Abstract
Soil salinization has become more and more serious. Exogenous nitric oxide (NO) could act in alleviating toxicity in plants. In this study, we aimed to explore the growth, photosynthetic pigments, water-solubles content, proteins, antioxidant enzyme parameters, and element contents of two types of wild barley (Hordeum brevisubulatum (Trin.) Link.), wild-type barley and Saertu wild barley cultivars, in response to salt stress. Both cultivars were first treated with 300 µmol·L−1 NaCl (increased in 25 µmol·L−1 increments to 300 µmol·L−1 for 7 days). Next, plants were sprayed with different NO concentrations on days 1, 5, 10, 15 and 20 after the NaCl concentration reached 300 µmol·L−1. The results showed that salt stress alone decreased all growth parameters [plant height (HT), fresh weight (FW), total dry weight (DW)], the photosynthetic pigment and protein contents, and the activity of the antioxidant enzymes [ascorbic acid peroxidase (APX), peroxidase (POD), catalase (CAT), superoxide dismutase (SOD)], and significantly increased the K+ and Na+ elements contents, water solubles and malondialdehyde (MDA) compared with the controls. However, the inhibitory effects of salt stress in both wild barley cultivars were alleviated by exogenous NO treatment, and 200 µmol·L−1 NO was the most effective concentration to improve plant growth, ion element levels and the physiology of wild barley under high salt stress. Altogether, this study demonstrated the beneficial role of exogenous NO donor, sodium nitroprusside, on wild barley under salinity stress and the underlying physiological and biochemical mechanisms.
Introduction
Soil salinization is considered to be one of the severe stress factors among various abiotic stresses. Salt stress has caused extensive losses of agricultural production [Citation1] with its negative effect on plant growth, development and productivity [Citation2]. Salt stress can damage the physiological processes of plants through the accumulation of excessive reactive oxygen species (ROS), ion toxicity, and osmotic stress, thereby disrupting the balance of nutrition, photosynthesis and hormones and impairing the antioxidant defense system [Citation3, Citation4].
Nitric oxide (NO) is involved in the regulation of multiple responses to various abiotic and biotic stresses in plants [Citation5]. Numerous studies have provided evidence that NO is a vital signaling mediator in plant responses to different types of stressors [Citation6, Citation7], especially drought stress [Citation8] and heat stress, disease resistance [Citation9] and apoptosis [Citation10]. Previous studies have demonstrated that an artificial NO donor is relevant to ethylene emission [Citation8], and ethylene can be regarded as a signal molecule for physiological growth and development, biochemical interactions and defense responses against plant abiotic and biotic stresses (including salt) [Citation11]. Besides, NO could regulate the antioxidant enzymes at the activity and gene expression level under some stress conditions, leading to either an increase or decrease in the redox status of cells. NO itself is a reactive nitrogen species, and its role (protective or toxic) in various cell types depends on the concentration, plant species and development stage [Citation12].
Wild barley (Hordeum brevisubulatum (Trin.) Link.), a perennial grass of the Gramineae family, has the characteristics of high yield, good palatability,and wide adaptability. Moreover, it has the advantages of salt tolerance, cold tolerance, drought resistance, and the ability to grow on barren soil. It is widely used for livestock, fiber products, improvement of heavy metal-contaminated soil, habitats for wildlife populations, recreation and beautification [Citation13]. Yin et al. [Citation14] have explored the changes in wild barley seedlings in terms of their physiological biochemistry, and they found that the wild barley exhibited a unique salt tolerance mechanism, which enabled it to adapt to high-salt conditions [Citation14]. As has been proved previously, wild barley can grow normally when the NaCl concentration is less than 350 mmol·L−1 [Citation15,Citation16]. This provides a reference for the understanding of the physiological mechanism of salt tolerance in wild barley. NO beneficial effects on maintaining ion homeostasis in the cytoplasm of plants under salt stress were determined. It has been shown that exogenous NO significantly changed the antioxidant enzyme activities under salt stress [Citation17]. Consistently, a previous study has pointed out that the activities of antioxidant enzymes [superoxide dismutase (SOD), peroxidases (POD), catalases (CAT)] and glutathione reductase in different plant species are elevated by exogenous NO treatment, especially under salt stress conditions [Citation18]. Furthermore, enzymatic proteins and NO directly affect the gene expression of antioxidant enzymes. Therefore, overexpression of SOD, cytosolic ascorbic acid peroxidase (APX), CAT and glutathione reductase genes in maize leaves under the influence of sodium nitroprusside (SNP, an NO donor) was analyzed [Citation19]. NO also helps plants cope with salt stress via reducing ion leakage [Citation20]. Phosphorus (P), potassium (K), magnesium (Mg) and calcium (Ca) are four essential macronutrients for plant growth and development. As has been reported previously, plants under saline pressure could maintain the ion homeostasis by NO via enhancing K+ uptake and reducing Na+ uptake [Citation21]. It is well-established that NO is a modulator of many developmental processes in plants [Citation22]. Moreover, SNP is an important NO donor in plant biology [Citation23]. However, the specific role of NO in wild barley under salinity stress remains elusive.
In this study, we aimed to investigate the effects of the exogenous NO donor SNP on regulating plant growth, carbohydrate concentration, lipid peroxidation and nutrients in two wild barley cultivars under salinity stress. Moreover, the role of NO in alleviating salt damage was explored, along with its underlying physiological and biochemical mechanisms.
Materials and methods
Plant materials and growth conditions
The wild-type barley and Saertu wild barley were used for the experiments. Saertu wild barley is a new high-yielding variety (National Forage Variety Approval Committee, variety registration number 550) cultivated from the wild barley species of Songnen saline-alkaline grassland by the Grassland Science Laboratory of Northeast China Agricultural University. Seeds of the same weight (0.15 g) were sown in sand-filled plastic pots (10 cm in diameter, 9 cm in depth) in the greenhouse of Northeast Agricultural University (Harbin, China) on Sep. 17, 2018.
The plants were sufficiently watered and fertilized every other day with half-Hoagland solution [Citation24] with a pH of 6.6 and an electrical conductivity (EC) of 1.5 dS m −1 (16 mm NaCl). Grasses were cut to 5.0–6 cm high once a week. The average air temperature and photosynthetic photon flux density (PPFD) in the greenhouse were 20°C ± 1.5 °C and 400 mmol m−2 s−1, respectively.
SNP and salt treatments
Before the initiation of salinity treatments, all plants were cut to 5–6 cm in height (the height of the smallest accession), which facilitated later measurements on the tissues produced after the imposition of salinity stress. Before salinity treatments, SNP was added to the hydroponic solution for 3 d (Nov. 24–26, 2018). The SNP concentration was 0, 50, 100, 200, 300 or 500 μmol·L−1 in each 10-mL pot ().
Table 1. The experimental design.
For salinity treatments on Nov. 27, 2018, half-Hoagland solution amended with NaCl at concentrations of 0 (control) and 300 mmol·L−1 NaCl (approximately 1.5 and 25.2 dS m−1, respectively) were used. To avoid saline shock in plants, the saline concentration was gradually attained by increasing the NaCl concentration of irrigation water by 25 mmol·L−1 each day until reaching 300 mmol·L−1 NaCl. Subsequently, the grasses were exposed to 300 mmol·L−1 NaCl treatment on Dec. 03, 2018. SNP was added once to the hydroponic solution at 1 d (Dec. 03, 2018), 5 d (Dec. 07, 2018), 10 d (Dec. 12, 2018) or 15 d (Dec. 17, 2018) during the stress period. Salt treatments ended on Dec. 22, 2018, when severe damage and leaf color loss occurred in the submerged grasses (visual observation). We observed that this salinity concentration and duration could produce salinity tolerance in Saertu wild barley and avoid saline accumulation in the sand media; irrigation was applied manually to each pot until free drainage occurred.
Photosynthetic pigment content in leaves
The plant height (HT) in each pot was recorded to determine the growth during the stress period. A portion of the shoot was immediately frozen in liquid nitrogen and stored at −80 °C for subsequent use. A portion of the leaf was randomly selected for chlorophyll and carotenoid (Car) extraction. Chlorophyll a, chlorophyll b, and Car were extracted from leaves by soaking leaf samples (approximately 20 mg) into dimethyl sulfoxide (DMSO, 10 mL) in the dark for 48 h. The absorbance value at 665 nm, 649 nm and 480 nm was recorded by a spectrophotometer (Thermo ScientificTM, MA, USA) and chlorophyll a, chlorophyll b, and Car concentrations were calculated using the method described by Wellburn [Citation25].
Water-soluble carbohydrate (WSC) concentration
The total WSC concentration was measured using the anthrone method as described by Liu and Jiang [Citation26]. Briefly, WSC was extracted from the fine powder of dry shoot tissues (approximately 20 mg) using double-distilled water (1 mL). Next, the extract was shaken for 10 min and centrifuged at 11,000×g for 10 min, with the supernatant collected. The extraction was done repeatedly three more times, and the supernatant was pooled. A 1-mL aliquot of the extract was mixed with 7 mL of freshly prepared anthrone [200 mg anthrone + 100 mL 72% (w/w) H2SO4], which was then placed in a boiling water bath for 8 min. After cooling, the absorbance value at 625 nm was read by a spectrophotometer (Thermo ScientificTM, MA, USA). A standard curve was generated using glucose in a range of 0–200 μg mL−1.
Measurement of water-soluble protein
To extract the water-soluble protein, we ground the frozen shoot tissues into a fine powder using liquid nitrogen. The shoot powder (approximately 100 mg) was mixed with 1.5 mL extraction buffer [50 mmol·L−1 potassium phosphate, 1 mmol·L−1 ethylenediaminetetraacetic acid (EDTA), and 1% polyvinylpyrrolidone (PVP), pH = 7.8]. The mixture was centrifuged at 15,000 g for 2 × 15 min at 4 °C, with the supernatant collected for enzyme assay. The water-soluble protein content was determined according to the method described by Bradford [Citation27].
Lipid peroxidation measurement
The malondialdehyde (MDA) content (an end product of lipid peroxidation) was detected for lipid peroxidation measurement, with some modifications. Briefly, 0.5 mL aliquot of the supernatant was mixed with 2 mL of 20% trichloroacetic acid containing 0.5% thiobarbituric acid. The mixture was heated at 95 °C for 30 min, quickly cooled, and then centrifuged at 10,000 g for 10 min. The absorbance value at 532 nm and 600 nm was read by a spectrophotometer (Thermo ScientificTM, MA, USA). The MDA concentration was calculated using an extinction coefficient of 155 mm−1 cm −1.
Antioxidant enzyme measurement
The activities of SOD, CAT, POD and APX were evaluated based on the methods described by Liu and Jiang [Citation26] and Zhang and Kirkham [Citation28]. Total SOD activity was measured via recording the rate of p-nitro blue tetrazolium chloride (NBT) reduction at an absorbance of 560 nm by a spectrophotometer (Thermo ScientificTM, MA, USA). The assay medium was supplemented with 50 mmol·L−1 phosphate buffer (pH = 7.8), 13 mmol·L−1 methionine, 75 mmol·L−1 NBT, 2 mmol·L−1 riboflavin, 0.1 mmol·L−1 EDTA and 20–40 mL enzyme extract. The reaction mixture was illuminated under 80–90 mmol m−2 s−1 for 10 min. A reaction mixture lacking enzyme developed the maximum color and exhibited NBT reduction. Additionally, the control reaction mixture was placed in the dark. One unit of SOD activity was defined as the amount of enzyme that caused 50% inhibition of the NBT reduction rate. The APX activity was assayed via recording the decrease in absorbance value at 290 nm for 1 min by a UV spectrophotometer (Thermo ScientificTM, MA, USA). The reaction mixture (1.5 mL) contained 50 mmol·L−1 potassium phosphate buffer (pH = 7.0), 0.5 mmol·L−1 ascorbic acid, 0.1 mmol·L−1 EDTA, 0.1 mmol·L−1 H2O2 and 0.15 mL crude extract. The reaction was initiated by the addition of 0.1 mmol·L−1 H2O2. The POD activity was determined by the increase in absorbance at 470 nm for 1 min. The assay contained 20 mmol·L−1 guaiacol (50 mL), 10 mmol·L−1 phosphate buffer (2.83 mL, pH = 7.0) and enzyme extract (0.1 mL). The reaction was initiated by the addition of H2O2. The CAT activity was determined by the decrease in absorbance at 240 nm for 1 min by a UV spectrophotometer (Thermo ScientificTM, MA, USA). The assay solution contained 50 mmol·L−1 phosphate buffer (pH = 7.0), 15 mmol·L−1 H2O2, and enzyme extract (0.1 mL). The reaction was initiated by the addition of enzyme extract.
Measurement of elemental contents
The shoots and roots were separately harvested, washed several times with distilled water and dried (80 °C, 48 h) to a constant weight, followed by extraction using 100 mmol·L−1 acetic acid in a water bath with constant temperature (90 °C) for 2 h. The liquid was allocated into two groups, and Na+ and K+ concentrations in the shoots and roots were detected using an M410 flame photometer (Sherwood Scientific, Cambridge, UK).
Data analyses
The experiment was a split-plot design with three kinds of treatments (control, salt and salt + SNP). Each treatment was done in three replicates (three pots). Graphs were created using Excel 2016. IBM SPSS Statistics 19.0 was used for statistical analysis. All data were analyzed by analysis of variance using the least significant difference (LSD) test at the 5% level.
Results
Plant growth
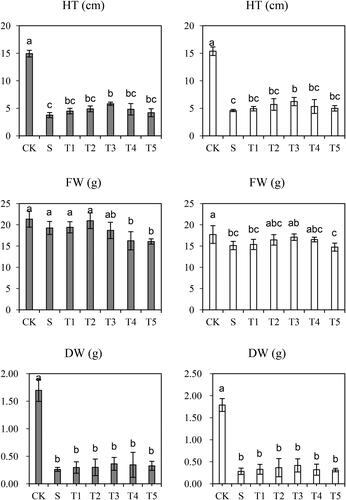
As shown in , after salt treatment, both cultivars showed notably decreased indexes [including the HT, FW, total DW], compared with their non-treated controls. Under the salt stress condition, the HT, FW and total DW were decreased by 74.7%, 9.8% and 84.5%, respectively, in Saertu wild barley, compared with 70.1%, 14.6% and 84.2% accordingly in the wild-type barley. Furthermore, after treatment with 50 µmol·L−1 NO, the inhibitory effects of salt stress were effectively alleviated, as manifested by a lesser decrease in HT (19.4%), FW (0.8%) and total DW (12.6%) in Saertu wild barley as compared to plants that were treated with salt only; similarly, the HT, FW and total DW were decreased to a lesser extent by 7.2%, 1.7%, and 15.9%, respectively in the wild-type barley as compared to plants that were treated with salt only. Therefore, we identified that the application of NO (50 µmol·L−1, 100 µmol·L−1 and 200 µmol·L−1) to salt-treated plants can increase the HT, FW and total DW in two wild barley varieties. However, after excessive NO treatment, this mitigating effect was gradually decreased and these plant growth indexes were decreased again. After treatment of 300 µmol·L−1 NO, the HT and DW were decreased, and the mitigating effect disappeared in the 500 µmol·L−1 NO group.
Figure 1. Effects of salt (S) and salt + SNP treatment (T) on plant height (HT), fresh weight (FW) and dry weight (DW) in two wild barley cultivars.
The gray bars indicate Saertu wild barley variety, and the white bars indicate the wild-type barley variety. Bars showed as mean values ± SD (n = 3). CK, non-treated control; T1–T5, see . Same lowercase letters indicate non-significant differences.
Photosynthetic pigment content
Under the salt stress condition, the concentrations of chlorophyll a, chlorophyll b, Car, and total chlorophyll a + b were remarkably decreased compared with those in the control cultivars of wild-type barley (). The chlorophyll b concentration was decreased in Saertu wild barley, with no significant change. After NO was given to the salt-treated plants, the inhibitory effects of salt stress on plants were alleviated, accompanied by a lesser decrease in the content of chlorophyll a. The 50 µmol·L−1 and 100 µmol·L−1 NO treatments increased chlorophyll a by 10.4% and 14.9%, respectively in Saertu wild barley and 1.3% and 5.2%, respectively in the wild-type barley. The concentrations of the photosynthetic pigments of chlorophyll a, chlorophyll b, chlorophyll a + b and Car were decreased after treatment with a high concentration of NO in the salt-treated plants of both cultivars. From all of the above, NO in a low concentration was more effective in alleviating salt stress than that in a high concentration.
Table 2. Effects of salt stress and NO on photosynthetic pigment content in both cultivars according to split-plot analysis of variance.
Lipid peroxidation
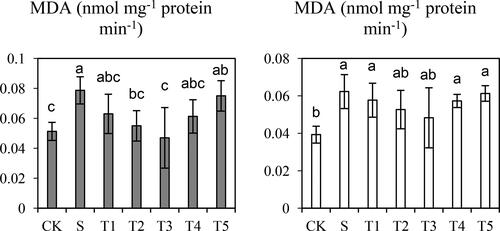
The MDA concentration was higher by 34.8% in Saertu wild barley and 35.9% in the wild-type barley under salt stress, as compared to that in the control group (). After treatment with low NO concentrations (50–200 µmol·L−1), the MDA level was considerably decreased in both cultivars compared with the salt-treated group. In detail, the addition of 50 µmol·L−1 NO led to an MDA decrease of 24.9% and 8.0% in Saertu wild barley and wild-type barley, respectively, compared with the salt-treated group. The MDA concentration showed a declining trend with the increase in NO concentration. The MDA level was the lowest with the treatment of 200 µmol·L−1 NO, and then the MDA level increased again.
Figure 2. Effects of salt (S) and Salt + SNP treatment (T) on Malondialdehyde (MDA) Concentration in two wild barley cultivars.
The gray bars indicate Saertu wild barley variety, and the white bars indicate the wild-type barley variety. Bars showed as mean values ± SD (n = 3). CK, non-treated control; T1–T5, see . Same lowercase letters indicate non-significant differences.
Antioxidant enzyme activities
Compared with plants under normal conditions, the SOD activity in barley was significantly decreased by 15% in Saertu wild barley and 13.5% in the wild-type barley under salt stress without NO treatment (). After NO treatment was applied, the inhibitory effect of salt stress alone was alleviated; however, NO in a high concentration showed no impact on the suppression or promotion of SOD activity. As shown in , the wild-type barley showed greater salt tolerance than Saertu wild barley.
Figure 3. Effects of salt (S) and Salt + SNP treatment (T) on SOD, APX, POD and CAT in two wild barley cultivars.
The gray bars indicate Saertu wild barley variety, and the white bars indicate the wild-type barley variety. Bars showed as mean values ± SD (n = 3). CK, non-treated control; T1–T5, see . Same lowercase letters indicate non-significant differences.
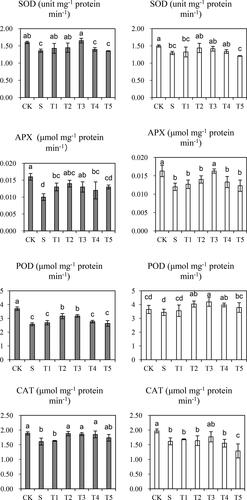
APX activity was notably decreased in both cultivars after salt stress treatment (). Our results showed that APX activity was decreased by 37.5% in Saertu wild barley and 26.4% in the wild-type barley. Moreover, the APX activity was dramatically increased after NO treatment in both cultivars, as compared to that in the control group. However, the APX activity began to decline when the NO concentration reached 200 µmol·L−1 in Saertu wild barley and 300 µmol·L−1 in the wild-type barley.
Salt stress could contribute to the decrease in POD activity in both cultivars, with a greater change detected in Saertu wild barley (nearly 0.5-fold decrease in POD activity). However, the inhibitory effect was markedly alleviated by the application of NO of low concentration. The POD activity began to decline when the NO concentration reached 300 µmol·L−1 in both cultivars.
As shown in , CAT was noticeably decreased in both cultivars under salt stress. Similarly, in Saertu wild barley, when the NO concentration reached 300 µmol·L−1, the CAT activity was increased by approximately 8% compared with that in the plants under salt stress. Additionally, CAT activity was remarkably decreased in the wild-type barley under both salt stress and 50 µmol·L−1 NO treatment. No considerable change was observed in other treatment groups.
Compatible osmolyte content
The effects of salt treatment on WSC and protein accumulation were analyzed in both cultivars (). As shown by our results, salt stress considerably upregulated the WSC level but downregulated the protein expression. The WSC concentration was increased by 62.7% in Saertu wild barley and 78.1% in the wild-type barley. Furthermore, when the NO concentration reached 100 µmol·L−1, WSC concentration was increased by 24.3% and 16.7% in Saertu wild barley and the wild-type barley, respectively.
Figure 4. Effects of Salt (S) and Salt + SNP treatment (T) on water-soluble carbohydrate (WSC) and Protein Content in two wild barley cultivars.
The gray bars indicate Saertu wild barley variety, and the white bars indicate the wild-type barley variety. Bars showed as mean values ± SD (n = 3). CK, non-treated control; T1–T5, see . Same lowercase letters indicate non-significant differences.
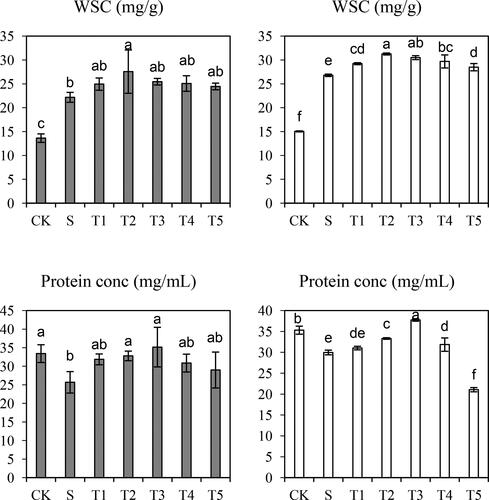
After the combined treatment of NO of low concentration and salt, WSC content was continuously increased. When the NO concentration reached 200 µmol·L−1, the WSC level was decreased. Additionally, the protein expression was increased after NO treatment compared with that in the salt-treated group, which showed a maximum with 200 µmol·L−1 NO treatment, resulting in an increase of 36.9% in Saertu wild barley and 26.0% in the wild-type barley.
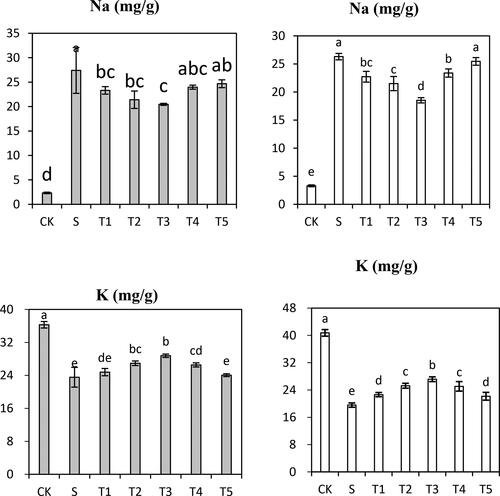
Element content
As shown in , the accumulation of the macronutrient K+ was remarkably suppressed under the salt condition. The amount of K+ was decreased when plants were treated with 300 µmol·L−1 NaCl, as manifested by the decrease in K+ amount of 35.1% in Saertu wild barley and 51.9% in the wild-type barley compared with that in each control group. Moreover, the Na+ concentration was increased by 10.7-fold in Saertu wild barley and 6.9-fold in the wild-type barley under salt stress. The effects of salt on macronutrients were relieved by the addition of NO of low concentration. The mitigating effects of 200 µmol·L−1 of NO for K+ and Na+ were estimated to be 18.1% in Saertu wild barley and 29.8% in the wild-type barley. However, these mitigating effects were inhibited after treatment with a high concentration of NO. Salt stress-caused damage was not aggravated until the NO concentration reached 500 µmol·L−1, and 200 µmol·L−1 NO showed the greatest mitigating effect on salt stress.
Figure 5. Effects of Salt (S) and Salt + SNP treatment (T) on Na+ and K+ Content in in two wild barley cultivars.
The gray bars indicate Saertu wild barley variety, and the white bars indicate the wild-type barley variety. Bars showed as mean values ± SD (n = 3). CK, non-treated control; T1–T5, see . Same lowercase letters indicate non-significant differences.
Discussion
Growth impairment in response to salt stress
Salt stress leads to the lack of nutrient elements in plants, disrupting their metabolism and normal growth. It has been reported that increasing salinity levels severely slow down the emergence and seedling growth of all wheat cultivars [Citation29, Citation30]. In this study, NaCl-treated plants showed notably decreased growth-related indexes (HT, FW and total DW) for both cultivars, which is mainly attributed to the osmotic pressures and water loss [Citation31]. In the present study, after the addition of NO to the salt-treated plants, the growth parameters were increased at different levels for both cultivars. Previous studies have shown consistent results, which further confirmed that NO could increase the salt tolerance of plants [Citation32, Citation33]. However, the positive effect of NO on salt stress is limited in this study. Under high NO concentrations, the mitigating effect of NO began to decrease or even showed no influence on the toxicity of salt stress. Furthermore, NO addition could not recover the decrease in water content and FW under salt treatments in both cultivars. Taken together, NO did not protect the barley from salt stress simply by reducing the water loss. Therefore, more complex molecular mechanisms need to be discussed.
Effect of salt stress on WSC and protein parameters
Plants exhibit various strategies with a wide range of biochemical adaptations under salt stress, one of which is the synthesis of organic permeates with low molecular weight for osmo-protection, such as sugars [Citation34]. In this process, NO is considered to be a regulatory signaling molecule. However, its mechanism under salt stress has not been fully explained. In this experiment, it was noted that the WSC content was increased in the salt-treated plants for both cultivars, which confirmed the physiological protection strategy of the plant. As a previous study has mentioned [Citation35], the WSC content continues to increase after NO treatment, indicating that NO promotes the organic permeates synthesis as a signaling molecule. However, the WSC content was slightly increased with the addition of NO, compared to the salt treatment, and even decreased under a high concentration of NO. These results indicate that the increased WSC synthesis was not enough to overcome the salt stress-caused water loss in this study. Moreover, NO with high concentration also exhibited toxicity.
A previous study has pointed out that the protein contents in Ceratophyllum demersum were decreased under salt stress [Citation36]. In the present study, the protein content was significantly decreased under salt stress and increased after treatment of NO with low concentration. The results showed that the appropriate concentration of NO could increase the accumulation of water-soluble protein and the activity of antioxidant enzymes (such as SOD, APX, POD, and CAT) that protect the plant from abiotic stress [Citation37–42]. However, NO with high concentration did not exhibit this effect.
Effect of salt stress on photosynthetic pigments
It has been shown that salt stress mainly disorganizes chloroplast grana stacking and reduces stomatal density, size and aperture as well as the photosynthetic capacity [Citation43]. Under salt stress, the chlorophyll and carotenoid content of the leaves were decreased in both cultivars, causing leaf senescence and decreased stress resistance. This effect may be caused by the oxidation of pigments in the chloroplast, and the pigment–protein complexes being not stable under salt stress [Citation44]. As has been evidenced previously, salt stress could increase the accumulation of NaCl in chloroplasts of higher plants, thereby suppressing the photosynthetic electron activity in photosynthesis and eventually decreasing the growth rate [Citation43]. The addition of NO increased the chlorophyll content under stress, which indicated that NO protected the leaf cell structure under salt stress. Consistently, it has been demonstrated that similar results are found in cotton and other plants [Citation1, Citation45]. NO, a key signaling molecule in plant physiology, can enhance the photosynthesis of plants and inhibit the synthesis of ROS [Citation46–48]. Song et al. [Citation49] cloned the AtCHX3 gene from Arabidopsis thaliana, which can encode a putative Na/H reverse transporter that regulates pH in chloroplasts and affects chloroplast development. Therefore, the activation of this gene may be another mechanism explaining the NO effect on the toxicity of salt stress, which needs further validation. According to the experimental results, 100 µmol·L−1 NO was the most suitable concentration for relieving chlorophyll and Car reduction; however, excessive NO concentration was not effective in these two cultivars.
Effect of salt stress on antioxidant enzymes
MDA accumulation was widely used as the indicator of lipid peroxidation, which results from the oxidative damage caused by abiotic or biotic stress [Citation35]. In a prior work, Hussain et al. has reported that the wheat seedlings with higher salinity tolerance showed lower lipid peroxidation [Citation22]. In our experiment, the MDA content was remarkably increased under salt stress in both cultivars compared with that in the control, indicating the occurrence of lipid peroxidation in plants under salt stress, which may ultimately result in membrane damage and plant cell death. NO treatment lowered the MDA content as compared with salt treatment, which indicated that NO can effectively alleviate the oxidative damage of salt in plants. However, NO in high concentration exhibited no efficient protection on the barley plants.
Accumulating studies have demonstrated that salt stress can generate excess ROS in plant cells, which could induce severe oxidative damage to plant tissues, cells and biomolecules (such as lipids, proteins and DNA) [Citation38, Citation39]. In the meanwhile, plants activate their antioxidant systems (including enzymatic and non-enzymatic mechanisms) to eliminate the excessive ROS and protect themselves. The antioxidant enzymes include SOD, APX, POD and CAT [Citation40]. SOD, the first line of enzymatic antioxidant system in the plant, can convert highly toxic ROS (superoxide radicals) into H2O2, while APX, POD and CAT can further detoxify excessive H2O2 or organic peroxides [Citation50]. However, in this study, the activity of these four antioxidant enzymes (SOD, APX, POD and CAT) was notably decreased under the salt treatment, as compared to that in the control. This result was different from the previous studies, which indicated that the activity of antioxidant enzymes was induced for protecting the plant under salt treatment [Citation37, Citation38]. This contradiction might be attributed to the higher NaCl concentration we used in the present study compared with the previous studies, which caused a higher content of ROS accumulation that probably could not be effectively scavenged by the antioxidant enzymes and non-enzymatic antioxidants and would damage the enzymatic antioxidant system in turn. The above reason may also explain the increase in MDA under salt treatment. However, the content of antioxidant enzymes was increased after pretreatment with a low concentration of NO, even though the NO concentration was still lower than that in the control. It has been reported that exogenous NO caused significant changes in the antioxidant enzyme activities under salt stress and alleviated plant oxidant damage [Citation41]. Wendehenne et al. [Citation42] have also indicated that NO protects cells from ROS-mediated cellular damage via increasing the level of cytoprotective proteins, such as CAT and SOD [Citation42]. In this study, after NO treatment, the oxidant damage was alleviated through the plants’ enhanced ability to eliminate ROS under salt stress and decrease the degree of membrane lipid peroxidation.
Effects of salt stress on element contents
It has been reported that salt-sensitive plants fail to maintain K+ content under high salinity conditions. Under salt stress, a high concentration of Na+ could inhibit the uptake of other ions (including macronutrients and micronutrients) by roots and their transport into the shoot, which leads to deficiency of nutrients in plants [Citation51]. Furthermore, a previous study has pointed out that two poplar species display a net K+ efflux after exposure to salt shock (100 mmol·L−1 NaCl) [Citation52]. Salt stress dramatically increased Na+ concentration and decreased K+ concentration in both barley cultivars in this study. Our results were consistent with the findings in switchgrass [Citation53] and cucumber [Citation51]. However, NO with low concentration decreased the Na+ content and increased the K+ content in both cultivars. Consistently, a previous study has indicated that the overexpression of PvNAC1 in Switchgrass could enhance the tolerance to salt stress through increased K+ and decreased Na+ accumulation and enhanced ROS scavenging ability [Citation53]. Therefore, NO pretreatment may activate the expression of specific genes related to ion transport, thereby keeping the ionic equilibrium in plant cells.
Conclusions
In summary, this study revealed that salt stress alone could decrease the plant growth indexes and the antioxidant enzyme activity via disbalancing the ionic equilibrium and inducing unrecoverable oxidative damage in both cultivars. NO with low concentration could effectively alleviate the toxicity of salt stress; however, a high concentration of NO exerted no obvious benefit on alleviating the toxicity in the plants under salt stress and even exhibited toxicity itself. Altogether, NO appears to be a biofunctional modulator in plant cells to handle salt stress. These results may be helpful for a deeper understanding of the mechanism of NO in the biology of plants.
Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability
The data that support the findings reported in this study are available from the corresponding author upon reasonable request.
Additional information
Funding
References
- Liu S, Dong Y, Xu L, et al. Effects of foliar applications of nitric oxide and salicylic acid on salt-induced changes in photosynthesis and antioxidative metabolism of cotton seedlings. Plant Growth Regul. 2014;73(1):67–78.
- Iqbal N, Umar S, Khan NA, et al. A nw perspective of phytohormones in salinity tolerance: regulation of proline metabolism. Environ Exp Bot. 2014;100:34–42.
- Alam P, Albalawi TH, Altalayan FH, et al. 24-Epibrassinolide (EBR) confers tolerance against NaCl stress in soybean plants by up-regulating antioxidant system, ascorbate-glutathione cycle, and glyoxalase system. Biomolecules. 2019;9(11):640.
- Ahanger MA, Alyemeni MN, Wijaya L, et al. Potential of exogenously sourced kinetin in protecting solanum lycopersicum from NaCl-induced oxidative stress through up-regulation of the antioxidant system, ascorbate-glutathione cycle and glyoxalase system. PLoS One. 2018;13(9):e0202175.
- Kaya C, Higgs D, Ashraf M, et al. Integrative roles of nitric oxide and hydrogen sulfide in melatonin-induced tolerance of pepper (Capsicum annuum L.) plants to iron deficiency and salt stress alone or in combination. Physiol Plant. 2020;168(2):256–277.
- Mostofa MG, Fujita M, Tran LSP. Nitric oxide mediates hydrogen peroxide- and salicylic acid-induced salt tolerance in rice (Oryza sativa L.) seedlings. Plant Growth Regul. 2015;77(3):265–277.
- Ziogas V, Tanou G, Filippou P, et al. Nitrosative responses in citrus plants exposed to six abiotic stress conditions. Plant Physiol Biochem. 2013;68:118–126.
- Vendrell KM, Pech H, Romojaro J, et al. Iology and biotechnology of the plant hormone ethylene II. Dordrecht: Springer, 1997.
- Delledonne M, Xia Y, Dixon RA, et al. Nitric oxide functions as a signal in plant disease resistance. Nature. 1998;394(6693):585–588.
- Pedroso MC, Magalhaes JR, Durzan D. A nitric oxide burst precedes apoptosis in angiosperm and gymnosperm callus cells and foliar tissues. J Exp Bot. 2000;51(347):1027–1036.
- Zhao MG, Tian QY, Zhang WH. Nitric oxide synthase-dependent nitric oxide production is associated with salt tolerance in Arabidopsis. Plant Physiol. 2007;144(1):206–217.
- Groß F, Durner J, Gaupels F. Nitric oxide, antioxidants and prooxidants in plant defence responses. Front Plant Sci. 2013;4:419.
- Quast DH, Otto W. Golf course turf management. New York, NY: McGraw-Hill; 2004.
- Yin LJ, Zhu L. Study on salt tolerance of wild barley seedling. Acta Prataculturae Sin. 1991;1:142–148.
- Ik A, Mar B, Saa A. Amelioration of salt induced toxicity in pearl millet by seed priming with silver nanoparticles (agnps): the oxidative damage, antioxidant enzymes and ions uptake are major determinants of salt tolerant capacity - sciencedirect. Plant Physiol Biochem. 2020;156:221–232.
- Sun Y, Cui GW, Zhang C, et al. Effect of NaCl stress on ultrastructure of mesophyll cells in Hordeum brevisubulatum. Chin J Grassl. 2015;37:102–106.
- Siddiqui MH, Al-Whaibi MH, Basalah MO. Role of nitric oxide in tolerance of plants to abiotic stress. Protoplasma. 2011;248(3):447–455.
- Ahanger MA, Aziz U, Alsahli AA, et al. Influence of exogenous salicylic acid and nitric oxide on growth, photosynthesis, and ascorbate-glutathione cycle in salt stressed Vigna angularis. Biomolecules. 2019;10(1):42.
- Aying Z, Mingyi J, Jianhua Z, et al. Nitric oxide induced by hydrogen peroxide mediates abscisic acid-induced activation of the mitogen-activated protein kinase Cascade involved in antioxidant defense in maize leaves. New Phytol. 2007;175(1):36–50.
- Marvasi M. Potential use and perspectives of nitric oxide donors in agriculture. J Sci Food Agric. 2017;97(4):1065–1072.
- Hasanuzzaman M, Oku H, Nahar K, et al. Nitric oxide-induced salt stress tolerance in plants: ROS metabolism, signaling, and molecular interactions. Plant Biotechnol Rep. 2018;12(2):77–92.
- Hussain S, Khaliq A, Tanveer M, et al. Aspirin priming circumvents the salinity-induced effects on wheat emergence and seedling growth by regulating starch metabolism and antioxidant enzyme activities. Acta Physiol Plant. 2018;40:68.
- Neha S, Sathish B. Nitric oxide regulates lateral root formation through modulation of ACC oxidase activity in sunflower seedlings under salt stress. Plant Signal Behav. 2018;13:1–7.
- Hoagland DR, Arnon DI. The water culture method for growing plants without soil. Los Angeles. CA: The College of Agriculture, University of California; 1950.
- Wellburn AR. The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J Plant Physiol. 1994;144(3):307–313.
- Liu M, Jiang Y. Genotypic variation in growth and metabolic responses of perennial ryegrass exposed to short-term waterlogging and submergence stress. Plant Physiol Bioch. 2015;95:57.
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254.
- Zhang J, Kirkham MB. Lipid peroxidation in sorghum and sunflower seedlings as affected by ascorbic acid, benzoic acid, and propyl gallate. J Plant Physiol. 1996;149(5):489–493.
- Khaliq A, Zia-Ul-Haq M, Aslam F, et al. Salinity tolerance in wheat cultivars is related to enhanced activities of enzymatic antioxidants and reduced lipid peroxidation. Clean Soil Air Water. 2015;43(8):1248–1258.
- El-Esawi MA, Alaraidh IA, Alsahli AA, et al. Bacillus firmus (SW5) augments salt tolerance in soybean (Glycine max L.) by modulating root system architecture, antioxidant defense systems and stress-responsive genes expression. Plant Physiol Biochem. 2018;132:375–384.
- Desoky EM, Elrys AS, Rady MM. Integrative moringa and licorice extracts application improves Capsicum annuum fruit yield and declines its contaminant contents on a heavy metals-contaminated saline soil. Ecotoxicol Environ Saf. 2019;169:50–60.
- Zhu T, Deng XG, Tan WR, et al. Nitric oxide is involved in brassinosteroid-induced alternative respiratory pathway in Nicotiana benthamiana seedlings’ response to salt stress. Physiol Plant. 2016;156(2):150–163.
- Kim DY, Hong MJ, Seo YW. Role of wheat trHb in nitric oxide scavenging. Mol Biol Rep. 2014;41(9):5931–5941.
- Messedi D, Slama I, Laabidi N, et al. Effect of nitrogen deficiency, salinity and drought on proline metabolism in Sesuvium portulacastrum. In: Öztürk M, Waisel Y, Khan MA, Görk G, editors. Biosaline agriculture and salinity tolerance in plants. Basel: Birkhäuser Basel; 2006. p. 65–72.
- Tian X, He M, Wang Z, et al. Application of nitric oxide and calcium nitrate enhances tolerance of wheat seedlings to salt stress. Plant Growth Regul. 2015;77(3):343–356.
- Dogan M, Saygideger S. Pysiological effects of NaCl on Ceratophyllum demersum L., a submerged rootless aquatic macrophyte. Iran J Fish Sci. 2018;17:346–356.
- Guo M, Liu X, Wang J, et al. Investigation on salt-response mechanisms in Arabidopsis thaliana from UniProt protein knowledgebase. J Plant Interact. 2019;14(1):21–29.
- Kaur H, Sirhindi G, Bhardwaj R, et al. 28-homobrassinolide regulates antioxidant enzyme activities and gene expression in response to salt- and temperature-induced oxidative stress in Brassica juncea. Sci Rep. 2018;8(1):8735.
- Dinler BS, Antoniou C, Fotopoulos V. Interplay between GST and nitric oxide in the early response of soybean (Glycine max L.) plants to salinity stress. J Plant Physiol. 2014;171(18):1740–1747.
- Ahmad P, Jaleel CA, Salem MA, et al. Roles of enzymatic and nonenzymatic antioxidants in plants during abiotic stress. Crit Rev Biotechnol. 2010;30(3):161–175.
- Kaya C, Akram NA, Ashraf M. Influence of exogenously applied nitric oxide on strawberry (Fragaria × ananassa) plants grown under iron deficiency and/or saline stress. Physiol Plant. 2019;165(2):247–263.
- Wendehenne D, Durner J, Klessig DF. Nitric oxide: a new player in plant signalling and defence responses. Curr Opin Plant Biol. 2004;7(4):449–455.
- Aftab T, Khan MMA, da Silva JAT, et al. Role of salicylic acid in promoting salt stress tolerance and enhanced artemisinin production in Artemisia annua L. J Plant Growth Regul. 2011;30:425–435.
- Sudhir P, Murthy SDS. Effects of salt stress on basic processes of photosynthesis. Photosynt. 2004;42(4):481–486.
- Kohli SK, Khanna K, Bhardwaj R, et al. Assessment of subcellular ros and no metabolism in higher plants: multifunctional signaling molecules. Antioxidants. 2019;8(12):641.
- Piacentini D, Ronzan M, Fattorini L, et al. Nitric oxide alleviates cadmium- but not arsenic-induced damages in rice roots. Plant Physiol Biochem. 2020;151:729–742.
- Scheler C, Durner J, Astier J. Nitric oxide and reactive oxygen species in plant biotic interactions. Curr Opin Plant Biol. 2013;16(4):534–539.
- Siddiqui M, Alamri S, Alsubaie Q, et al. Exogenous nitric oxide alleviates sulfur deficiency-induced oxidative damage in tomato seedlings. Nitric Oxide. 2020;94:95–107.
- Song CP, Guo Y, Qiu Q, et al. A probable Na+(K+)/H + exchanger on the chloroplast envelope functions in pH homeostasis and chloroplast development in Arabidopsis thaliana. Proc Natl Acad Sci U S A. 2004;101(27):10211–10216.
- Ma C, White J, Dhankher O, et al. Metal-based nanotoxicity and detoxification pathways in higher plants. Environ Sci Technol. 2015;49(12):7109–7122.
- Zhang F, Wang Y, Liu C, et al. Trichoderma harzianum mitigates salt stress in cucumber via multiple responses. Ecotoxicol Environ Saf. 2019;170:436–445.
- Yastreb TO, Kolupaev YE, Karpets YV, et al. Effect of nitric oxide donor on salt resistance of Arabidopsis jin1 mutants and wild-type plants. Russ J Plant Physiol. 2017;64(2):207–214.
- Wang J, Zhang L, Wang X, et al. PvNAC1 increases biomass and enhances salt tolerance by decreasing Na + accumulation and promoting ROS scavenging in switchgrass (Panicum virgatum L.). Plant Sci. 2019;280:66–76.

