Abstract
The new coronavirus disease-19 (COVID-19) pandemic has rapidly spread all around the world, eliciting many questions and doubts about the pathogenesis of the disease and treatment. Mortality has been related to a prothrombotic state. Risk factors for the infection and for severe forms of COVID-19 have still to be defined. According to data collected, women appear to be less prone to severe forms of the disease and their mortality was lower than for men. The role of female hormones in the modulation of inflammation may be the reason behind this gender gap.
Considering the prothrombotic state activated by the virus, hormone therapies have been placed under investigation as possible increasing risk factors for severe forms. Moreover, new vaccines and their rare thrombotic side effects have increased the concern about this issue.
The goal of this review is to go over the mechanisms that lead up to thrombosis during COVID-19, trying to explain the possible reasons why women seem to be naturally protected. The expert opinions about whether to continue/discontinue hormonal therapies are reviewed. Moreover, available data about the so-called ‘vaccine induced immune thrombotic thrombocytopaenia’ caused by vaccines against COVID-19 are discussed.
新型冠状病毒肺炎、性别和雌孕激素, 我们知道什么? 摘要
新型冠状病毒肺炎(COVID-19)在全球范围内迅速蔓延, 引发了人们对其发病机制和治疗方法的诸多疑问。COVID-19导致死亡的机制与血栓前状态有关。感染的高危因素和疾病的重症表现仍有待确定。根据目前的数据, 女性似乎不容易进展为重症, 死亡率也低于男性。造成这种性别差异的原因可能与女性激素的炎症调节作用有关。由于病毒可能激活血栓前状态, 激素替代治疗是否可能会增加患重症的风险, 值得进一步研究。新疫苗及其罕见的血栓副作用使人们对这一问题更加关注。本综述旨在回顾COVID-19形成血栓的机制, 并试图解释女性可能存在内源性保护的原因。对于是否继续/停止激素治疗的专家意见进行了总结。并对COVID-19疫苗引起所谓的“疫苗诱导免疫血栓性血小板减少症”的证据进行了讨论。
Introduction
The novel Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV2) has spread around the world since December 2019, causing the well-known coronavirus disease-2019 (COVID-19) pandemic. Since the outbreak of COVID-19 in December 2019 in Wuhan, China, the infection has rapidly become the biggest pandemic of the century, with more than 185 million global cases and over 4 million deaths around the world by July 2021 [Citation1].
There are still many open questions about this new disease, and the search for an effective therapy is actively ongoing. The pathogenesis of the disease and its different manifestations are also difficult to explain. By now, it is generally accepted that SARS-CoV2 causes a more severe illness and kills more men than women [Citation2], and being a pre-menopausal woman seems to be a protective factor for COVID-19 infection. A significant number of deaths from COVID-19 infection have been attributed to a prothrombotic state, thus to an increased risk of venous thromboembolism (VTE). For this reason, doubts about whether to discontinue or limit the use of combined hormonal contraceptives (CHC) during the pandemic has emerged.
Much data has accumulated over the more than one year from the beginning of this pandemic. The goal of this review is to make an update and to discuss how female hormones can positively influence the inflammation mechanism triggered by viral infections, with particular attention to the use of hormonal contraceptives during COVID-19. Moreover, a comment on the thrombotic effect of the new vaccines will be given.
Sex differences in the epidemiology of COVID-19
The mechanisms of death in COVID-19 remain to be clarified, but the most likely hypothesis relates to the activation of a dysregulated inflammatory reaction that leads to multisystem organ pathology in a subset of patients [Citation3]. This mechanism may be at the basis of gender differences in morbidity and mortality of COVID-19. Men with COVID-19 are more at risk for severe symptoms and death, independent of age and other comorbidities such as diabetes, hypertension, and cardiovascular diseases [Citation4]. This observation for COVID-19 is in line with previous results for other infections. The predilection of viral infections for men was already observed in previous similar epidemics such as the severe acute respiratory syndrome (SARS) and the Middle East respiratory syndrome (MERS) [Citation5].
Sex-disaggregated data at a global level (last update June 2021) suggest that the ratio of positive cases between males and females is similar, but the number of men hospitalised is 1.1 times greater than women; intensive care unit (ICU) admissions are 1.8 times higher with males than with females, and the confirmed cases of death are 1.5 times that of women of every age [Citation6]. During the first months of the pandemic, many series of data were reported. In Italy, women represented 18% of all COVID-19 admissions to ICU in the Lombardy region [Citation7]. Among 1099 COVID-19 hospitalised cases in Wuhan, China, 42% were women; with only 32% of severe cases in females [Citation2]. Other series of data in different settings provided similar observations [Citation8]. Marina S. and Piemonti L. in 2020 analysed the data of six European countries about number of confirmed cases and deaths stratified by age and sex. They found a clear age-dependent pattern of the male to female ratio of cumulative incidence of cases in all countries analysed: even if the rate of confirmed SARS-CoV2 infection among females compared to males between the age of 10 and 50 years was higher, a higher preliminary fatality rate among males compared to females emerged in all age groups above 20 years old (not peer reviewed article, available at: https://papers.ssrn.com/sol3/papers.cfm?abstract_id=3576790).
Interestingly, there is also an age-related pattern in infection rate and fatality. Seeland et al. identified a sex- and age-specific distribution of the COVID-19 incidence rate: premenopausal women (in the 20-55-year age range) presented the highest frequency of SARS-CoV2 infection, but their fatality rate compared to age-matched men was lower [Citation9]. We collected sex- and age-disaggregated data about the number of deaths for COVID-19 per 100,000 population in different European countries from an online dataset (https://globalhealth5050.org/the-sex-gender-and-covid-19-project/the-data-tracker) and we represented the calculated male to female mean death ratio for 10-year age groups in . These results support the hypothesis of an important role for female hormones in this disease, suggesting that biological sex differences may be at the basis of the different death rate.
Figure 1. Sex- and age- disaggregated data on deaths from COVID-19 in Europe. Data on mortality in males and females in Europe is shown in the graph. Data are derived from a specific dataset available at https://globalhealth5050.org/the-sex-gender-and-covid-19-project/the-data-tracker [Citation6]. As a compromise between accurate age- and sex-stratification and sufficient data density, we included in the analyses only European countries with sex-disaggregated data about number of deaths per 100,000 population divided into 10-year age groups. The final selection included data from: Czech Republic, Denmark, England, France, Hungary, Israel, Italy, Poland, Portugal, Spain, Switzerland, Wales. The mean about separate males and females’ mortality of all the countries selected was done. Then, the ratio between males and females’ mortality per 100,000 population was calculated for every 10-year age group.
![Figure 1. Sex- and age- disaggregated data on deaths from COVID-19 in Europe. Data on mortality in males and females in Europe is shown in the graph. Data are derived from a specific dataset available at https://globalhealth5050.org/the-sex-gender-and-covid-19-project/the-data-tracker [Citation6]. As a compromise between accurate age- and sex-stratification and sufficient data density, we included in the analyses only European countries with sex-disaggregated data about number of deaths per 100,000 population divided into 10-year age groups. The final selection included data from: Czech Republic, Denmark, England, France, Hungary, Israel, Italy, Poland, Portugal, Spain, Switzerland, Wales. The mean about separate males and females’ mortality of all the countries selected was done. Then, the ratio between males and females’ mortality per 100,000 population was calculated for every 10-year age group.](/cms/asset/dc79bc21-2b0a-4575-985a-26e980f0a598/iejc_a_2000959_f0001_b.jpg)
COVID-19: from inflammation to venous thromboembolism
The pathogenic mechanisms of this new disease have still to be clarified, but our knowledge increases daily. The most likely current hypothesis is that SARS-CoV2 enters the cells and stimulates an early antiviral response with the activation of the innate and then the adaptive immune response. Inflammation leads to endothelial damage and to an hypercoagulative status ().
Figure 2. Mechanisms of tissue damage by SARS-CoV2 and sex-hormone defence mechanisms. (A) Severe acute respiratory syndrome coronavirus 2 (SARS-CoV2) spike proteins recognise the host cell via the angiotensin-converting enzyme 2 (ACE2) and the serine protease Transmembrane Protease Serine 2 (TMPRSS2), leading to virus entry. SARS-CoV2 infection can enhance the ACE2 and TMPRSS2 messenger ribonucleic acid (mRNA) gene expression. Immune cells infiltrate the lungs, causing hyperactivation of monocytes and macrophages, with production of pro-inflammatory cytokines, tissue necrosis factor-β (TNF-β) and chemokines. The exaggerated activation of the immune response leads to the so-called ‘cytokine storm’, and this situation causes lymphopenia and lung injury. Tissue damage ultimately leads to the activation of the extrinsic pathway of the coagulation cascade. (B) Both oestradiol (E2) and progesterone (P4) can modulate innate and adaptive immune response by binding their nuclear receptors. Both hormones can inhibit macrophage activation, thus reducing the secretion of pro-inflammatory cytokines and the migration of neutrophils and monocytes into inflamed tissues and can stimulate the production of anti-inflammatory cytokines and the expansion of T regulatory (Treg) cells. Moreover, E2 can enhance the production of antibodies by B cells, and it inhibits the increasing expression of TMPRSS2 in infected cells, possibly reducing the virus content inside the cells. Created with BioRender.com. ERs: oestrogen receptors; PR: progesterone receptor; TF: tissue factor.
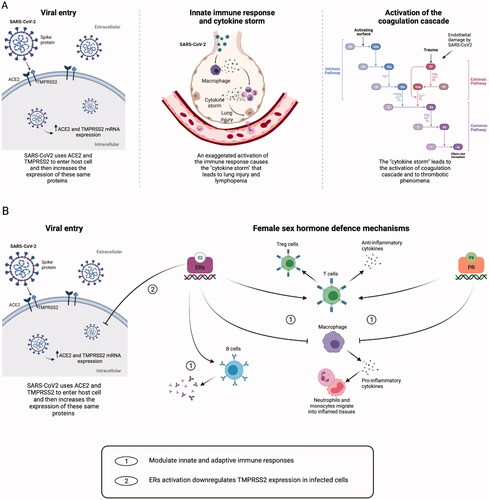
SARS-CoV2 spike proteins have two domains. The first one binds the angiotensin-converting enzyme 2 (ACE2) expressed in the host cell surface; the second domain is cleaved by the host cell serine protease Transmembrane Protease Serine 2 (TMPRSS2). These two steps are necessary to allow the virus entry into the host cell [Citation10,Citation11]. ACE2 is encoded by the X chromosome [Citation12]; SARS-CoV2 infection per se is capable of enhancing the ACE2 and TMPRSS2 messenger ribonucleic acid (mRNA) gene expression [Citation13].
Severe COVID-19 outcomes are associated with delayed and exaggerated innate immune responses: patients with COVID-19 do not die from damage caused by the virus replication; they die instead because of the effects induced by the immune response that lead to the so-called ‘cytokine storm’ [Citation3,Citation14,Citation15]. Immune cells infiltrate the lungs, causing hyperactivation of monocytes and macrophages, with production of pro-inflammatory cytokines, tumour necrosis factor α (TNF-α) and chemokines. This situation causes lymphopenia (which is related with COVID-19 severity) and lung injury [Citation15], leading to acute respiratory distress syndrome (ARDS) and multiorgan failure.
The cytokine storm may ultimately lead to the activation of the coagulation cascade, causing thrombotic phenomena () [Citation3]. Some indicators of these increased prothrombotic effects are the identification of fibrinous thrombi in small pulmonary areas, regardless of their involvement in lung injury, endothelial tumefaction and a large number of megakaryocytes in the pulmonary capillaries [Citation16]. Anticoagulant therapy may be associated with a better prognosis in severe COVID-19 patients with sepsis-induced coagulopathy [Citation17].
Oestradiol and immune response in women
The immune response is likely to play a critical role in COVID-19 disease and females usually develop heightened immune responses compared to males [Citation14]. The X chromosome contains the largest number of immune-related genes [Citation18], and this may be related to sex-based differences in immune responses. Females benefit from having two X chromosomes and being a mosaic of X-linked genes, randomly expressing alleles inherited from their mother or father [Citation19]. The outcome and survival rates from infections or sepsis are usually better in females than in males. Females, however, respond more aggressively to self-antigens and they are more susceptible to autoimmune diseases [Citation20].
The steroid hormones, oestradiol (E2) and progesterone (P4), at high physiological concentrations are also powerful immunomodulators [Citation21]. The variation in sex steroids concentration that occurs during life contributes to differences in immune profiles and disease susceptibility patterns at different ages [Citation12]. Oestrogen receptors (ERs) are expressed in all immune cells, and different peripheral blood mononuclear cells express a different balance between ERα and ERβ [Citation21]. E2 can modulate innate immunity by suppressing the production of proinflammatory cytokines and interleukins (ILs), IL-6, IL-1β, tumour necrosis factor-α - TNF-α, by monocytes and macrophages and the migration of innate immune cells into inflamed areas (e.g., neutrophils and monocytes). Moreover, E2 stimulates the production of anti-inflammatory cytokines (IL-4, IL-10 and interferon-γ) by CD4+ T-helper cells and decreases the production of proinflammatory IL-17. The expansion of regulatory T cells and the production of antibodies by B cells are also enhanced by E2 () [Citation14,Citation20].
Several lines of research have shown that oestrogen exhibits antiviral properties, which have been studied against influenza A virus, human immunodeficiency virus (HIV), hepatitis C virus (HCV), Ebola and human cytomegalovirus [Citation5,Citation14].
P4 is another important immunomodulator and anti-inflammatory hormone. Its receptors are widely distributed in immune cells: it inhibits pro-inflammatory IL-12 and IL-1β production by macrophages, and it favours anti-inflammatory actions of CD4+ T-helper cells, similarly to E2 () [Citation14]. Studies in mice have demonstrated that luteal-phase levels of P4 could protect from influenza A virus pneumonia, reducing inflammation and promoting pulmonary tissue repair [Citation22].
Thus, female sex hormones can enhance and modulate innate and adaptive immunity, representing the major causing factor of the gender-bias existing in the infection and the course of the COVID-19 disease.
Oestrogen signalling is critical for cellular protection from SARS-CoV2, too. A recent study showed that E2 was able to modulate ACE2 gene-expression levels in differentiated airway epithelial cells [Citation23]. However, preclinical evidence that ACE2 expression is regulated in a sex-dependent manner has not yet been validated and further studies are needed [Citation24]. Lemes et al. found that the in vitro activation of ERs in SARS-CoV2 infected cells may cause the downregulation in TMPRSS2 gene expression (), suggesting that this mechanism may be related to the reduction of virus content inside the cells found after the activation of ERs. This seems to be a defensive mechanism activated by infected cells, considering that E2 didn’t cause changes in ACE2 and TMPRSS2 expression in non-infected cells [Citation13].
Post-COVID-19 syndrome
A post-COVID-19 syndrome has recently attracted attention, which consists of the persistence of symptoms after recovering from the initial acute COVID-19 [Citation25–28]. In particular, the most common symptoms are fatigue, dyspnoea, neurological disorders, anosmia/dysgeusia, and they resulted to be significantly associated with disease severity at onset [Citation27].
Studies suggest that the overall incidence of post-COVID-19 syndrome may range from 40% to 60% after 6 months from recovery, but knowledge about this syndrome is still poor. Curiously, first data suggest that this syndrome may have a higher prevalence in women [Citation27,Citation28]. However, sex-disaggregated data are lacking, as well as information about the pathogenesis of this long-term syndrome. Currently, no conclusions about these gender differences may be drawn.
Female natural hormones: potential benefit of their administration
Considering that the natural protection against severe forms of the disease derives from mechanisms elicited by female hormones, some researchers tried to assess the possible benefits of administering E2 during COVID-19 infection. Seeland et al. [Citation9] analysed real-world evidence in a subset of 16,891 post-menopausal women classified according to the use of oestradiol therapy. They found a significant difference in death rates among post-menopausal women receiving hormone-replacement therapy with E2 versus non-users: the fatality risk for women >50 years receiving E2 treatment is reduced by more than 50%, from 6.6 to 2.3%. These data suggest a protective role of oestrogens. Other authors have suggested using oestrogens in the treatment of COVID-19 after analysing experimental evidence of well-established actions of this hormone in the immune modulation mechanisms [Citation29,Citation30]. However, up to this review, we have not been able to identify any prospective, randomised study evaluating the effect of oestrogen treatment on the severity of the disease either among males or post-menopausal women presenting with any form of the disease.
What to do with combined hormonal contraception during the COVID-19 pandemic?
It is more complex to establish if what is known for natural hormones is valid also for CHC in pre-menopausal women, mainly due to the variability of oestrogens (natural and synthetic) and of routes of administration used. The magnitude of the protective effect of CHC usage among women in reproductive age versus non-users seems to be small, mostly because circulating hormone levels are already high [Citation9].
Despite the apparent protective effect of female hormones, in this last year, the safety of CHCs during COVID-19 pandemic has been doubted due to their well-known pro-thrombotic effect. Many contradictory messages have been given to the public by media and researchers. However, currently there are no studies that demonstrate an increased risk of VTE and of severe forms of COVID-19 in CHC users versus non-users during SARS-CoV2 infection. In May 2020, the Italian Society of Contraception (SIC) published their guidelines for the management of hormonal contraception during this pandemic. The aim was to avoid unnecessary discontinuation of pill intake, especially considering that no data were available in support of an increased thromboembolic risk in this particular population. They recommended not to discontinue contraception (in particular CHCs) in asymptomatic and mild/moderate forms of COVID-19 [Citation31]. Moreover, the effect of pill discontinuation on the normalisation of coagulation parameters requires about two months to realise. Thus, some authors suggested to add an anticoagulant therapy versus stopping CHC usage in women with COVID-19 [Citation32]. During hospitalisation, CHCs must be interrupted, and re-initiation can be performed immediately after recovery [Citation31]. These suggestions were corroborated by the fact that recent meta-analyses didn’t find an association between COVID-19 and an increased risk of VTE in the general population. In the review by Mai et al., seven studies (41,768 patients) evaluated VTE in COVID-19 cohorts compared to non-COVID-19 cohorts. The overall risk of VTE (RR 1.18; 95% CI 0.79–1.77; p = 0.42; I2=54%), pulmonary embolism (RR 1.25; 95% CI 0.77–2.03; p = 0.36; I2 = 52%) and deep venous thrombosis (RR 0.92; 95% CI 0.52–1.65; p = 0.78; I2 = 0%) did not significantly differ between COVID-19 and non-COVID-19 cohorts. An increased risk of VTE among COVID-19 versus non-COVID-19 cohorts emerged when only patients hospitalised within the ICU were considered (RR 3.10; 95% CI 1.54–6.23), which was not observed in cohorts of predominantly non-ICU patients (RR 0.95; 95% CI 0.81–1.11) [Citation33]. Moreover, according to a study by Jiménez et al. [Citation34], the clinical characteristics associated with a higher risk of VTE during COVID-19 were older age and having a history of VTE, recent surgery/immobilisation and hypertension; D-dimer >5000 ng/mL was also a strong predictor. The use of hormonal contraceptives was not reported [Citation34]. All this evidence confirms the hypothesis that women can continue to use their hormonal contraceptive without the need to move to a progestogen-only contraception.
In agreement with the World Health Organisation guidelines [Citation35], the SIC maintains that the coronavirus pandemic has not changed the indications and the contraindications for the use of the different hormonal contraceptive methods. Where it is not possible to collect an appropriate medical history and conduct a physical examination, the physician may consider prescribing a progesterone only pill (POP) until an outpatient visit is possible.
The recommendations from the Spanish Menopause Society, Sociedad Española de Ginecología y Obstetricia and Sociedad Española de Trombosis y Haemostasia are more restrictive [Citation36]. For perimenopausal women using CHC, they suggest withdrawing CHC, also in women with mild symptoms and confirmed COVID-19 infection and adding prophylactic low molecular weight heparin when there are persistent respiratory symptoms, also if there are not criteria for hospitalisation. If contraception is required for medical reasons, they suggest to switching to progesterone-only methods [Citation36]. However, even if COVID-19 may be a risk factor for VTE, as previously described data does not support the need to change the contraceptive method in mild/moderate forms of COVID-19 [Citation33,Citation34].
If the goal is to use a combination with a low or no impact on coagulation, we must be aware that preparations containing natural oestrogens induced similar or less changes in the overall activity of the coagulation system than those containing ethinylestradiol (EE) [Citation37,Citation38]. This may be a weapon for the clinician to address the problem of the increased risk of VTE when anti-androgenic progestins are needed. In a study comparing 17β-oestradiol (17βE2) + nomegestrol acetate (NOMAC) versus EE + levonorgestrel (LNG), the first combination induced significantly fewer changes in the overall activity of the coagulation system. In particular, mean changes in prothrombin fragment 1 + 2 and antithrombin were close to zero, suggesting minimal effects on thrombin turnover, while D-dimer levels, a degradation product of fibrin which correlates with the thrombus burden, decreased rather than increased [Citation39]. Similar results were observed with a preparation with oestradiol valerate (E2V) and dienogest [Citation40]. In the study by Dinger et al. the rate of VTE events with E2V + dienogest was comparable to other EE-containing CHCs (crude HR 0.8, 95% CI 0.4–1.6), but the difference became significant when the data were corrected for age, body mass index, duration of use, and family history of VTE (adjusted HR was 0.5 [95% CI, 0.2–1.0]). However, the risk of VTE was similar to a pill containing EE and a second-generation progestin (LNG) [Citation37]. Recently, the study by Reed et al. showed that 17βE2 + NOMAC was not associated with a higher risk of VTE or arterial thromboembolism compared to a EE + LNG pill [Citation38].
It is important to avoid unnecessary discontinuation because of the risk of undesired pregnancies, that could represent a further risk factor for VTE. An Italian group investigated the effects of social distancing during SARS-CoV2 pandemic, administering an online questionnaire to women taking long- or short-term hormonal contraceptives [Citation41]. Women taking long-term contraceptives didn’t show significant differences, while unmarried and non-cohabiting women regularly taking short-term contraceptives (CHC or POP) generally discontinued the treatment, despite continuing to have sexual intercourse. Among this latter group of women, more than 30% went through unexpected pregnancies, all of them requiring voluntary termination of pregnancy [Citation41].
In recent months, other articles and guidelines were published about this issue, the majority of them confirming the fact that clinicians can continue to prescribe and use CHCs, including those containing EE, during this pandemic [Citation42,Citation43]. By now, all these recommendations reflect the opinions of expert groups, but scientific studies designed to define whether and when continuing, discontinuing, or switching to another type of contraceptive during COVID-19 disease are still lacking.
The dilemma about COVID-19 vaccines and combined hormonal contraceptives
In recent months, new concerns have arisen since the worldwide diffusion of the novel COVID-19 vaccines. At present, the European Medicines Agency (EMA) has approved four vaccines against COVID-19, and more than 3 billion doses of vaccines had been administered globally by July 2021 [Citation44].
The main concern is about the vaccination with the recombinant adenoviral vector encoding the spike protein antigen of SARS-CoV2 (ChAdOx1 nCov-19, Vaxzevria, previously AstraZeneca). Since the end of February 2021, rare cases of unusual thrombotic thrombocytopenia have been reported after ChAdOx1 nCoV-19 vaccination.
At the beginning of this ‘vaccine era’, in Norway, this vaccine was administered to healthcare professionals younger than 65 years of age [Citation45]. Within 10 days after receiving a first immunisation with ChAdOx1 nCoV-19, five health care workers, four of them women aged 32–54 years of age, presented with thrombosis in unusual sites and concomitant severe thrombocytopenia, among a total of 132,686 first doses administered. Among these, four had severe cerebral venous thrombosis with major cerebral haemorrhage, three with fatal outcomes [Citation45]. A common denominator in all five patients was a high level of antibodies to platelet factor 4 (PF4)–polyanion complexes, similar to those produced during ‘heparin-induced thrombocytopenia’ [Citation46], even if none of these patients had received heparin before the onset of symptoms. These findings suggest that a syndrome similar to autoimmune heparin-induced thrombocytopenia may occur after administration of ChAdOx1 nCoV-19 [Citation45]. The name ‘vaccine-induced immune thrombotic thrombocytopenia’ (VITT) has been proposed to call this emerging post-vaccination syndrome [Citation45,Citation47].
Based on a report by the Society of Thrombosis and Haemostasis Research, it has been hypothesised that post-vaccination antibodies are formed against platelet antigens as a part of the immune stimulation process. These antibodies trigger massive platelet activation against PF4, resulting in immune thrombotic thrombocytopenia [Citation48]. Cross-reaction between the vaccine and platelets or PF4 has also been highlighted as a potential contributing factor in the pathogenesis of this syndrome especially knowing that adenovirus can bind to platelets resulting in platelet activation [Citation45].
A similar experience was reported in Austria and Germany. By March 2021, 11 patients were found to have one or more thrombotic complications beginning 5–16 days after vaccination with ChAdOx1 nCov-19. The median age was 36 years old and 9 out of 11 patients were female. None of the patients had received heparin before the onset of symptoms or the diagnosis of thrombosis. Also in these cases, VITT seemed to be at the basis of the thrombotic events with thrombocytopenia [Citation47].
A common denominator of the cases of VITT reported in these few published studies is the onset of thrombosis in unusual sites. Currently, there are still few studies about this side effect and low patient numbers. The incidence of this side effect has still to be quantified, but it is considered very rare but obviously severe.
Based on the EMA report of April 7th, 2021, there were 169 cases of thrombosis in the cerebral veins and 53 cases of thrombosis in the abdominal veins, with 18 fatal cases, following the administration of 34 million doses of Vaxzevria vaccine in Europe and United Kingdom (UK), i.e., 6.5 events/million subjects who received at least one dose of vaccine. The absolute estimated risk is 1/100,000 subjects who received the vaccine [Citation49].
In Italy by April 26th, 2021, 34 cases of venous thrombosis in atypical areas have been reported, 18 of them associated with thrombocytopenia. The estimated incidence was 0.45/100,000 people who received the vaccine: this is a lower incidence than the one reported in UK and Europe, probably as a consequence of the lower number of doses of vaccine administered [Citation50]. More recently, similar observations were done for the Ad26.COV.2.S vaccine (Janssen, Johnson & Johnson) [Citation51].
The mechanisms behind VITT are completely different from those of COVID-19 itself, as described above. The majority of these VITT complications occurred within the first five to twenty-one days following vaccination and occurred mainly in women under 55 years of age [Citation52]. At the present time, the possible occurrence after the second dose cannot be ruled out. Moreover, why this situation occurs with very high predominance in women has still not been clarified. The thrombotic complication in unusual sites seems to be related to the production of autoantibodies. It may be of interest to observe that the incidence of autoimmune diseases is higher in women [Citation14,Citation53].
In this context, many doubts arose about the concomitant presence of other prothrombotic risk factors during vaccination against COVID-19. A pre-existing hypercoagulable or autoimmune condition may favour the onset of this particularly rare side effect, with the vector vaccine serving as a trigger resulting in cascade of thrombosis and thrombocytopenia. Considering the small number of cases described in literature at this point, it’s hard to establish real correlations. In the cohort of 11 patients with suspected VITT described by Greinacher et al., only one patient was found to have pre-existing von Willebrand disease, anticardiolipin antibodies and factor V Leiden [Citation47]. Scully et al. found no previous prothrombotic medical condition in the 23 patients presenting with possible VITT [Citation12]. This clearly shows the difficulty of finding possible risk factors for the onset of VITT.
Recently, the researcher’s lens also stopped over the CHCs and HRTs. At present, there are no data studying any potential correlation between these hormonal therapies and the onset of VITT or other vaccine-related side effects, thus there is no indication to discontinue hormonal therapy before the administration of the vaccine. The mechanisms underlying thrombosis events in VITT, which are described above, are different from those described during hormonal contraception.
The EMA [Citation54], the Agenzia Italiana del Farmaco (AIFA) [Citation55] and the International Society on Thrombosis and Haemostasis (ISTH) [Citation56] recently concluded that unusual blood clots with low blood platelet counts should be listed as a very rare side effect of the vaccine. However, they agree that currently no specific risk factors have been identified, and that the benefits of the vaccine continue to outweigh the risks for people who receive it. As for all vaccines, EMA will continue to monitor Vaxzevria’s safety and effectiveness and to provide the public with the latest information [Citation54]. A study which may clarify the risk factors and the mechanisms beside the development of this rare side effect is necessary.
Conclusion
There are currently few certainties in this complex situation. It is certain that the COVID-19 pandemic has revolutionised people’s lives, so it is necessary to better identify the risk and protective factors behind this disease. Women seem to be relatively protected from the virus compared to men, and it may be postulated that sex hormones may contribute to this defence. However, no randomised studies are yet available to give answers to these questions. Why some vaccines cause this very rare but severe thrombotic side effect in some people is even less clear but mandatory to find out, figuring out a world where this vaccine will become routine.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Dong E, Du H, Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis. 2020;20(5):533–534.
- Guan W-J, Ni Z-Y, Hu Y, China Medical Treatment Expert Group for Covid-19, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720.
- Mehta P, McAuley DF, Brown M, HLH Across Speciality Collaboration, UK, et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033–1034.
- Jin J-M, Bai P, He W, et al. Gender differences in patients with COVID-19: focus on severity and mortality. Front Public Heal. 2020;8:152.
- Pirhadi R, Sinai Talaulikar V, Onwude J, et al. Could estrogen protect women from COVID-19? J Clin Med Res. 2020;12(10):634–639.
- Global Health 5050. COVID-19 sex-disaggregated data tracker n.d. https://globalhealth5050.org/the-sex-gender-and-covid-19-project/the-data-tracker/.
- Grasselli G, Zangrillo A, Zanella A, COVID-19 Lombardy ICU Network, et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy region, Italy. JAMA. 2020;323(16):1574–1581.
- Richardson S, Hirsch JS, Narasimhan M, The Northwell COVID-19 Research Consortium, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York city area. JAMA. 2020;323(20):2052–2059.
- Seeland U, Coluzzi F, Simmaco M, et al. Evidence for treatment with estradiol for women with SARS-CoV-2 infection. BMC Med. 2020;18(1):369.
- Hoffmann M, Kleine-Weber H, Schroeder S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271–280.e8.
- Dalan R, Bornstein SR, El-Armouche A, et al. The ACE-2 in COVID-19: foe or friend? Horm Metab Res. 2020;52(5):257–263.
- Scully EP, Haverfield J, Ursin RL, et al. Considering how biological sex impacts immune responses and COVID-19 outcomes. Nat Rev Immunol. 2020;20(7):442–447.
- Lemes RMR, Costa AJ, Bartolomeo CS, et al. 17β-estradiol reduces SARS-CoV-2 infection in vitro. Physiol Rep. 2021;9(2):1–8.
- Mauvais-Jarvis F, Klein SL, Levin ER. Estradiol, progesterone, immunomodulation, and COVID-19 outcomes. Endocrinology. 2020;161(9):1–8.
- Chen G, Wu D, Guo W, et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest. 2020;130(5):2620–2629.
- Dolhnikoff M, Duarte‐Neto AN, Monteiro ARA, et al. Pathological evidence of pulmonary thrombotic phenomena in severe COVID-19. J Thromb Haemost. 2020;18(6):1517–1519.
- Tang N, Bai H, Chen X, et al. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020;18(5):1094–1099.
- Bianchi I, Lleo A, Gershwin ME, et al. The X chromosome and immune associated genes. J Autoimmun. 2012;38(2–3):J187–192.
- Klein SL, Flanagan KL. Sex differences in immune responses. Nat Rev Immunol. 2016;16(10):626–638.
- Trenti A, Tedesco S, Boscaro C, et al. Estrogen, angiogenesis, immunity and cell metabolism: solving the puzzle. IJMS. 2018;19(3):859.
- Phiel KL, Henderson RA, Adelman SJ, et al. Differential estrogen receptor gene expression in human peripheral blood mononuclear cell populations. Immunol Lett. 2005;97(1):107–113.
- Hall OJ, Limjunyawong N, Vermillion MS, et al. Progesterone-based therapy protects against influenza by promoting lung repair and recovery in females. PLOS Pathog. 2016;12(9):e1005840.
- Stelzig KE, Canepa-Escaro F, Schiliro M, et al. Estrogen regulates the expression of SARS-CoV-2 receptor ACE2 in differentiated airway epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2020;318(6):L1280–1281.
- Bienvenu LA, Noonan J, Wang X, et al. Higher mortality of COVID-19 in males: sex differences in immune response and cardiovascular comorbidities. Cardiovasc Res. 2020;116(14):2197–2206.
- Huang C, Huang L, Wang Y, et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397(10270):220–232.
- Goërtz YMJ, Van Herck M, Delbressine JM, et al. Persistent symptoms 3 months after a SARS-CoV-2 infection: the post-COVID-19 syndrome? ERJ Open Res. 2020;6(4):00542-2020.
- Peghin M, Palese A, Venturini M, et al. Post-COVID-19 symptoms 6 months after acute infection among hospitalized and non-hospitalized patients. Clin Microbiol Infect. 2021;27(10): 1507–1513.
- Ghosn J, Piroth L, Epaulard O, et al. Persistent COVID-19 symptoms are highly prevalent 6 months after hospitalization: results from a large prospective cohort. Clin Microbiol Infect. 2021;27:1041.e1–e1041.e4.
- Calderone A, Menichetti F, Santini F, et al. Selective estrogen receptor modulators in COVID-19: a possible therapeutic option? Front Pharmacol. 2020;11:1085.
- Breithaupt-Faloppa AC, Correia C de J, Prado CM, et al. 17β-Estradiol, a potential ally to alleviate SARS-CoV-2 infection. Clinics. 2020;75:e1980.
- Fruzzetti F, Cagnacci A, Primiero F, et al. Contraception during coronavirus-Covid 19 pandemia. Recommendations of the board of the Italian Society of Contraception. Eur J Contracept Reprod Health Care. 2020;25(3):231–232.
- Ferreira-Filho ES, de Melo NR, Sorpreso ICE, et al. Contraception and reproductive planning during the COVID-19 pandemic. Expert Rev Clin Pharmacol. 2020;13(6):615–622.
- Mai V, Tan BK, Mainbourg S, et al. Venous thromboembolism in COVID-19 compared to non-COVID-19 cohorts: a systematic review with meta-analysis. Vascul Pharmacol. 2021; 139:106882.
- Jiménez S, Miró Ò, Llorens P, Spanish Investigators on Emergency Situations TeAm (SIESTA) Network, et al. Incidence, risk factors, clinical characteristics and outcomes of deep venous thrombosis in patients with COVID-19 attending the emergency department: results of the UMC-19-S8. Eur J Emerg Med. 2021;28(3):218–226.
- World Health Organization (WHO). Coronavirus disease (COVID-19): contraception and family planning 2020. https://www.who.int/news-room/q-a-detail/coronavirus-disease-covid-19-contraception-and-family-planning.
- Ramírez I, De la Viuda E, Baquedano L, et al. Managing thromboembolic risk with menopausal hormone therapy and hormonal contraception in the COVID-19 pandemic: recommendations from the Spanish Menopause Society, Sociedad Española de Ginecología y Obstetricia and Sociedad Española de Trombosis y Hemostasia. Maturitas. 2020;137:57–62.
- Dinger J, Do Minh T, Heinemann K. Impact of estrogen type on cardiovascular safety of combined oral contraceptives. Contraception. 2016;94(4):328–339.
- Reed S, Koro C, DiBello J, et al. Prospective controlled cohort study on the safety of a monophasic oral contraceptive containing nomegestrol acetate (2.5mg) and 17β-oestradiol (1.5mg) (PRO-E2 study): risk of venous and arterial thromboembolism. Eur J Contracept Reprod Heal Care. 2021;0:1–8.
- Gaussem P, Alhenc-Gelas M, Thomas J-L, et al. Haemostatic effects of a new combined oral contraceptive, nomegestrol acetate/17β-estradiol, compared with those of levonorgestrel/ethinyl estradiol. Thromb Haemost. 2011;105(03):560–567.
- Junge W, Mellinger U, Parke S, et al. Metabolic and haemostatic effects of estradiol valerate/dienogest, a novel oral contraceptive: a randomized, open-label, single-centre study. Clin Drug Investig. 2011;31(8):573–584.
- Caruso S, Rapisarda AMC, Minona P. Sexual activity and contraceptive use during social distancing and self-isolation in the COVID-19 pandemic. Eur J Contracept Reprod Health Care. 2020;25(6):445–448.
- FSRH Clinical Effectiveness Unit. FSRH Clinical Effectiveness Unit statement: use of combined hormonal contraception during the Covid-19 pandemic. 2020. https://www.fsrh.org/documents/fsrh-clinical-effectiveness-unit-statement-use-of-combined/
- Lete I. Combined hormonal contraception and COVID-19. Eur J Contracept Reprod Health Care. 2021;26(2):128–131.
- World Health Organization. WHO coronavirus (COVID-19) dashboard. Geneva: World Health Organization; 2021. https://covid19.who.int.
- Schultz NH, Sørvoll IH, Michelsen AE, et al. Thrombosis and thrombocytopenia after ChAdOx1 nCoV-19 vaccination. N Engl J Med. 2021;384(22):2124–2130.
- Greinacher A, Selleng K, Warkentin TE. Autoimmune heparin-induced thrombocytopenia. J Thromb Haemost. 2017;15(11):2099–2114.
- Greinacher A, Thiele T, Warkentin TE, et al. Thrombotic thrombocytopenia after ChAdOx1 nCov-19 vaccination. N Engl J Med. 2021;384(22):2092–2101.
- Oldenburg J, Klamroth R, Langer F, et al. Diagnosis and management of vaccine-related thrombosis following AstraZeneca COVID-19 vaccination: guidance statement from the GTH. Hamostaseologie. 2021;41(03):184–189.
- European Medicines Agency (EMA). AstraZeneca’s COVID-19 vaccine: EMA finds possible link to very rare cases of unusual blood clots with low blood platelets; April 7, 2021. https://www.ema.europa.eu/en/news/astrazenecas-covid-19-vaccine-ema-finds-possible-link-very-rare-cases-unusual-blood-clots-low-blood.
- Agenzia Italiana del Farmaco (AIFA). Quarto Rapporto AIFA sulla sorveglianza dei vaccini COVID-19 [Fourth AIFA report on COVID-19 vaccines surveillance]; May 10, 2021. https://www.aifa.gov.it/-/quarto-rapporto-aifa-sulla-sorveglianza-dei-vaccini-covid-19.
- European Medicines Agency (EMA). COVID-19 vaccine Janssen: EMA finds possible link to very rare cases of unusual blood clots with low blood platelets; April 20, 2021. https://www.ema.europa.eu/en/news/covid-19-vaccine-janssen-ema-finds-possible-link-very-rare-cases-unusual-blood-clots-low-blood.
- European Medicines Agency (EMA). COVID-19 vaccine safety update; 14 April 2021. https://www.ema.europa.eu/en/documents/covid-19-vaccine-safety-update/covid-19-vaccine-safety-update-comirnaty-january-2021_en.pdf.
- Carrel L, Brown CJ. When the Lyon(ized chromosome) roars: ongoing expression from an inactive X chromosome. Phil Trans R Soc B. 2017;372(1733):20160355.
- European Medicines Agency (EMA). AstraZeneca’s COVID-19 vaccine: EMA to provide further context on risk of very rare blood clots with low blood platelets; April 14, 2021. https://www.ema.europa.eu/en/news/astrazenecas-covid-19-vaccine-ema-provide-further-context-risk-very-rare-blood-clots-low-blood.
- Agenzia Italiana del Farmaco (AIFA). AstraZeneca’s COVID-19 vaccine: EMA to provide further context on risk of very rare blood clots with low blood platelets; April 9, 2021. https://www.aifa.gov.it/en/-/vaccino-covid-19astrazeneca-ema-approfondisce-ulteriormente-il-rischio-di-casi-molto-rari-di-trombi-associati-a-piastrinopenia.
- International Society on Thrombosis and Haemostasis (ISTH). Statement from the ISTH on reports indicating blood clots associated with the AstraZeneca vaccine; April 9, 2021. https://www.isth.org/news/559981/.
