Abstract
Background
The study objective was to assess the effect of vutrisiran, an RNA interference therapeutic that reduces transthyretin (TTR) production, in patients with hereditary transthyretin (ATTRv) amyloidosis with polyneuropathy.
Methods
HELIOS-A was a phase 3, global, open-label study comparing the efficacy and safety of vutrisiran with an external placebo group (APOLLO study). Patients were randomized 3:1 to subcutaneous vutrisiran 25 mg every 3 months (Q3M) or intravenous patisiran 0.3 mg/kg every 3 weeks (Q3W) for 18 months.
Results
HELIOS-A enrolled 164 patients (vutrisiran, n = 122; patisiran reference group, n = 42); external placebo, n = 77. Vutrisiran met the primary endpoint of change from baseline in modified Neuropathy Impairment Score +7 (mNIS+7) at 9 months (p = 3.54 × 10−12), and all secondary efficacy endpoints; significant improvements versus external placebo were observed in Norfolk Quality of Life-Diabetic Neuropathy, 10-meter walk test (both at 9 and 18 months), mNIS+7, modified body-mass index, and Rasch-built Overall Disability Scale (all at 18 months). TTR reduction with vutrisiran Q3M was non-inferior to within-study patisiran Q3W. Most adverse events were mild or moderate in severity, and consistent with ATTRv amyloidosis natural history. There were no drug-related discontinuations or deaths.
Conclusions
Vutrisiran significantly improved multiple disease-relevant outcomes for ATTRv amyloidosis versus external placebo, with an acceptable safety profile.
ClinicalTrials.gov
NCT03759379
Introduction
Hereditary transthyretin (ATTRv; v for variant) amyloidosis, also known as hATTR amyloidosis, is a rare, rapidly progressive, debilitating, and fatal disease caused by transthyretin (TTR) gene variants [Citation1–4]. Misfolded TTR accumulates as amyloid deposits in multiple organs and tissues [Citation5–8], resulting in a heterogeneous clinical presentation including sensory, motor, and autonomic polyneuropathy, and cardiomyopathy [Citation2,Citation9–11]. ATTRv amyloidosis, which affects approximately 50,000 people worldwide [Citation12], has an aggressive course in which disease progression is associated with increased symptom severity, decreased quality of life (QOL), loss of physical function, and death [Citation4,Citation13]. Median survival is 4.7 years following diagnosis [Citation14], with reduced survival (3.4 years) for patients presenting with cardiomyopathy [Citation15–17].
Effective, early treatment of multisystem organ dysfunction by targeting underlying disease pathophysiology is crucial to reducing the burden and progression of ATTRv amyloidosis. Treatment strategies include those which stabilize the tetrameric structure of TTR and those which reduce pathogenic TTR protein levels through silencing of the TTR gene, either by harnessing the natural process of RNA interference (RNAi) or by using antisense oligonucleotides [Citation18–21].
Vutrisiran is an RNAi therapeutic that reduces serum TTR levels by reducing synthesis of variant and wild-type TTR [Citation22,Citation23]. The vutrisiran small interfering RNA (siRNA) is directed to the liver, the primary site of TTR synthesis, by conjugation to a triantennary N-acetylgalactosamine (GalNAc) ligand that binds the asialoglycoprotein receptor expressed on the surface of hepatocytes [Citation22–25]. The vutrisiran siRNA–GalNAc conjugate features an enhanced stabilization chemistry (ESC) design for increased potency and high metabolic stability allowing for once every 3 months (Q3M) subcutaneous (SC) injection. In a phase 1 study in healthy volunteers, vutrisiran was generally well tolerated and elicited robust and durable TTR reduction [Citation23]. Vutrisiran was recently approved for the treatment of the polyneuropathy of ATTRv amyloidosis in adults [Citation22].
Patisiran utilizes the same RNAi approach as vutrisiran to target variant and wild-type TTR synthesis [Citation20,Citation26,Citation27]. The patisiran siRNA is encapsulated in a lipid nanoparticle formulation that directs it to the liver following intravenous (IV) administration [Citation28]. Patisiran was approved based on the pivotal phase 3, randomized, double-blind, placebo-controlled, 18-month APOLLO study, which demonstrated improvements in polyneuropathy and all secondary endpoints compared with placebo, in patients with ATTRv amyloidosis with polyneuropathy [Citation20]. Patisiran 0.3 mg/kg every 3 weeks (Q3W) is now approved in more than 30 countries for the treatment of ATTRv amyloidosis with polyneuropathy [Citation29,Citation30]. Here, we report efficacy and safety data from the 18-month treatment period of the HELIOS-A study, a phase 3, open-label, randomized study evaluating vutrisiran in patients with ATTRv amyloidosis with polyneuropathy.
Methods
Trial design
HELIOS-A was a phase 3, global, randomized, open-label study conducted at 57 sites in 22 countries (registered as NCT03759379 at ClinicalTrials.gov November 30, 2018). The study protocol and amendments were approved by relevant Institutional Review Boards or Independent Ethics Committees. Written informed consent was obtained from each participant. The study was conducted in accordance with all applicable regulatory requirements, the current guidelines of Good Clinical Practice, and principles originating from the Declaration of Helsinki.
Patients
Patients were aged 18–85 years with a diagnosis of ATTRv amyloidosis with a documented TTR variant and neuropathy (baseline Neuropathy Impairment Score [NIS] of 5–130), a polyneuropathy disability (PND) score of ≤ IIIb, a Karnofsky Performance Status score of ≥60%, and adequate liver and renal function. Patients who had received previous gene-silencing therapy were excluded. Previous use of TTR stabilizers was permitted but patients must have completed a wash-out period (14 days for tafamidis; 3 days for diflunisal) prior to study drug dosing. Patients with prior liver transplantation or likely to undergo liver transplantation during the 18-month treatment period and those with a New York Heart Association heart failure class > II were excluded.
Randomization and treatment
Eligible patients were randomized 3:1 to treatment with vutrisiran 25 mg SC Q3M or patisiran 0.3 mg/kg IV Q3W, for those in the reference group, for 18 months. Randomization is described in the Supplementary Methods. Following the randomized treatment period, all patients were eligible to receive vutrisiran in an open-label treatment extension phase.
Patients randomized to patisiran received premedication approximately 60 min before each infusion (details in the Supplementary Methods) to minimize the risk of infusion-related reactions (IRRs). Patients who received vutrisiran did not require premedication. All patients were instructed to take the recommended daily allowance of vitamin A. The placebo group of the APOLLO study [Citation20], which had similar endpoints and eligibility criteria to HELIOS-A, was used as an external placebo control for the primary endpoint and most secondary and exploratory endpoints.
Outcomes
The primary endpoint was the change in neuropathy impairment from baseline as measured by a modified Neuropathy Impairment Score +7 (mNIS+7) compared with the placebo group of the APOLLO study (external placebo group) at Month 9. Secondary endpoints were also compared with the external placebo group and are listed here in the prespecified hierarchical order for statistical testing: change from baseline in QOL (total score on the Norfolk Quality of Life-Diabetic Neuropathy questionnaire [Norfolk QOL-DN]) at Month 9; gait speed (10-meter walk test [10-MWT]) at Month 9; mNIS+7 at Month 18; Norfolk QOL-DN total score at Month 18; 10-MWT at Month 18; nutritional status (modified body mass index [mBMI]) at Month 18; and disability (Rasch-built Overall Disability Scale [R-ODS] score) at Month 18. In addition, non-inferiority in serum TTR level percent reduction through Month 18 in the vutrisiran group compared with the within-study patisiran group was a secondary endpoint. Further details on efficacy endpoints, and amendments made to mitigate the potential impact of the COVID-19 pandemic, are included in the Supplementary Methods. Safety outcomes were recorded throughout the study and are detailed in the Supplementary Methods.
Statistical analyses
A sample size of approximately 160 patients was selected to provide >90% power to establish the superiority of vutrisiran over external placebo for the original co-primary endpoints, using a 0.05 significance level (further detail in the Supplementary Methods).
The primary population for efficacy analysis was the modified intent-to-treat (mITT) population (randomized patients who received any amount of study drug; same patients as the safety population). TTR percent reduction analysis was performed in the TTR per-protocol population (mITT patients with a non-missing TTR assessment at baseline and ≥1 trough TTR assessment with adequate treatment compliance between Months 6 and 18). A predefined cardiac subpopulation (patients with baseline left ventricular wall thickness ≥1.3 cm and no aortic valve disease or hypertension in their medical history), analogous to the APOLLO cardiac subpopulation, was analyzed to determine the effect of vutrisiran treatment on cardiac manifestations. Assessments in the cardiac subpopulation are exploratory and are not reported here.
Efficacy endpoints at Month 9 were assessed with an analysis of covariance model with multiple imputation and at Month 18 with a mixed-effects model for repeated measures (details in the Supplementary Methods). Secondary endpoints were analyzed in the prespecified hierarchical order described above to control the overall type I error. Sensitivity analyses performed for change from baseline in mNIS+7 and Norfolk QOL-DN at Month 9 utilized a propensity score method to account for differences in patient baseline characteristics (including those between the HELIOS-A vutrisiran group and the external placebo group). Further details are given in the Supplementary Methods. Non-inferiority of vutrisiran versus within-study patisiran in TTR percent reduction was declared if the lower limit of the 95% confidence interval (CI) for the treatment difference was greater than −10%. For all primary and secondary endpoints (excluding reduction in serum TTR levels), the comparison between vutrisiran and within-study patisiran treatments was summarized descriptively and not tested statistically.
Analyses of adverse events (AEs) are presented for events that are considered treatment-emergent, which is defined as any AE with onset during or after the administration of the study drug through 28 days following the last dose of patisiran or 84 days following the last dose of vutrisiran. In addition, any event considered drug-related is also considered treatment-emergent. Deaths are reported regardless of treatment-emergent status.
Results
Patient disposition
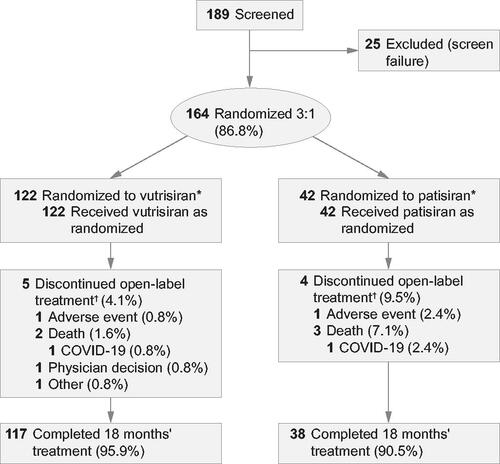
Between February 2019 and March 2020, 164 patients were randomized, received treatment, and were included in the mITT population (vutrisiran, n = 122; patisiran, n = 42; ). In the vutrisiran and patisiran groups, 117 (95.9%) and 38 (90.5%) patients, respectively, completed the randomized 18-month treatment period; the primary reason for study discontinuation was death which occurred in 2/122 (1.6%) and 3/42 (7.1%) patients, respectively (Supplementary Table S1).
Figure 1. Patient disposition. *Modified intent-to-treat population: all patients who were randomized and received at least one dose of the study drug. †Numbers of discontinuations to the end of 18 months. One patient in each treatment group discontinued due to suspected or confirmed diagnosis of COVID-19 or due to the impact of the global COVID-19 pandemic, reported in addition to the primary reason for treatment discontinuation. There were two deaths due to COVID-19, one in each treatment arm.
Baseline demographics and disease characteristics
The patient population enrolled included a wide range of disease severity and was representative of the global population with this disease. Baseline characteristics were similar across treatment groups in HELIOS-A and APOLLO placebo groups (). Overall, the patient group was 64.6% male with a median (interquartile range [IQR]) age of 60 years (18) and a median (IQR) time since ATTRv amyloidosis diagnosis of 2.22 years (4.15); 45.1% of patients had the V30M TTR variant; patients with 26 different TTR variants were included in the HELIOS-A study (Supplementary Table S2). The majority of patients had previous treatment with TTR stabilizers, including 61.5% of the vutrisiran group. The HELIOS-A vutrisiran group had a greater proportion of patients with PND I/II and NIS <50 than the external placebo group (n = 77), although the two populations had widely overlapping characteristics and were clinically comparable ().
Table 1. Baseline demographics and clinical characteristics.
Primary outcome
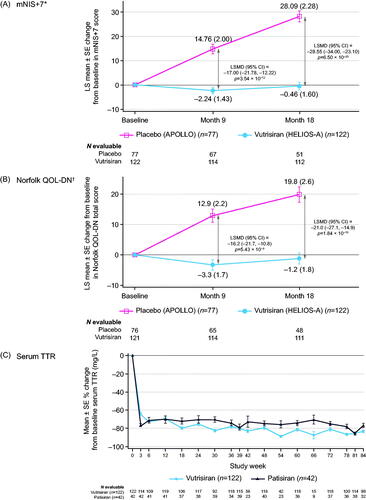
Vutrisiran treatment resulted in statistically significant improvement in mNIS+7 at Month 9 versus the external placebo group (least squares [LS] mean change from baseline: −2.24 [vutrisiran] and +14.76 [placebo]; LS mean difference [95% CI]: −17.00 [–21.78, −12.22], p = 3.54 × 10−12), meeting the primary endpoint (). The treatment effect of vutrisiran on mNIS+7 at Month 9 was validated by sensitivity analyses. At Month 9, 50.4% of patients in the vutrisiran group showed improvement in mNIS+7 (decrease from baseline) versus 18.2% in the external placebo group (odds ratio [OR] [95% CI]: 4.8 [2.4, 9.5]; nominal p = 4.64 × 10−6; Supplementary Figure S1A).
Figure 2. Key efficacy and pharmacodynamic endpoints assessing the effect of vutrisiran. LS mean change from baseline in (A) mNIS+7, (B) Norfolk QOL-DN, and (C) percent change from baseline in serum TTR levels with vutrisiran and patisiran through 18 months of the HELIOS-A study. The mNIS+7 and Norfolk QOL-DN data were calculated using the modified intent-to-treat population; the reduction in serum TTR was calculated using the TTR per-protocol population. *Higher scores of mNIS+7 indicate more neuropathy impairment (range, 0–304). At baseline, the mean (±SD) mNIS+7 was 60.6 (36.0) in the vutrisiran group and 74.6 (37.0) in the external placebo group. Data at 9 months are from the ANCOVA/multiple imputation model and data at 18 months are from the MMRM model. †Higher scores of Norfolk QOL-DN indicate worse quality of life (range, –4 to 136). At baseline, the mean (±SD) Norfolk QOL-DN score was 47.1 (26.3) in the vutrisiran group and 55.5 (24.3) in the external placebo group. Data at 9 months are from the ANCOVA/multiple imputation model and data at 18 months are from the MMRM model. ANCOVA: analysis of covariance; CI: confidence interval; LS: least squares; LSMD: least squares mean difference; MMRM: mixed-effects model for repeated measures; mNIS+7: modified Neuropathy Impairment Score +7; Norfolk QOL-DN: Norfolk Quality of Life-Diabetic Neuropathy; SD: standard deviation; SE: standard error; TTR: transthyretin.
Secondary outcomes
Significant improvement in mNIS+7 with vutrisiran compared with the external placebo group was also observed at Month 18, with an LS mean change from baseline: −0.46 for vutrisiran and 28.1 for external placebo (LS mean difference [95% CI]: −28.55 [−34.00, −23.10], p = 6.50 × 10−20) (; ). The treatment effect favoring vutrisiran at Months 9 and 18 was consistent across all prespecified patient subgroups and subcomponents of mNIS+7 (Supplementary Figures S2 and S3). At Month 18, 48.3% of patients in the vutrisiran group showed improvement in mNIS+7 versus 3.9% in the external placebo group (OR [95% CI]: 22.9 [6.8, 76.9]; nominal p = 7.53 × 10−11; Supplementary Figure S1B).
Table 2. Select secondary and exploratory endpoints in the mITT population.
Vutrisiran treatment also significantly improved the total Norfolk QOL-DN score compared with the external placebo group at Month 9 (LS mean change from baseline: −3.3 [vutrisiran] and +12.9 [placebo]; LS mean difference [95% CI]: −16.2 [–21.7, −10.8], p = 5.43 × 10−9) and Month 18 (LS mean change from baseline: −1.2 [vutrisiran] and 19.8 [placebo]; LS mean difference [95% CI]: −21.0 [–27.1, −14.9], p = 1.84 × 10−10) (; ). As with mNIS+7, the treatment effect for Norfolk QOL-DN at Months 9 and 18 was consistent across all prespecified subgroups (Supplementary Figure S4) and individual domains of the score (Supplementary Figure S5). At Month 9, 53.4% of patients in the vutrisiran group showed improvement (decrease from baseline) in Norfolk QOL-DN score versus 23.4% in the external placebo group (OR [95% CI]: 4.0 [2.1, 7.8], nominal p = 1.92 × 10−5; Supplementary Figure S1A). By Month 18, the percentage of patients showing improvement in Norfolk QOL-DN score was 56.8% vs 10.4% in the vutrisiran and external placebo groups, respectively (OR [95% CI]: 11.3 [5.0, 25.7], nominal p = 9.37 × 10−11; Supplementary Figure S1B).
Significant improvements with vutrisiran treatment compared with the external placebo group were observed for all other secondary endpoints, including 10-MWT at Months 9 and 18, mBMI at Month 18, and R-ODS at Month 18 (). The mean changes from baseline for primary and secondary efficacy endpoints in the within-study patisiran group (Supplementary Table S3) were similar to those in the vutrisiran group.
Pharmacodynamics
Vutrisiran treatment resulted in rapid (≤3 weeks) and sustained reduction in serum TTR levels over 18 months, similar to what was observed in the within-study patisiran group (). Following 18 months of vutrisiran treatment, steady-state mean (SD) peak and trough serum TTR reductions from baseline were 87.6% (15.7%) and 81.0% (21.0%), respectively (Supplementary Table S4).
TTR reduction with vutrisiran was statistically non-inferior to within-study patisiran in the TTR per-protocol population (secondary endpoint), assessed by mean trough serum TTR levels over 18 months. The fluctuation between median steady-state peak and trough values was lower with vutrisiran (peak-trough = Δ; 91.6–86.2%=5.4%) compared with patisiran (88.3–78.2%=10.1%) (Supplementary Table S4), which was reflected in the reduced variability in TTR reduction (smaller standard error) observed at most time points with vutrisiran ().
Serum TTR reduction with vutrisiran was also similar across all patient subgroups. As expected from previous studies, serum vitamin A levels were reduced in parallel with reductions in serum TTR levels in both treatment groups [Citation31].
Adverse events
During the 18-month treatment period, AEs were reported in 119 (97.5%) patients in the vutrisiran group, with the majority mild or moderate in severity (). There were two (1.6%) deaths in the vutrisiran group and three (7.1%) deaths in the patisiran group, none of which were considered drug-related (Supplementary Table S1). One death in each group was due to COVID-19. The non-COVID-19 deaths, one in the vutrisiran group and two in the patisiran group, were seen in patients with non-V30M TTR variants who had medical histories of cardiac disease. Three (2.5%) patients in the vutrisiran group discontinued treatment, and also stopped study participation, due to AEs by Month 18 (two of which were due to death). AEs leading to discontinuation included acute cardiac failure, COVID-19 pneumonia, and iliac artery occlusion (each n = 1; 0.8%), none of which were considered related to vutrisiran.
Table 3. Summary of adverse events.
Two (1.6%) patients experienced serious AEs considered related to vutrisiran (one dyslipidemia and one urinary tract infection). AEs occurring in ≥10% of patients receiving vutrisiran included falls, pain in extremity, diarrhea, peripheral edema, urinary tract infection, arthralgia, and dizziness; all of which, except pain in extremity and arthralgia, occurred at a similar or lower rate than in the external placebo group (). There were no cardiac AEs related to vutrisiran in the safety population.
Five patients (4.1%) who received vutrisiran reported mild and transient injection site reactions (ISRs). In total, 5/836 (0.6%) injections led to ISRs. IRRs, which are reported with patisiran due to their different mode of administration, occurred in 10 (23.8%) patients who received patisiran.
There were no safety signals regarding liver function tests, hematology, or renal function related to vutrisiran. A total of 4 (3.3%) vutrisiran-treated patients developed anti-drug antibodies (ADAs). ADA titers were low and transient with no evidence of an effect on clinical efficacy, safety, or pharmacodynamic parameters of vutrisiran.
The study took place during the COVID-19 pandemic. Five patients in the vutrisiran group missed efficacy assessments due to COVID-19: two at both 9 and 18 months, two at 9 months (who remained on study until 18 months), and one at 18 months. In addition, 1/836 (0.1%) and 27/1065 (2.5%) doses were missed, and 18/836 (2.2%) and 3/1065 (0.3%) doses were delayed due to COVID-19 in the vutrisiran and patisiran groups, respectively (Supplementary Table S5).
Discussion
In the HELIOS-A study, vutrisiran met the primary and all secondary efficacy endpoints at 9 and 18 months, demonstrating significant improvements in neuropathy impairment, QOL, gait speed, nutritional status, and disability compared with the external placebo group. Furthermore, for assessments of neuropathy and QOL, improvement relative to baseline was observed in approximately half of vutrisiran-treated patients, demonstrating reversal of disease manifestations. The improvements with vutrisiran treatment were apparent after 9 months of treatment, suggesting that patients can derive clinical benefit over this timeframe. Vutrisiran was generally well tolerated and demonstrated an acceptable safety profile, with the majority of AEs being mild or moderate in severity and generally consistent with those expected as a consequence of ATTRv amyloidosis [Citation4,Citation13]. In vutrisiran-treated patients, there was a low mortality rate and a low rate of treatment discontinuation due to AEs, and none of these events were considered to be related to vutrisiran treatment.
ATTRv amyloidosis affects multiple systems, necessitating the analysis of a range of endpoints to thoroughly assess the disease burden [Citation20,Citation21,Citation32]. In this study, vutrisiran improved a wide range of disease-relevant endpoints compared with an external placebo. These beneficial effects of vutrisiran were observed alongside an acceptable safety profile, affording further confidence in the RNAi therapeutic approach for this disease. Indeed, improvements in efficacy outcomes with vutrisiran were generally similar to those observed with patisiran, although no statistical testing was conducted as HELIOS-A was not designed to compare the two treatments. Together, the findings from HELIOS-A build on the previously reported experience with patisiran to afford further confidence in the RNAi therapeutic approach for this disease [Citation20].
TTR reduction with vutrisiran Q3M SC injection was demonstrated to be non-inferior to patisiran Q3W IV infusion, confirming the potency and stability associated with the ESC-siRNA design. In addition to allowing for infrequent dosing, this ESC design also resulted in a reduced peak-to-trough fluctuation of TTR reduction in the vutrisiran group compared with the patisiran group. Notably, the conjugation of the siRNA to GalNAc enables SC administration of vutrisiran without the need for premedication, in contrast to patisiran infusion [Citation20]. Importantly, there were few (4%) ISR AEs reported. These features allow for a reduced burden of care for patients, along with the acceptable safety and efficacy data previously described. The benefits of the infrequent, Q3M SC regimen were particularly apparent in HELIOS-A as the study was conducted during the COVID-19 pandemic, which posed a challenge to administering treatment in clinical trials. However, the pandemic had minimal impact on vutrisiran dosing. Increased compliance is likely to be clinically valuable as the long-term real-world benefits of chronically administered pharmacotherapies are better realized when patient compliance is high [Citation33,Citation34]. Furthermore, the infrequent Q3M SC dosing of vutrisiran may result in a lower burden on the healthcare system. The ongoing extension period of HELIOS-A will assess long-term safety and efficacy with continued Q3M vutrisiran treatment, or an alternative every 6 months dosing regimen.
An important limitation of this study was the use of an external placebo control with an open-label design rather than a within-trial placebo group in a double-blind study. An external placebo group was primarily chosen to allow a more efficient trial design in which all patients could receive active treatment. Furthermore, the populations in HELIOS-A and APOLLO were expected to be similar due to similarities in recruitment criteria. While some differences in baseline characteristics were observed, with the HELIOS-A vutrisiran group having a greater proportion of patients with PND I/II and NIS <50 than the external placebo group, access to patient-level data from the external placebo group allowed for differences in baseline characteristics to be accounted for during the analyses. The validity of this approach is supported by the propensity score analysis, a prespecified sensitivity analysis that incorporates baseline variables covering potential differences between the two study populations. Future analyses of these data looking at the impact of vutrisiran compared with external placebo across different quartiles of baseline disease severity may further support the interpretation.
In the HELIOS-A phase 3 study, vutrisiran treatment improved neuropathy and QOL in addition to a range of important disease manifestations in patients with ATTRv amyloidosis, with an acceptable safety profile. These data are consistent with those reported in the phase 3 APOLLO study of patisiran, affording further confidence in this therapeutic approach, while providing an infrequent SC route of administration. The long-term safety and efficacy of vutrisiran will continue to be investigated in the ongoing extension period of this trial.
| Abbreviations | ||
| 10-MWT | = | 10-meter walk test |
| ADA | = | anti-drug antibody |
| AE | = | adverse event |
| ATTRv | = | hereditary transthyretin (v for variant) |
| ANCOVA | = | analysis of covariance |
| CI | = | confidence interval |
| ESC | = | enhanced stabilization chemistry |
| GalNAc | = | N-acetylgalactosamine |
| hATTR | = | hereditary transthyretin-mediated |
| IQR | = | interquartile range |
| IRR | = | infusion-related reaction |
| ISR | = | injection site reaction |
| IV | = | intravenous |
| LS | = | least squares |
| LSMD | = | least squares mean difference |
| mBMI | = | modified body mass index |
| mITT | = | modified intent-to-treat |
| MMRM | = | mixed-effects model for repeated measures |
| mNIS+7 | = | modified Neuropathy Impairment Score +7 |
| NIS | = | Neuropathy Impairment Score |
| Norfolk QOL-DN | = | Norfolk Quality of Life-Diabetic Neuropathy |
| NT-proBNP | = | N-terminal pro-brain natriuretic peptide |
| OR | = | odds ratio |
| PND | = | polyneuropathy disability |
| Q3M | = | every 3 months |
| Q3W | = | every 3 weeks |
| QOL | = | quality of life |
| R-ODS | = | Rasch-built Overall Disability Scale |
| RNAi | = | RNA interference |
| SC | = | subcutaneous |
| SD | = | standard deviation |
| SE | = | standard error |
| siRNA | = | small interfering RNA |
| TTR | = | transthyretin. |
Supplemental Material
Download MS Word (31.8 KB)Supplemental Material
Download MS Word (1.9 MB)Acknowledgments
The authors would like to thank the patients and their families for their participation in the HELIOS-A study. In addition, they would like to thank Rebecca Shilling for her contribution to the study and manuscript content, Emre Aldinc for his contribution to the manuscript content, Rick Blakesley for his support in data analysis, and the members of the HELIOS-A Collaborators group for their work on the study (The HELIOS-A Collaborators list is available here). Editorial assistance was provided by Ed Childs of Adelphi Communication Ltd, and funded by Alnylam Pharmaceuticals.
Disclosure statement
Prof. Adams reports consultancy for Alnylam Pharmaceuticals, Eidos, and Pfizer Inc. Prof. Tournev reports speakers bureau/lecturer fees from Ewopharma, Genesis Pharma, Pfizer Inc., Roche, and Sanofi. Also, advisory board/committee and consultancy fees from Alnylam, Novartis, Pfizer, and Roche and research grants from Pfizer, Roche, and Sanofi. Dr. Taylor has received honoraria from Pfizer Inc. Dr. Coelho reports no financial disclosures. Institution was paid per protocol for the participation in the trials sponsored by Alnylam. Prof. Planté-Bordeneuve reports no financial disclosures. Dr. Berk reports consultancy for Akcea Therapeutics, Corino Therapeutics, Intellia Therapeutics, and Ionis Pharmaceuticals. Also, research funding from Pfizer Inc., and consultancy and research funding from Alnylam Pharmaceuticals, Eidos Therapeutics, and Ionis Pharmaceuticals. Dr. González-Duarte has received honoraria from Alnylam Pharmaceuticals and Pfizer Inc. Prof. Gillmore reports consultancy for Alnylam Pharmaceuticals. Dr. Low reports no financial disclosures. Prof. Sekijima reports honoraria and research funding from Alnylam Pharmaceuticals and Pfizer Inc. Dr. Obici reports speakers bureau fees from Akcea Therapeutics, Alnylam Pharmaceuticals, Pfizer Inc., and SOBI. Drs. Chen, Badri, and Arum report being employees of Alnylam Pharmaceuticals. Dr. Vest reports being an employee of Alnylam Pharmaceuticals, and reports ownership of equity in Alnylam Pharmaceuticals. Prof. Polydefkis reports consultancy for Akcea, Alnylam Pharmaceuticals, Biogen-Idec, Pfizer Inc., and Vertex Pharmaceuticals.
Data availability statement
De-identified individual participant data that support these results will be made available in a secure-access environment 12 months after study completion and when the product and indication have been approved for no less than 12 months in the US and the EU. Access will be provided contingent upon the approval of a research proposal and the execution of a data sharing agreement. Requests for access to data can be submitted via the website www.vivli.org.
Correction Statement
This article has been corrected with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Hawkins PN, Ando Y, Dispenzeri A, et al. Evolving landscape in the management of transthyretin amyloidosis. Ann Med. 2015;47(8):625–638.
- Mohty D, Damy T, Cosnay P, et al. Cardiac amyloidosis: updates in diagnosis and management. Arch Cardiovasc Dis. 2013;106(10):528–540.
- Hanna M. Novel drugs targeting transthyretin amyloidosis. Curr Heart Fail Rep. 2014;11(1):50–57.
- Adams D, Coelho T, Obici L, et al. Rapid progression of familial amyloidotic polyneuropathy: a multinational natural history study. Neurology. 2015;85(8):675–682.
- Kelly JW. Amyloid fibril formation and protein misassembly: a structural quest for insights into amyloid and prion diseases. Structure. 1997;5(5):595–600.
- Klimtchuk ES, Prokaeva T, Frame NM, et al. Unusual duplication mutation in a surface loop of human transthyretin leads to an aggressive drug-resistant amyloid disease. Proc Natl Acad Sci USA. 2018;115:E6428–E6436.
- Koike H, Katsuno M. Ultrastructure in transthyretin amyloidosis: from pathophysiology to therapeutic insights. Biomedicines. 2019;7(1):11.
- Mangione PP, Verona G, Corazza A, et al. Plasminogen activation triggers transthyretin amyloidogenesis in vitro. J Biol Chem. 2018;293(37):14192–14199.
- Shin SC, Robinson-Papp J. Amyloid neuropathies. Mt Sinai J Med. 2012;79(6):733–748.
- Conceição I, Gonzalez-Duarte A, Obici L, et al. "Red-flag" symptom clusters in transthyretin familial amyloid polyneuropathy. J Peripher Nerv Syst. 2016;21(1):5–9.
- Adams D, Koike H, Slama M, et al. Hereditary transthyretin amyloidosis: a model of medical progress for a fatal disease. Nat Rev Neurol. 2019;15(7):387–404.
- Gertz MA. Hereditary ATTR amyloidosis: burden of illness and diagnostic challenges. Am J Manag Care. 2017;23(7 Suppl):S107–S112.
- Adams D, Suhr OB, Hund E, et al. First European consensus for diagnosis, management, and treatment of transthyretin familial amyloid polyneuropathy. Curr Opin Neurol. 2016;29(Suppl. 1):S14–S26.
- Swiecicki PL, Zhen DB, Mauermann ML, et al. Hereditary ATTR amyloidosis: a single-institution experience with 266 patients. Amyloid. 2015;22(2):123–131.
- Sattianayagam PT, Hahn AF, Whelan CJ, et al. Cardiac phenotype and clinical outcome of familial amyloid polyneuropathy associated with transthyretin alanine 60 variant. Eur Heart J. 2012;33(9):1120–1127.
- Gertz MA, Kyle RA, Thibodeau SN. Familial amyloidosis: a study of 52 North American-born patients examined during a 30-year period. Mayo Clin Proc. 1992;67(5):428–440.
- Castaño A, Drachman BM, Judge D, et al. Natural history and therapy of TTR-cardiac amyloidosis: emerging disease-modifying therapies from organ transplantation to stabilizer and silencer drugs. Heart Fail Rev. 2015;20(2):163–178.
- Berk JL, Suhr OB, Obici L, et al. Repurposing diflunisal for familial amyloid polyneuropathy: a randomized clinical trial. JAMA. 2013;310(24):2658–2667.
- Coelho T, Maia LF, Martins da Silva A, et al. Tafamidis for transthyretin familial amyloid polyneuropathy: a randomized, controlled trial. Neurology. 2012;79(8):785–792.
- Adams D, Gonzalez-Duarte A, O'Riordan WD, et al. Patisiran, an RNAi therapeutic, for hereditary transthyretin amyloidosis. N Engl J Med. 2018;379(1):11–21.
- Benson MD, Waddington-Cruz M, Berk JL, et al. Inotersen treatment for patients with hereditary transthyretin amyloidosis. N Engl J Med. 2018;379(1):22–31.
- Alnylam Pharmaceuticals Inc. US prescribing information: AMVUTTRA (vutrisiran) injection, for subcutaneous use. 2022. [cited June 28, 2022]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/215515s000lbl.pdf.
- Habtemariam BA, Karsten V, Attarwala H, et al. Single-dose pharmacokinetics and pharmacodynamics of transthyretin targeting N-acetylgalactosamine-small interfering ribonucleic acid conjugate, vutrisiran, in healthy subjects. Clin Pharmacol Ther. 2021;109(2):372–382.
- Soprano DR, Herbert J, Soprano KJ, et al. Demonstration of transthyretin mRNA in the brain and other extrahepatic tissues in the rat. J Biol Chem. 1985;260(21):11793–11798.
- Holmgren G, Steen L, Ekstedt J, et al. Biochemical effect of liver transplantation in two Swedish patients with familial amyloidotic polyneuropathy (FAP-met30). Clin Genet. 1991;40(3):242–246.
- Adams D, Suhr OB, Dyck PJ, et al. Trial design and rationale for APOLLO, a phase 3, placebo-controlled study of patisiran in patients with hereditary ATTR amyloidosis with polyneuropathy. BMC Neurol. 2017;17(1):181.
- Solomon SD, Adams D, Kristen A, et al. Effects of patisiran, an RNA interference therapeutic, on cardiac parameters in patients with hereditary transthyretin-mediated amyloidosis. Circulation. 2019;139(4):431–443.
- Coelho T, Adams D, Silva A, et al. Safety and efficacy of RNAi therapy for transthyretin amyloidosis. N Engl J Med. 2013;369(9):819–829.
- Alnylam Pharmaceuticals Inc. US prescribing information: ONPATTRO (patisiran) lipid complex injection, for intravenous use. 2020. [cited February 17, 2022]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/210922s007lbl.pdf.
- European Medicines Agency. Summary of product characteristics: Onpattro 2 mg/mL concentrate for solution for infusion. 2018. [cited February 17, 2022]. Available from: https://www.ema.europa.eu/documents/product-information/onpattro-epar-product-information_en.pdf.
- Zhang X, Goel V, Attarwala H, et al. Patisiran pharmacokinetics, pharmacodynamics, and exposure-response analyses in the phase 3 APOLLO trial in patients with hereditary transthyretin-mediated (hATTR) amyloidosis. J Clin Pharmacol. 2020;60(1):37–49.
- Suanprasert N, Berk JL, Benson MD, et al. Retrospective study of a TTR FAP cohort to modify NIS + 7 for therapeutic trials. J Neurol Sci. 2014;344(1-2):121–128.
- Jackevicius CA, Mamdani M, Tu JV. Adherence with statin therapy in elderly patients with and without acute coronary syndromes. JAMA. 2002;288(4):462–467.
- Strauch B, Petrák O, Zelinka T, et al. Precise assessment of noncompliance with the antihypertensive therapy in patients with resistant hypertension using toxicological serum analysis. J Hypertens. 2013;31(12):2455–2461.
