ABSTRACT
Objectives: Coenzyme Q10, incorporated in DOPC lyposomes or naturally present in liver bovine mitochondria or in human blood plasma, was reacted with nitrogen dioxide •NO2 or with a •NO/•NO2 mixture.
Methods and Results: The reaction course was monitored by Electron Paramagnetic Resonance (EPR) spectroscopy and in all cases the formation of a di-tert-alkyl nitroxide was observed, deriving from the addition of •NO2 to one of the double bonds, most likely the terminal one, of the isoprenic chain. The rate constant for nitroxide formation was also determined by EPR spectroscopy and an initial rate of ca. 7 × 10−8 M s−1 was obtained.
Introduction
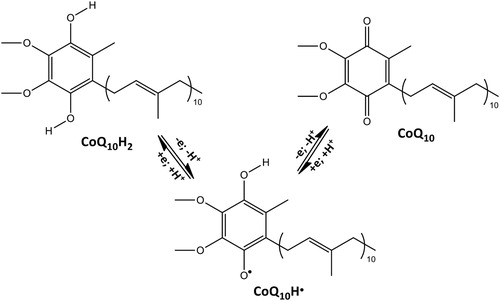
Coenzyme Q10 (CoQ10) is a component of the electron transport chain present in most eukaryotic cells and primarily in the mitochondria. It participates to the aerobic cellular respiration, generating ATP [Citation1,Citation2]. In the interconversion from the reduced CoQ10H2 to the oxidized CoQ10 (Scheme 1) electrons are transferred to the enzyme complexes of the chain responsible for the oxidative phosphorylation and ATP production. It follows that organs with the highest energy requirements, such as heart, liver and kidney, have the highest CoQ10 concentration [Citation3–5]. The relevance of CoQ10 is not limited to its role in the electron transport chain but also to its antioxidant capacity. In fact, the reduced form CoQ10H2 is an efficient chain breaking antioxidant able to inhibit lipid peroxidation by reacting with carbon- and oxygen-centred radicals [Citation6–8] and by recycling Vitamin E from its one electron oxidation product, the tocopheroxyl radical [Citation9,Citation10]. The interaction of CoQ10 and CoQ10H2 with superoxide anion (O2−•) may also be important [Citation7,Citation11] because O2−• is the proximal radical produced during oxidative stress within mitochondria [Citation12]. Moreover, CoQ10H2 can react also with Reactive Nitrogen Species (RNS) which are both endogenous products and dangerous pollutants. CoQ10H2/nitrogen monoxide (nitric oxide, •NO) reactivity was kinetically studied [Citation13] as well as CoQ10H2 oxidation by peroxynitrite (−OONO) [Citation14]. In both cases, nitrosative damage was prevented by the one electron oxidation of CoQ10H– anion to CoQ10H• ubisemiquinone radical by •NO or –OONO: these reactions are not simple and are part of a complex mechanism with several implications for mitochondrial function and integrity.
Recently, we studied the reactivity of CoQ10 and of other model compounds toward nitrogen monoxide, in the presence and in the absence of oxygen, and observed the formation of nitration products [Citation15]. In particular, they were dinitro compounds and nitroalcohols all deriving from the addition of •NO2 to the double bonds of the isoprenic chain. Even if addition of •NO2 to a double bond is a well-described process [Citation16], we demonstrated for the first time that such reaction effectively occurs also between CoQ10 and nitrogen dioxide (•NO2). Noteworthy was the formation of a di-tert-butyl nitroxide, whose three lines signal [Citation17] was clearly visible when the reaction mixtures were analysed by Electron Paramagnetic Resonance (EPR) spectroscopy. In fact, it was the first time that a nitroxide radical, already observed in the reaction between •NO2 with alkenes [Citation18], was obtained starting from CoQ10.
These findings prompted us to deepen the understanding of nitrogen dioxide (or of nitrogen monoxide/nitrogen dioxide mixture) reactivity toward CoQ10, naturally contained or incorporated, in biological systems. In this paper, we describe the reactions carried out on liposomes with incorporated CoQ10, and on mitochondria and plasma, in which CoQ10 is naturally present. Data concerning the rate constant of nitroxide formation in the reaction of CoQ10 with •NO2 are also reported.
Materials and methods
Materials
Coenzyme CoQ10, lead (IV) nitrate Pb(NO3)2 and all the other reagents and solvents were purchased from Sigma-Aldrich and used without further purification. 1,2-Dioleoyl-sn-glycero-3-phosphocholine (DOPC) was purchased from Avanti Polar Lipids as chloroform solution.
Preparation of liposomes
Liposomes were prepared by the ‘thin film hydration’ method. Appropriate amounts of chloroform solutions of DOPC (200 mM) and CoQ10 (2 mM) were mixed in a 200:1 molar ratio. The solvent was slowly evaporated with a stream of nitrogen and the thin film obtained was dried for at least 2 h under reduced pressure. This dried film was then resuspended by vortex agitation in the required amount of 5 mM PB (pH 7.4) to a 50 mM DOPC and 0.25 mM CoQ10 final concentration and incubated overnight to swell and stabilize. The resulting liposomes were sonicated by a probe sonicator (Sonic Vibracell) for 10 min at 50% amplitude.
Preparation of liver bovine mitochondria
Bovine liver mitochondria were isolated from 100 to 150 g fresh liver of adult bovine supplied by a local slaughterhouse. Mitochondria were purified from liver tissue, clean washed and homogenized in homogenization buffer pH 7.5 (1:10 w:v), containing 75 mM sucrose, 225 mM mannitol, 1 mM EDTA, 5 mM Hepes and 0.5 mg/mL bovine serum albumin fatty acid free. The obtained homogenate was centrifuged for 10 min at 600 g at 4°C. Subsequently, the supernatant was centrifuged for 20 min at 1200 g at 4°C. The mitochondrial pellet was washed two times at 2800 g for 10 min at 4°C and purified mitochondria were carefully resuspended in 1 mL ice-cold homogenization buffer [Citation19]. The mitochondrial protein content was determined by the Bradford protein assay [Citation20]. Purity of isolated mitochondria was checked by measuring Glo I activity, a cytosolic marker, in the mitochondrial suspension. Enzymatic activity was determined spectrophotometrically at 240 nm using 1.0 mM GSH/methylglyoxal hemithioacetal as substrate in 100 mM sodium phosphate buffer, pH 6.6. The hemithioacetal is generated in situ by pre-incubation of 2 mM methylglyoxal with 2 mM GSH in 100 mM sodium phosphate buffer pH 6.6 at 37°C and this step is essential to avoid conditions where the formation of the hemithioacetal is rate limiting.
Preparation of plasma
Human blood was withdrawn from a volunteer (one of the authors), collected into heparinized tube and kept in the refrigerator overnight. Plasma was obtained by spontaneous decantation and used without any treatment. In the case of orally administrated CoQ10, 3 × 100 mg CoQ10 pills were administered every 5 h starting at 8.00 am by the volunteer and the withdrawing was performed at 10.00 pm. Typical CoQ10 plasma levels were 0.8 μg/mL before and 3 μg/mL after CoQ10 administration as verified by HPLC using the method described in [Citation21].
General procedures
Reactions with •NO2
Nitrogen dioxide was obtained from the thermal decomposition of Pb(NO3)2 and bubbled into the sample (liposomes, mitochondria or plasma) for 2 min under stirring. The reaction mixture was immediately transferred into a Pasteur pipette and inserted into the EPR cavity for the measurements.
Reaction with •NO/•NO2.
The •NO/•NO2 mixture was obtained from nitrous acid decomposition in a three-way distillation receiver equipped with three receiver flasks. Sodium nitrite (0.5 g) was poured in one of the flasks, acetic acid (2 mL) in the second and the biological sample (1 mL) in the third one. The apparatus was closed in the presence of air and acetic acid was poured into the NaNO2 compartment avoiding the contact with the sample. From this mixture nitrous acid was formed, but it decomposed into nitrogen monoxide which reacted with oxygen giving a •NO/•NO2 mixture. The sample (liposomes or mitochondria) was exposed to this gaseous atmosphere for 30 min before EPR measurements.
EPR measurements
EPR spectra were recorded on a Bruker EMX EPR spectrometer equipped with an XL microwave frequency counter, Model 3120 for the determination of g-factor and on a Varian E4 spectrometer. The spectra were recorded with the following instrumental settings: modulation frequency 100 kHz, modulation amplitude 0.4 G, sweep width 80 G, microwave power 5 mW, time constant 1.28 s, receiver gain 5 × 103, number of scans 50.
Liposomes: 300 μL of •NO2 or •NO/•NO2 treated liposomes suspension were transferred into a Pasteur pipette and the spectrum recorded. To the same sample, 200 μL of CHCl3 were added and the spectrum was recorded again. 300 μL of untreated liposomes were used as a control.
Mitochondria: 300 μL of •NO2 or •NO/•NO2 treated mitochondria suspension were transferred into a Pasteur pipette and the spectrum recorded. 500 μL of these treated mitochondria suspensions were treated with a Triton X solution and extracted with CHCl3 (2 × 4 mL). The collected chloroform solution was reduced to 2 mL volume under reduced pressure and then submitted to EPR spectroscopy.
Plasma: nitrogen dioxide was bubbled into 500 μL of plasma until complete solidification of the sample. The triturated solid was extracted with CHCl3 (2 × 4 mL), the volume reduced to 2 mL under reduced pressure and then submitted to EPR spectroscopy.
Kinetic measurements
EPR spectra were recorded on a Bruker Elexsys at room temperature. •NO2 solution was obtained by bubbling •NO2 (thermal decomposition of Pb(NO3)2) in cyclohexane for 30 s; its concentration was determined by weighting the solution before and after bubbling. •NO2 100 mM solution (100 μL) was added to a 6.5 mM solution of CoQ10 (200 μL) in an NMR tube (final concentration [CoQ10] = 4.3 mM, [•NO2] = 36 mM), vortexed and introduced in the EPR cavity 65 s after mixing; 500 scans (1 s per scan) were recorded with a delay of 2 s between each scan. Nitroxide concentration was determined from a calibration curve obtained from TEMPO solutions (10−6–10−4 M).
Results
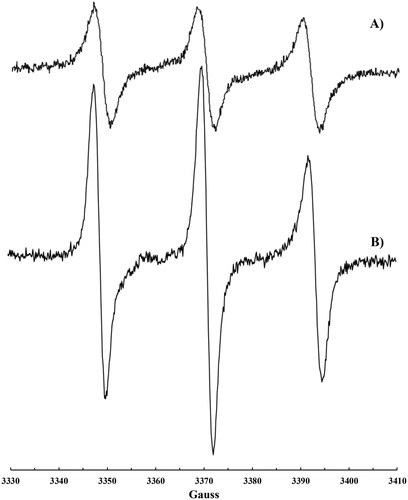
Before considering the biological systems object of this study, the reaction between a chloroform solution of CoQ10 and •NO2 or a mixture of •NO/•NO2 was repeated and followed by EPR spectroscopy. Nitrogen dioxide (•NO2) was produced by thermal decomposition of lead (IV) nitrate [Pb(NO3)2], whereas •NO/•NO2 mixture was obtained by spontaneous decomposition of nitrous acid generated form sodium nitrite and acetic acid in the presence of oxygen (see experimental). In fact, •NO2 is rapidly formed (k = 1.1 × 106 M−1 s−1) [Citation22] from the reaction between •NO and oxygen. The typical three lines EPR signal already reported by us [Citation15] characterized by aN = 15.4 Gauss, g-factor 2.0062(3) was recorded, and it was identical to those shown in Figures 1(c), 2(c) and 3(b).
Reaction of •NO2 or •NO/•NO2 mixture with CoQ10-containing liposomes
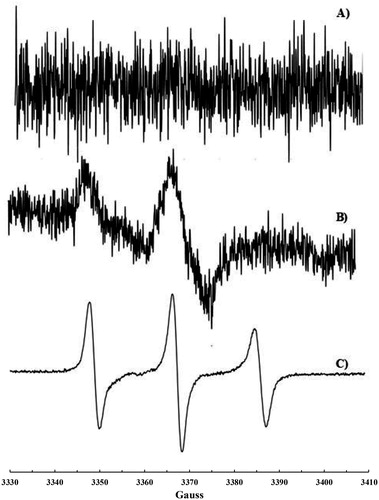
1,2-Dioleoyl-sn-glycero-3-phosphocholine (DOPC) liposomes were prepared and supplemented with CoQ10. No EPR signal was recorded on these liposomes before exposure to •NO2 or •NO/•NO2 (a). After exposure to •NO2 or •NO/•˙NO2 mixture, a very weak signal was recorded and shown in (b): it is the typical signal of a nitroxide immobilized in a high viscosity region such as the lipid bilayer of DOPC liposomes. Chloroform was then added to dissolve the lipid and extract the nitroxide formed. A very well-resolved three lines signal (aN = 15.58 G, g = 2.0062(3)) was recorded (c) and assigned to a di-tert-alkyl nitroxide formed upon addition of •NO2 to the carbon–carbon double bond of the isoprenic chain according to the mechanism already described [Citation15] and reported in Scheme 2.
Reaction of •NO2 or •NO/•NO2 mixture with mitochondria
The same kind of experiment was carried out on isolated bovine liver mitochondria. Also in this case, the typical signal for an immobilized nitroxide (b) was detected when mitochondria suspensions were introduced into the EPR cavity after treatment with •NO2 or •NO/•NO2 mixture. The addition of Triton X-100 solution to the suspension resulted in mitochondria lysis and extraction of the di-tert-alkyl nitroxide with chloroform was then possible. A well-resolved three lines EPR signal (aN = 15.65 G, g = 6.0062(3)) was recorded (c), even if a second radical species may be glimpsed in this spectrum, likely another nitroxide of the same type formed upon reaction of •NO2 with a different double bond of the isoprenic chain. In fact, the overlapping of more signals was observed when CoQ10 solutions were reacted with increasing amounts of nitrogen dioxide [Citation15]. EPR measurements were carried out also before treatment with •NO2. A very weak signal was recorded (a) likely due to a naturally occurring nitroxide formed upon reaction between CoQ10 (present in mitochondria) and endogenously produced •NO2.
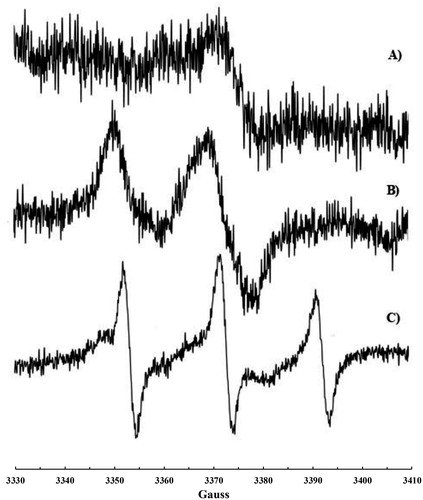
Reaction of •NO2 or •NO/•NO2 mixture with human plasma
Nitroxide formation (aN = 15.41 G, g-factor 2.0062(5), a) was observed also upon exposure of plasma to •NO2 or to a •NO/•NO2 mixture. In this case, the addition of CHCl3 was necessary to dissolve the solid formed immediately after the addition of •NO2 to plasma. A more intense signal (b) was recorded on plasma samples if some CoQ10 was orally administrated to the volunteer in the days before the blood withdrawing.
Kinetic measurements
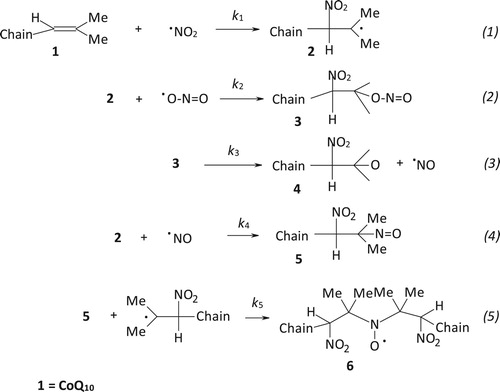
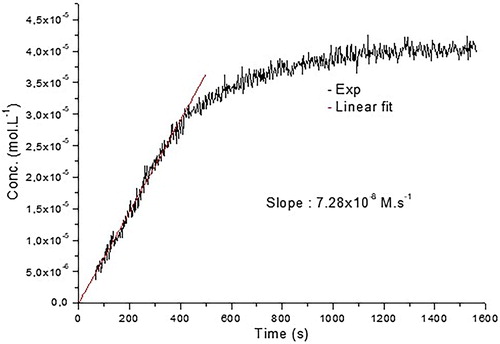
An EPR signal was observed upon mixing of cyclohexane solutions of CoQ10 and •NO2 (final concentration [CoQ10] = 4.3 mM, [•NO2] = 36 mM, at t = 0) and monitored over time (). The evolution of nitroxide 6 concentration showed a linear increase within the first 400 s with a slope of 7.28 × 10−8 M s−1 resulting from a limiting step in the cascade reactions reported in Scheme 2.
Discussion
The obtained results confirm the previous findings that •NO2 may react with the double bonds of the CoQ10 isoprenic chain [Citation15], though a very complex process with formation of nitroxide 6 together with other nitration products. Moreover, they demonstrate that such reactions may take place also in biological systems such as liposomes (upon incorporation of CoQ10) or in mitochondria and blood where CoQ10 is naturally occurring. The various steps necessary to justify the formation of the nitroxide are reported in Scheme 2 and some considerations can be drawn concerning their kinetics. The addition of •NO2 to the terminal double bond of the isoprenic chain (Equation 1, Scheme 2), which is the most accessible and less sterically hindered, has a calculated (DFT) activation energy of 7.9 kcal [Citation15], and the rate constant for this reaction should be in the range of 104–106 M−1 s−1 in agreement with the value of 106 M−1 s−1 reported for the addition of •NO2 to double bonds of arachidonic acid [Citation23]. Reactions occurring in steps 2, 4 and 5 are very fast, being free radical couplings (steps 2 and 4) and radical addition to a nitroso compound (step 5) with a rate constant of about 106 M−1 s−1 [Citation24]. It follows that the slowest reaction in the proposed process should be the decomposition of the alkyl nitrite compound 3 in step 3 with formation of •NO. This hypothesis is confirmed by the slow decomposition rate constants reported for some alkyl nitrites (1 mM in 0.1 M phosphate buffer at 37°C) in the range 2.7 × 10−8–1 × 10−7 M s−1 [Citation25]. Since step 3 is the limiting reaction, nitroso compound 5, formed upon addition of •NO to radical 2, should always be at a low concentration compared to 2 and hence nitroxide 6 should form with a rate (7.28 × 10−8 M s−1 as determined in the kinetic experiment) close to alkyl nitrites decomposition rate [Citation25]. Although the formation of 6 is slow and derives from a complex process with competitive side reactions such as that of •NO with O2 or the self-coupling of radical 2 (not considered in that study), we believe that the formation of this nitroxide is an indirect proof of the possible reaction of •NO2 with the isoprenic chain of CoQ10. Moreover, the fact that an EPR signal, even if very weak, likely due to a nitroxide like 6 was recorded on untreated mitochondria and that CoQ10 is present in various organs [Citation26] could make this reaction relevant for biological media.
Acknowledgements
The authors wish to thank Università Politecnica delle Marche for financial support and Dr. Giovanna Mobbili for liposomes preparation.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Ernster L, Dallner G. Biochemical, physiological and medical aspects of ubiquinone function. Biochim Biophys Acta. 1995;1271:195–204. doi: 10.1016/0925-4439(95)00028-3
- Dutton PL, Ohnishi T, Darrouzet E, et al. Coenzyme Q: molecular mechanism in health and disease. Kagan VE, Quinn PJ, editors. Boca Raton: CRC Press; 2000. p. 65–82.
- Okamoto T, Matsuya T, Fukunaga Y, et al. Human serum ubiquinol-10 levels and relationship to serum lipids. Int J Vitam Nutr Res. 1989;59(3):288–292.
- Aberg F, Appelkvist EL, Dallner G, et al. Distribution and redox state of ubiquinones in rat and human tissues. Arch Biochem Biophys. 1992;295(2):230–234. doi: 10.1016/0003-9861(92)90511-T
- Shindo Y, Witt E, Han D, et al. Enzymic and non-enzymic antioxidants in epidermis and dermis of human skin. J Invest Dermatol. 1994;102(1):122–124. doi: 10.1111/1523-1747.ep12371744
- Frei B, Kim MC, Ames BN. Ubiquinol-10 is an effective lipid-soluble antioxidant at physiological concentrations. Proc Natl Acad Sci USA. 1990;87:4879–4883. doi: 10.1073/pnas.87.12.4879
- James AM, Smith RAJ, Murphy MP. Antioxidant and prooxidant properties of mitochondrial Coenzyme Q. Arch Biochem Biophys. 2004;423:47–56. doi: 10.1016/j.abb.2003.12.025
- Silvestri S, Orlando P, Armeni T, et al. Coenzyme Q10 and α-lipoic acid: antioxidant and pro-oxidant effects in plasma and peripheral blood lymphocytes of supplemented subjects. J Clin Biochem Nutr. 2015;57:21–26. doi: 10.3164/jcbn.14-130
- Kagan V, Serbinova E, Packer L. Antioxidant effects of ubiquinones in microsomes and mitochondria are mediated by tocopherol recycling. Biochem Biophys Res Commun. 1990;169(3):851–857. doi: 10.1016/0006-291X(90)91971-T
- Ernster L, Forsmark P, Nordenbrand K. The mode of action of lipid-soluble antioxidants in biological membranes: relationship between the effects of ubiquinol and vitamin E as inhibitors of lipid peroxidation in submitochondrial particles. BioFactors. 1992;3(4):241–248.
- Maroz A, Anderson RF, Smith ASJ, et al. Reactivity of ubiquinone and ubiquinol with superoxide and hydroperoxyl radical: implications for in vivo antioxidant activity. Free Radical Biol Chem. 2009;46:105–109. doi: 10.1016/j.freeradbiomed.2008.09.033
- Murphy MP. How mitochondria produce reactive oxygen species. Biochem J. 2009;417(1):1–13. doi: 10.1042/BJ20081386
- Poderoso JJ, Carreras MC, Schöpfer F, et al. The reaction of nitric oxide with ubiquinol: kinetic properties and biological significance. Free Radical Biol Med. 1999;26(7/8):925–935. doi: 10.1016/S0891-5849(98)00277-9
- Schöpfer F, Riobò NA, Carreras MC, et al. Oxidation of ubiquinol by peroxynitrite: implications for protection of mitochondria against nitrosative damage. Biochem J. 2000;349(1):35–42. doi: 10.1042/bj3490035
- Astolfi P, Charles L, Gigmes D, et al. Reaction of nitric oxide and nitrogen dioxide with coenzyme Q; involvement of the isoprenic chain. Org Biomol Chem. 2013;11:1399–1406. doi: 10.1039/c2ob27198b
- Golding P, Powell JL, Ridd JH. Reaction of nitrogen dioxide with hexenes. The mechanistic and structural factors controlling the product composition. J Chem Soc Perkin Trans. 1996;2:813–819. doi: 10.1039/p29960000813
- Alberti A, Benaglia M, Macciantelli D. Mechanistic studies of radical-based processes. Use and misuse of EPR spectroscopy. Org Lett. 2000;2(11):1553–1555. doi: 10.1021/ol005741j
- Park JSB, Walton JC. Reaction of nitric oxide and nitrogen dioxide with functionalised alkenes and dienes. J Chem Soc Perkin Trans. 1997;2:2579–2584. doi: 10.1039/a702295f
- Jassem W, Armeni T, Quiles JL, et al. Protection of mitochondria during cold storage of liver and following transplantation: comparison of the two solutions, University of Wisconsin and Eurocollins. J Bioenerg Biomemb. 2006;38:49–55. doi: 10.1007/s10863-006-9005-6
- Bradford MM. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3
- Mosca F, Fattorini D, Bompadre S, et al. Assay of coenzyme Q(10) in plasma by a single dilution step. Anal Biochem. 2002;305:49–54. doi: 10.1006/abio.2002.5653
- Goldstein S, Czapski G. Mechansim of the nitrosation of thiols and amines by oxygenated NO solutins: the nature of the nitrosating intermediates. J Am Chem Soc. 1996;118:3419–3425. doi: 10.1021/ja9536680
- Prütz WA, Mönig H, Butler J, et al. Reaction of nitrogen dioxide in aqueous model systems: oxidation of tyrosine units in peptides and proteins. Arch Biochem Biophys. 1985;243:125–134. doi: 10.1016/0003-9861(85)90780-5
- Alberti A, Macciantelli D. in Electron paramagnetic resonance – a practitioner’s toolkit. Brustolon M, Giamello EJ, editors. Wiley and Sons, 2009. p. 285–324.
- Nicolescu AC. Mechanism of nitric oxide release from organic nitrates and nitrites: implications in the activation of soluble guanylyl cyclase and inhibition of lipid peroxidation [PhD thesis]. Kingston (ON): Queen’s University; January 2004.
- Bhagavan HN, Chopra RK. Coenzyme Q10: absorption, tissue uptake, metabolism and pharmacokinetics. Free Radical. Res. 2006;40:445–453. doi: 10.1080/10715760600617843