1-2):35–42.
- Cho S, Lee Y, Lee S, et al. Enhanced cytotoxic and genotoxic effects of gadolinium following ELF-EMF irradiation in human lymphocytes. Drug Chem Toxicol. 2014;37(4):440–447.
- Akdag MZ, Dasdag S, Cakir DU, et al. Do 100 and 500 µT ELF magnetic fields alter beta amyloid protein, protein carbonyl and malondialdehyde in brain? Electromagn Biol Med. 2013;32(3): 363–372.
- Akdag MZ, Dasdag S, Uzunlar AK, et al. Can safe and long-term and extremely low frequency (50 Hz) magnetic field affect apoptosis, reproduction and oxidative stress? Int J Rad Biol. 2013;89(12):1053–1060.
- Yokus B, Akdag MZ, Dasdag S, et al. Extremely low frequency magnetic fields cause oxidative DNA damage in rats. Int J Radiat Biol. 2008;84:789–795.
- Aksen F, Akdag MZ, Ketani A, et al. Effect of 50-Hz 1-mT magnetic field on the uterus and ovaries of rats (electron microscopy evaluation). Med Sci Monit. 2006;12:215–220.
- Yokus B, Cakir DU, Akdag Z, et al. Oxidative DNA damage in rats exposed to extremely low frequency electromagnetic fields. Free Radical Res. 2005;39:317–323.
- Zmyslony M, Jajte J, Rajkowska E, et al. Weak (5 mT) static magnetic field stimulates lipid peroxidation in isolated rat liver microsomes in vitro. Elec Magnet. 1998;17:109–113.
- Yokus B, Akdag MZ, Dasdag S, et al. Extremely low frequency magnetic fields cause oxidative DNA damage in rats. Int J Radiat Biol. 2008;84(10):789–795.
- Akdag MZ, Dasdag S, Canturk F, et al. Does prolonged radiofrequency radiation emitted from Wi-Fi devices induce DNA damage in various tissues of rats? J Chem Neuroanat. 2016;75:116–122.
- Oshima M, Tsuji T. Functional tooth regenerative therapy: tooth tissue regeneration and whole-tooth replacement. Odontology. 2014;102:123–136.
- Srisuwan T, Tilkorn DJ, Al-Benna S, et al. Survival of rat functional dental pulp cells in vascularized tissue engineering chambers. Tissue and Cell. 2012;44(2):111–121.
- Kawashima N, Okiji T. Odontoblasts: specialized hard-tissue-forming cells in the dentin-pulp complex. Congenit Anom (Kyoto). 2016;56(4):144–153.
- Nakanishi T, Takegawa D, Hirao K, et al. Roles of dental pulp fibroblasts in the recognition of bacterium-related factors and subsequent development of pulpitis. Japan Dent Sci Rev. 2011;47(2):161–166.
- Chisini LA, Conde MC, Correa MB, et al. Vital pulp therapies in clinical practice: findings from a survey with dentist in southern. Braz Dent J. 2015;26(6):566–571.
- Ramazanzadeh BA, Sahhafian AA, Mohtasham N, et al. Histological changes in human dental pulp following application of intrusive and extrusive orthodontic forces. J Oral Sci. 2009;51(1):109–115.
- Demarco FF, Conde MC, Cavalcanti BN, et al. Dental pulp tissue engineering. Braz Dent J. 2011;22(1):3–13.
- Farges JC, Alliot-Licht B, Baudouin C, et al. Odontoblast control of dental pulp inflammation triggered by cariogenic bacteria. Front Psychol. 2013;4(326):1–3.
- Kaya S, Celik MS, Akdag MZ, et al. The Effects of extremely low frequency magnetic field and Mangan to the oral tissues. Biotechnol Biotechnol Equip. 2008;22(3):869–873.
- Han G, Hu M, Zhang Y, et al. Pulp vitality and histologic changes in human dental pulp after the application of moderate and severe intrusive orthodontic forces. Am J Orthod Dentofacial Orthop. 2013;144(4):518–522.
- Zamanian A, Hardiman CY. Electromagnetic radiation and human health: a review of sources and effects. High Freq Electron. 2005;4(3):16–26.
- Çiftçi ZZ, Kırzıoğlu Z, Nazıroğlu M, et al. Effects of prenatal and postnatal exposure of Wi-Fi on development of teeth and changes in teeth element concentration in rats. Biol Trace Elem Res. 2015;163(1–2):193–201.
- Dasdag S, Yavuz I, Bakkal M, et al. Effect of long term 900 MHz radio frequency radiation on enamel microhardness of rat's teeth. Oral Health Dent Manage. 2014;13(3):749–752.
- Kaya FA, Akdag MZ, Kaya CA, et al. Effects of extremely low frequency magnetic fields on periodontal tissues and teeth in rats. J Anim Vet Adv. 2011;10(22):3021–3026.
- Yavuz I, Akdag MZ, Dasdag S, et al. Influences of extremely low frequency magnetic fields on mineral and trace elements content of rat teeth. Afr J Biotechnol. 2008;7(21): 3811–3816.
- Ince B, Akdag Z, Bahsi E, et al. Can exposure to manganese and extremely low frequency magnetic fields affect some important elements in the rat teeth? Eur Rev Med Pharmacol Sci. 2012;16(6):763–796.
- Soderqvist F, Hardell L, Carlberg M, et al. Ownership and use of wireless telephones: a population-based study of Swedish children aged 7–14 years. BMC Public Health. 2007;7(105):1–9.
- Knave B. Electromagnetic fields and health outcomes. Ann Acad Med Singapore. 2001;30(5):489–493.
- Kaya S, MS Celik, MZ Akdag, et al. Effects of extremely low frequency magnetic field on pulp tissue of rats. J Anim Vet Adv. 2011;10(3):295–300.
- Kaya FA, Dasdag S, Kaya CA, et al. Effects of radiofrequency radiation by 900 MHz mobile phone on periodontal tissues and teeth in rats. J Anim Vet Adv. 2008;12(7):1644–1650.
- Adiguzel O, Dasdag S, Akdag MZ, et al. Effect of mobile phones on trace elements content in rat teeth. Biotechnol Biotechnol Equip. 2008;22(4):998–1001.
- Hsu SH, Chang JC. The static magnetic field accelerates the osteogenic differentiation and mineralization of dental pulp cells. Cytotechnology. 2010;62(2):143–155.
- Erdem Koç G, Kaplan S, Altun G, et al. Neuroprotective effects of melatonin and omega-3 on hippocampal cells prenatally exposed to 900 MHz electromagnetic fields. Int J Radiat Biol. 2016;92(10):590–595.
- Guo-Xiong ZW, Long-Xing N. Melatonin attenuates inflammation of acute pulpitis subjected to dental pulp injury. Am J Transl Res. 2015;7(1):66–78.
- Reiter RJ, Rosales-Corral SA, Liu XY, et al. Melatonin in the oral cavity: physiological and pathological implications. J Periodont Res. 2015;50:9–17.
- Haghnegahdar A, Khosrovpanah H, Andisheh-Tadbir A, et al. Design and fabrication of Helmholtz coils to study the effects of pulsed electromagnetic fields on the healing process in periodontitis: preliminary animal results. J Biomed Phys Eng. 2014;4(3):83–90.
- Lim K, Hexiu J, Kim J, et al. Effects of electromagnetic fields on osteogenesis of human alveolar bone-derived mesenchymal stem cells. Biomed Res Int. 2013;2013:296019.
- Duan Y, Wang Z, Zhang H, et al. The preventive effect of lotus seedpod procyanidins on cognitive impairment and oxidative damage induced by extremely low frequency electromagnetic field exposure. Food Funct. 2013;4(8):1252–1262.