Summary
The skin is a target organ of several hormones. Specific diseases appear in consequence of hypo- or hypersecretion of endocrine organs, particularly in the elderly patient. There, knowledge of skin alterations is important not only for dermatologists, but also for endocrinologists and other physicians, because a clinical diagnosis of the underlying disease is often possible. In this review, a number of representative skin diseases having an endocrinological basis are described. These include acanthosis nigricans, diseases due to alterations of androgen metabolism, carcinoid syndrome, diseases due to alterations of corticosteroid metabolism, diseases in association with diabetes mellitus, diseases due to alterations of estrogen metabolism, genetic syndromes including dermatological and endocrine symptoms, the glucagonoma syndrome, diseases due to dysfunctions of growth hormone secretion, diseases in association with Merkel cells of the skin, diseases due to dysfunctions of the thyroid gland, diseases to alteration of vitamin D metabolism, and vitiligo and disorders of pigmentation.
Acanthosis nigricans
Acanthosis (‘acantho’ meaning thorn) nigricans (black) (AN) is a reactive skin pattern seen in association with type 2 diabetes mellitus, obesity, cancer, and other systemic disorders. It is a rare disease due to the hyperactivity of different growth factors. However, Indians have high prevalence of AN Citation[1].
Acanthosis nigricans may precede type 2 diabetes mellitus; the prevalence increases with age Citation[2]. In diabetic patients, there is a statistically significant correlation of increasing severity of AN with increasing BMI. The association with obesity is presumably via insulin resistance and enhanced production of IGF-1 and IGF-2. Acanthosis nigricans is often a symptom of a malignant tumor, mainly in the gastrointestinal tract, but other tumors have also been described, e.g. of the endometrium Citation[3]. Acanthosis nigricans may precede the clinical diagnosis of the tumor; it regresses after treatment. It is unknown, however, whether the relapse of acanthosis nigricans also indicates a relapse of the tumor. It was also observed following bone marrow transplantation in lymphoblastic lymphoma; in this case it is likely that it was a graft versus host reaction.
The disease occurs in mutations of the receptors of epidermal growth factors (EGF) and the fibroblast growth factors (FGF) or by mutations of ligands themselves. On a molecular basis, the common pathogenic pathway of the disease is the activation of tyrosine kinases, which exert mitogenic and anti-apoptotic effects onto the keratinocytes Citation[4]. The earlier-used classification of acanthosis nigricans as malignant and benign types is not justified, since the clinical feature is common in all causes.
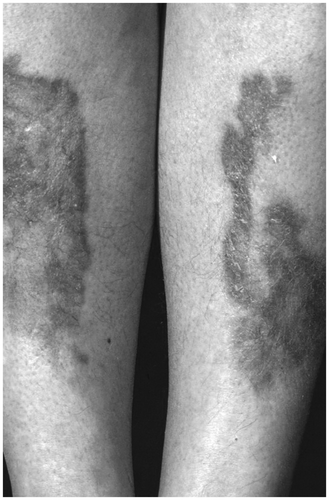
Lesions are gray-brown to black, rough, have thickened plaques and prominent skin lines, and occur most commonly in flexural areas (e.g. axillae, back and sides of neck, inguinal creases, inframammary) (). Also, palms and soles show hyperplasia of the relief as ‘tripe palms’. Histologically, there is hyperkeratosis, epidermal papillomatosis, and increased numbers of melanocytes. Acanthosis nigricans is frequently asymptomatic, but patients may present with lesions that are painful, disfiguring, malodorous, or macerated.
Figure 1. Acanthosis nigricans. Gray-brown to black lesions, rough, thickened plaques and prominent skin lines.
Treatment is difficult, with skin abrasion or topical calcipotriene as suggested treatment options Citation[5]. Treatment of the underlying disease (obesity, diabetes mellitus, intestinal tumor) is essential.
Androgens
Physiology
Testosterone is an important growth factor for the terminal hairs, the sebaceous glands, the follicle epithelia, the fibroblasts, and the melanocytes. It is, thus, the hormone with the most prominent effects on the skin.
The biochemical and molecular biological basis of the clinical effects is well understood. The skin and its appendages are capable of intense androgen metabolism. Nearly all of the enzymes necessary for the metabolism of steroids to testosterone, and those managing the degradation of testosterone, were found in the skin. Of particular importance is the 5-α-reductase, since the reduced form of testosterone, 5-α-dihydrotestosterone, is a closer ligand to the androgen receptor in the skin than testosterone itself Citation[6,7].
Testosterone is also essential for the development of distinct skin diseases ().
Table I. Effects of testosterone in skin diseases
In addition, there are some other clinical parameters indicating a normal androgen supply in the male:
The thickness of the skin is associated with the bone mineral density. Thus, the determination of skin thickness, which is easily achieved by ultrasound measurement, may serve as a prognostic factor in osteoporosis prediction instead of the direct measurement of bone mineral density, which requires different techniques Citation[8].
Measurement of a standardized skin fold allows an estimate of the lean body mass (LBM). LBM is negatively correlated to testosterone levels Citation[9].
Androgen deficiency (hypogonadism)
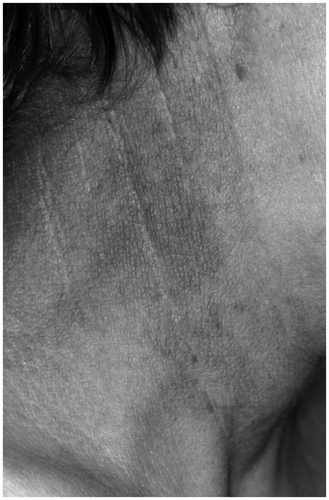
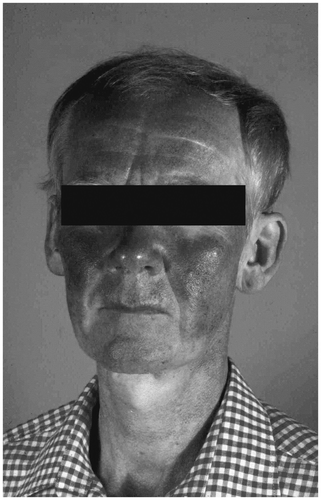
If hypogonadism is already present by prepubertal age, no pubertal signs occur during growth. In these men, the aging skin remains thin and smooth, and the pores remain small, which is particularly apparent on the face. The periocular wrinkles are notably small and crinkling (). Beard and other terminal hairs (axillae, pubes) are lacking. The reduction of scalp hair is delayed. Specific skin diseases do not occur Citation[10].
Figure 2. Skin in hypogonadism. The skin is thin and smooth; wrinkles are notably small and crinkling.
The normal decline of testosterone levels in the aging male is associated with a thinning of the skin; it becomes atrophic and loses elasticity, the surface becomes dry and produces less lipids. Sebum secretion declines, the cells of the sebaceous glands, a holocrine gland, increase their life cycle. However, hyperplasias of the glands as a consequence of the decreasing collagen synthesis also occur. The terminal hairs do not regress, but turn grey. In men supplemented with testosterone, the signs appear to a lesser extent. However, there are no controlled studies demonstrating the effect of testosterone substitution in the aging skin.
A special kind of hypogonadism is Klinefelter's syndrome, based on disomy of the x-chromosome (47, XXY). Also, a more frequent occurrence of the x-chromosome is known. Patients tend to develop androgen deficiency at an earlier age than healthy men. They also have a higher risk of developing connective tissue diseases, thrombophilia and osteoporosis. These diseases can be prevented by the substitution of testosterone Citation[11]. Men with Klinefelter's syndrome have a higher risk of developing cancer, particularly breast cancer (standardized mortality ratio 57.8 higher!). By way of compensation, they have a lower mortality from prostate cancer Citation[12].
Androgenic alopecia
Scalp hairs are also influenced by androgens, but in an opposite manner. In the hair follicle, testosterone is metabolized to 5α-dihydrotestosterone, which induces atrophy of the follicle and leads to androgenetic alopecia Citation[10]. The sensitivity of the individual hair follicle and the scalp hairs in general is determined genetically, whereby a number of genes are involved. Some gene polymorphisms of the androgen receptor were found. Men lacking 5a-reductase do not develop androgenetic alopecia.
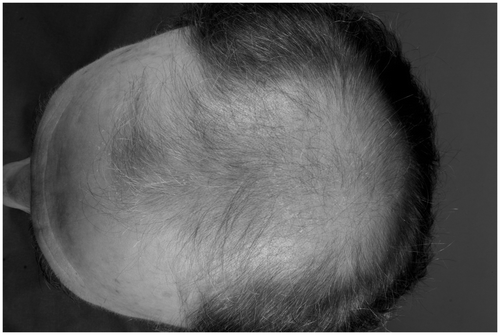
Androgenetic alopecia starts at different ages. It first involves the temples and the vertex region of the scalp. The hairless regions continue to grow until they unite. The end result is a bald scalp surrounded by a chaplet (). The individual hairs first become thinner, undergo a precocious telogen phase, and regrowth (anagen phase) becomes uncommon. At the end, the hair follicle disappears.
Figure 3. Androgenic alopecia. In an advanced stage, the bald skin of the scalp is surrounded by a chaplet.
A method for quantification of hair growth is TrichoScan Citation[13]. TrichoScan is able to analyze several parameters of hair growth. It can be used for clinical studies to compare placebo versus treatment, to compare different capacities of hair growth-promoting substances, to study androgenetic alopecia, and other forms of diffuse hair loss.
Androgenetic alopecia was, for a long time, considered an inevitable part of male life, and some experimental surgical procedures for treatment were prescribed. Most recently finasteride, the inhibitor of 5α-reductase, thereby inhibiting the conversion of testosterone to dihydrotestosterone (DHT), was successfully introduced as a proven treatment option. The treatment results in a significant improvement in subjective and objective assessments of hair growth and density. Finasteride is well tolerated with a favorable adverse event history. A long-term treatment is possible, but only half of men utilize it for years Citation[14].
Carcinoid syndrome
Carcinoid cells originate from the Kulchitsky cells of Lieberkühn's crypts (glandulae intestinales) of the small intestine. Most carcinoid tumors originate from the small intestine; they can spread to large parts of the intestinum, the stomach, the bronchus, and may metastasize to lymph nodes and the liver Citation[15]. However, carcinoid tumours can also arise in the esophagus, pancreas, liver, biliary tract, gallbladder, Meckel's diverticulum, ovary, otolaryngeal organs and the breast Citation[16].
The pathophysiological basis of the syndrome is an excessive secretion of serotonin and a malfunction of the tryptophan metabolism. In the normal metabolism, 1% of the daily intake of tryptophan is metabolized to serotonin, but in carcinoid syndrome this amount increases to about 60%. As a consequence, a tryptophan deficiency of other organs occurs. In the skin, this results in a pellagra-like appearance. Serotonin induces vasoconstriction and dilation of the arterioles, which clinically appears as a typical flush. In addition, histamine, kallikrein, prostaglandins and some vasoactive peptides may be produced by the carcinoid tumor, which intensifies the clinical symptoms.
Episodic flushing is the clinical hallmark of the carcinoid syndrome, and occurs in 85% of patients Citation[17] (). The typical flush begins suddenly and lasts 20 to 30 seconds. It primarily involves the face, neck, and upper chest. As the disease progresses, the episodes may last longer and the flushing may be more diffuse and cyanotic. Most flushing episodes occur spontaneously, but they can be provoked by eating, drinking alcohol, defecation, emotional events, palpation of the liver, and anesthesia. Due to prolonged and repeated vasodilation, venous telangiectasias may occur. The most debilitating component of this syndrome is the diarrhea. Some patients develop pellagra-like lesions with pigmentation and hyperkeratosis in the legs or the arms. These alterations respond well to the application of niacin Citation[18].
Figure 4. The typical flush, the clinical hallmark of the carcinoid syndrome, begins suddenly and lasts 20 to 30 seconds. It primarily involves the face, neck, and upper chest. (Courtesy of Prof. Dr Arnold, Marburg, Germany)
The diagnosis is fixed by the proof of a maximally increased urinary excretion of 5-hydroxyindol acetic acid (5-HIAA) up to 1000 mg per day Citation[19].
The therapy of the syndrome is the exstirpation of the tumor. If this is not possible, long-acting somatostatin analoga are the treatment of choice. Corticoids and beta-inhibitors are ineffective for symptomatic improvement.
Corticosteroids
Physiology
Nearly all cells of the organism need glucocorticosteroids for the maintenance of normal function Citation[20]. The concentration of cellular binding proteins (glucosteroid receptors) in the skin shows large variations between different sites. This possibly reflects the different sensitivity of different skin areas to topically applied glucocorticosteroids (e.g. the foreskin is highly sensitive, the abdominal skin is of low and the face is of moderate sensitivity). Cultivated keratinocytes or fibroblasts from different areas also show the different receptor densities. Corticosteroid receptors are also present in the endothelial cells of the skin vessels. The blanching effect due to vascular constriction of different glucocorticosteroids is parallel to the receptor affinity of the compound Citation[21].
After binding of glucosteroids to the receptor, the complexes bind to distinct DNA sequences and modulate the activity of certain genes Citation[22]. Some studies have suggested a positive association between obesity, hypertension, and insulin resistance, with alleles at the glucocorticoid receptor (GR) gene. Also, distinct mutations in the GR gene have been postulated as being relevant to the progression of type 2 diabetes and cardiovascular diseases, but they could not be proven exactly up to now Citation[23].
Addison disease
Addison's disease is the consequence of a hyposecretion of glucocorticoids (adrenal insufficiency). This is usually the result of a primary insufficiency of the adrenal gland following inflammation or autoimmune diseases. The typical manifestation is hyperpigmentation of the skin, due to the increased pituitary secretion of POMC peptides. The pigmentation is generalized, similar to sunburn and is more pronounced in scars, in skinfolds, palmae, nipples, perineum, genitalia, and in linea alba. The pigmentation sometimes occurs in the form of freckles. The hairs become darker, and nails show dark bands. Existing nevi also become darker. With sufficient treatment, the pigmentation resolves. The pigmentation may occur before signs of adrenal insufficiency are visible. Twelve per cent of patients show a vitiligo.
Cushing disease
Cushing's disease occurs as a consequence of hypersecretion of the adrenal gland. There are primary (adrenal) and secondary (hypophyseal) causes of hypersecretion. The typical signs are listed in .
Table II. Clinical signs in Cushing's disease
Cushing's disease may also include hepatic steatosis, diseases of the gall bladder, pulmonal insufficiency, osteoarthritis, proteinuria, increased hemoglobin concentration, and immunologic incompetence.
Systemically applied corticosteroids at a dose above the daily production of endogenous cortisol (more than 20 mg) usually lead to a clinical picture identical to that of endogenous hypersecretion. The clinical appearance is indistinguishable without data of the medical history.
Diseases due to topical corticosteroid overdose
Following long-term application of topical glucocorticosteroids striae distensae, thinning of the skin, telangiectasias and – in the face – a rosacea-like dermatitis may occur (). These unwanted side effects are unavoidable, connected to the anti-inflammatory effects of glucocorticoids, and are always more pronounced in the face than in other areas of the body, since they increase the degradation of collagen and elastic fibers induced by sunlight. Thus, the aging skin is put at higher risk than young skin.
Figure 5. Topical overdosage of corticosteroids. Following long-term application, development of telangiectasias and a rosacea-like dermatitis may occur, particularly on the face.
One of the aims of the continuing development of topical steroids is to improve the anti-inflammatory and immune suppressive effect by avoiding stronger side effects. However, an ideal steroid is yet to be discovered. It should permeate well through the stratum corneum and penetrate into the skin, but should not lead to enhanced concentrations in the blood serum. In order to attain this goal, the lipid solubility was increased by esterification. Other changes to the molecular structure, leading to an improvement in efficacy, mostly imply higher side effects. Typical compounds betamethasone dipropionate and clobetasol propionate may be included as possible options. Novel steroids such as budesonide, mometasone furoate, prednicarbate, 17,21-hydrocortisone-aceponate and hydrocortison-17-butyrat-21-propionat, methylprednisolone aceponate, alclometason dipropionate, and fluticasone propionate all have a good anti-inflammatory effect and lesser side effects Citation[24].
Diabetes mellitus
Diabetic dermopathy
Skin diseases in diabetes mellitus are the consequence of several influences dependent on the duration of diabetes and the quality of insulin therapy:
The skin of the diabetic patient is characterized by risk of bacterial and mycotic infections. If such diseases occur frequently in aging men, the exclusion of diabetes mellitus is mandatory. The frequency of skin diseases in diabetic patients is illustrated by the following: Wozniak and Bar Citation[25] undertook dermatological investigation of 500 diabetic outpatients and found obesity in 54% (273 persons), and lesions on skin or mucous membranes in 83.6% (418 persons). Signs of infections are erythema, warmth, tenderness, swelling, and abscess Citation[26].
The diabetic microangiopathy originates from the modification of blood flow characteristics. Histologically, capillaries and arterioles show thickening of the intima, focal deposits of PAS-positive material and extravasation of erythrocytes and leukocytes. The metabolism of the vascular walls is altered due to changes of the collagen types and the decrease of water-binding capacity. The permeation of granulocytes throughout the vascular wall is inhibited; the phagocytic activity of leukocytes is decreased. The tissue S(HSI)O2 is reduced in the skin of patients with diabetes, and this impairment is accentuated in the presence of neuropathy in the diabetic foot. Additionally, energy reserves of the foot muscles are reduced in the presence of diabetes Citation[27]. Subsequently, wound healing is also impaired. Diabetic dermopathy is often associated with angiopathy of other vascular areas Citation[28]. Screening for vascular disease includes peripheral pulses, skin temperature, skin thickness, and skin color Citation[26].
Neuropathy leads to decreased pain sensations, to changes in pressure and temperature sensations. Screening for diabetic neuropathy includes a detailed history, physical examination (vibration, pressure, pain, and temperature sensation), and may result in a quantitative signs score Citation[26].
The diabetic foot
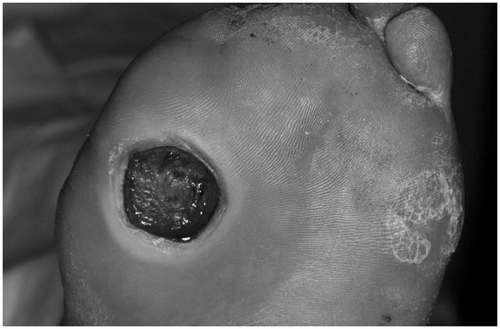
The main focus of skin diseases are the feet (). Fifteen per cent of individuals with diabetes develop a foot ulcer during their lifetime, and 85% of lower extremity amputations are preceded by an ulcer with or without infection. A strong correlation between diabetes-associated foot morbidity and morbid obesity was found Citation[29]. Skin defects and subsequent ulcers result from an interaction of a number of causes. The vulnerability of the diabetic skin promotes infections. Peripheral vascular disease appears to be more frequent in type 2 diabetes; the risk increases with the duration of the disease. Neuropathy is present in over 80% of patients with foot ulcers; it promotes ulcer formation by decreasing pain sensation and perception of pressure, as well as by causing muscle imbalance.
Figure 6. The diabetic foot. Chronic inflammations of the skin with defects and subsequent ulcers, showing recurrent infections.
Bacterial infections are mostly caused by staphylococcal aureus, and also by enterococcus and, to a lesser extent, E. coli. It is not unusual that multiple bacterial species are found; in a number of cases no common antibiotic is available. In up to 80% of severe infections anaerobic bacteria are found; they may be accompanied by general symptoms (‘Diabetic foot flu’). Treatment should be related to bacterial species and sensitivity. Wound debridement is mandatory. A relatively new treatment is vacuum therapy. Its use in foot and ankle surgery leads to a quicker wound closure and, in most patients, avoids the need for further surgery Citation[30].
Topical treatment is allowed only in slight or moderate infections; in all other cases, which are hazards for a possible amputation (cellulitis, lymphangitis, deep ulceration, necrosis, gangrene, osteomyelitis), only systemic antibiotics should be used. Foot infections and ulcer are risk factors for subsequent amputations. Osteomyelitis is frequent in diabetic feet; those patients in whom bone is visible in the ulcer are particularly in at risk. Short-contact application of topical tretinoin improved the healing of foot ulcers in patients with diabetes. The tretinoin therapy was generally well tolerated, without serious local or systemic adverse effects Citation[31].
In order to prevent ulcers, the rules for foot care in diabetes should be strictly followed (). If foot-care education in patients is sufficient and skin changes are intensively treated, patients should have significantly lower incidence of diseases. Also, training of health personnel significantly improved skin health in the observed diabetics Citation[32,33].
Table III. Diabetic foot care
The incidence of tinea and onychomycosis are also increased. Treatment should be with oral antimycotics; they are well tolerated, and there are no diabetes-specific side effects. They are far more effective than topical antimycotics, even if the nail substance is mechanically reduced Citation[34].
Necrobiosis lipoidica diabeticorum (NLD)
The pathogenesis of NLD is not known. Immune histological studies suggest the involvement of immunological mechanisms. The extent of the association of diabetes mellitus and NLD is discussed controversially. In a study by Freinkel and Freinkel Citation[28], only 10% of 171 patients identified as having NLD did not have diabetes. In rare cases, NLD occurs as part of a syndrome.
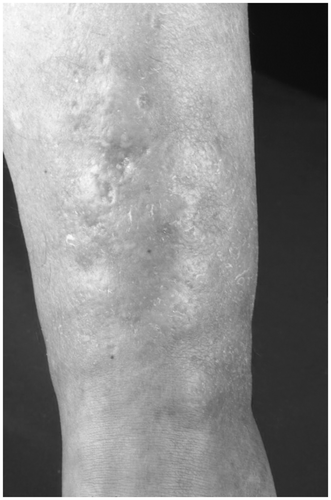
The lesion in NLD begins as a dark red, elevated nodule with an irregular border. Later, the skin becomes atrophic. The lesion becomes yellowish-brown; only the margin maintains the red color (). The epidermis is thin and shows geringe scaling. Enlarged vessels may be seen through the translucent skin. New lesions of NLD may be observed as a Koebner phenomenon. Subjective sensations are not reported. No sufficient treatment is known; corticosteroids or cryotherapy with liquid nitrogen are applied. Systemic application of small doses of ASS was assumed to be successful.
Adverse skin reactions to insulin
Insulin itself may cause side effects to the skin. Locally, itching, edema, stinging, pain or warmth and redness at the injection site, as well as generalized urticaria, may occur. Frequently lipotrophy and lipohypertrophy are seen. These may be caused by an altered kindetic of the injected insulin. This risk increases if the injection site is not frequently changed. Rapidly resorbed insulins may decrease the risk, but a regular change of the injection site is mandatory. Liposuction of the altered fat deposits is not effective. There are also allergic reactions to insulin and the preservatives Citation[35].
Patients using exogenous insulin have a lower risk of developing NMSC, and the protective effect of insulin use becomes more distinct with increasing age Citation[36].
Estrogens
Estrogen deficiency
Estrogens are essential for structure and function of the normal skin. The decrease of circulating estrogens in the female climacteric lead to specific alterations, and large studies were able to show partial resolution of the alteration by (topical or systemical) estrogen supplementation. Estrogens levels also decrease in the aging male, but it is unknown whether similar alterations are also due to a lack of estrogens Citation[37,38].
Estrogen effects on the skin are:
The life span of keratinocytes declines.
The epidermal water loss increases.
The activity of sebaceous glands and sweet glands declines.
The epidermis becomes atrophic and wrinkles arise (‘aging skin’).
The skin becomes more irritated by temperature, moisture, and mechanical traumata.
The water-binding capacity of the cutis declines; the skin loses tonus.
The collagen content of the cutis decreases by 1% per year of age.
The collagen fibers disentangle; the skin becomes flaccid, thin, and translucent.
The thickness of a skin fold declines. This effect is increased when diabetes is present.
The amount and complexity of elastic fibers declines.
Gynecomastia
Gynecomastia is the consequence of increased estrogen action in the male. The male breast tissue is estrogen sensitive, as it is in women. In this respect, not only enhanced estrogen levels are effective, but also an altered relation of testosterone to estrogens is of relevance.
Gynecomastia appears in different ages with different frequency. At the age of 14, about 40% of the boys have gynecomastia. In aging men, about 60% suffer from gynecomastia Citation[39]. In both groups gynecomastia is usually associated with obesity, but a specific cause has not yet been found. The risk of gynecomastia is greater in several groups: (i) men using drugs with estrogen-like action or promoting the aromatization of androgens; (ii) men with tumors arising from the testis or other organs which produce human chorionic gonadotropin; and (iii) men with hyperprolactinemia induced by prolactinoma or by dopamine-inhibiting drugs, or undertaking HIV therapy, where macromolecules of prolactin occur.
The clinical appearance of gynecomastia in the range of a palpable tumor under the mammilla up to a large pending breast with significant submammary fold is independent of the pathogenesis (). In the development of gynecomastia, pressure pain is often the first symptom. The mammilla is enlarged to more than 2.5 cm and hyperpigmented.
Figure 8. Gynecomastia. Large pending breast with significant submammary fold in a patient with Klinefelter's syndrome.
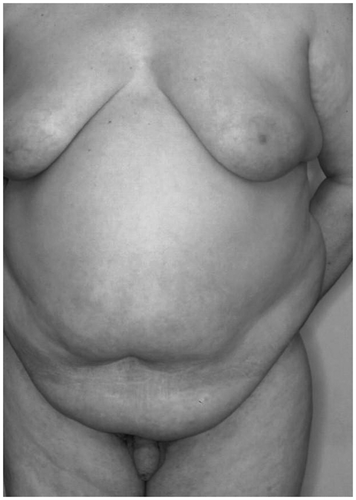
The differential diagnosis to the male breast carcinoma is essential, although its incidence is only 1% of the incidence of breast cancer in women. One-sided gynecomastia is suspicious; it should be clarified using mammography and fine-needle puncture.
The therapy of choice in gynecomastia, particularly if no endocrine or pharmacological causes are found, comprises surgical intervention. Usually, the skin is opened by a circular section along the margin of the mammilla, and the gland is then excised or extracted by suction. The intervention is easy to perform. A relapse may occur if the cause of gynecomastia persists. In male breast carcinoma, the radical excision, including axillary lymph nodes, is necessary.
Genetic syndromes of the skin with involvement of endocrine organs
Carney complex
Carney's complex (NAME syndrome) Citation[40,41] is an autosomal dominant disorder characterized by skin lesions such as lentigines and blue nevi on the face, neck and trunk, and by multiple tumors with endocrine activity (Sertoli cell tumor of the testis, and adrenal tumor) and without (cutaneous, mammary, and atrial myxomas).
About two-thirds of the cases of the syndrome occur in families. The diagnosis is usually confirmed at the end of the second decade of life. Nevi may be present at birth, but lentigines develop at the time of puberty. About 25% of patients have micronodular adrenal hyperplasia occurs leading to Cushing's syndrome.
There are three genetic loci for the Carney complex; the responsible disease gene has been identified for two of these. One of these encodes a regulatory protein of the protein kinase A. A mutation of this gene codes for a non-functioning protein, so that protein kinase A activity is enhanced. A third gene on chromosome 17 codes for the myosin heavy chain (MYH8) Citation[42].
POEMS syndrome (Crow-Fukase-syndrome)
This acronym stands for: polyneuropathy, organomegaly, endocrinopathy, monoclonal proteinuria, and skin diseases. Antibodies against pituitary tissue inhibiting hormone secretion appear to be the pathogenetic marker. Additional features described in this syndrome include sclerotic bone lesions, Castleman disease, papilledema, pleural effusion, edema, ascites, erythrocytosis, and thrombocytosis. This is why the term ‘osteosclerotic myeloma’ was coined Citation[43].
Peripheral neuropathy usually dominates the clinical picture. Fifty per cent of the patients in the series Citation[44] had organomegaly (hepatomegaly, splenomegaly, or lymphadenopathy). Two-thirds of the patients had at least one endocrine abnormality at presentation; the most common abnormality was hypogonadism. All of the patients had evidence of a monoclonal plasma cell proliferative disorder.
Skin changes with hyperpigmentation, hypertrichosis, acrocyanosis, plethora, hemangiomas or telangiectasia were noted in two-thirds of the patients. Telangiectasia and hemangiomas were observed in only 10% of the cases. Histopathologically, the hemangiomas resemble to renal glomeruli Citation[45].
In the series of 99 patients, only 29 had all five components of the syndrome. Thus, Dispenzieri et al. Citation[44] proposed that diagnosis of a POEMS syndrome should be made only when both major criteria (polyneuropathy and a monoclonal plasma cell disorder) are present, along with at least one of the other criteria. The course of POEMS syndrome is chronic; patients survive for many years in contrast to those with multiple myeloma. Overall median survival of the 99 patients was 13.7 years.
There is no standard treatment for this disorder. However, 74% of the patients had systemic chemotherapy. Alternatives are corticosteroids, plasmapheresis, or strontium-89. In younger patients, autologous hematopoietic cell transplantation is a consideration. The use of agents with anticytokine activity (interferon, retinoic acid, monoclonal antibodies) are anecdotal.
Glucagonoma syndrome
Glucagonoma syndrome is a rare disease resulting from a nearly always malignant pancreatic tumor. As a skin disease, the necrolytic migratory erythema is often one of the first presenting symptoms (). Cutaneous eruptions occur mainly in the groin, extremities, thighs, buttocks, and perineum. They begin as erythematous papules or plaques. Central clearing then occurs, leaving bronze-colored, indurated areas centrally, with blistering, crusting, and scaling at the borders. The affected areas are often pruritic and painful. Skin biopsies obtained from the edge of the lesions reveal superficial necrolysis, with separation of the outer layers of the epidermis, and perivascular infiltration with lymphocytes and histiocytes. Also the mucous membranes are affected, resulting in glossitis, angular cheilitis, stomatitis, and blepharitis Citation[46].
Figure 9. Necrolytic migratory erythema in glucagonoma. Bronze-colored, indurated areas with blistering, crusting, and scaling at the borders.
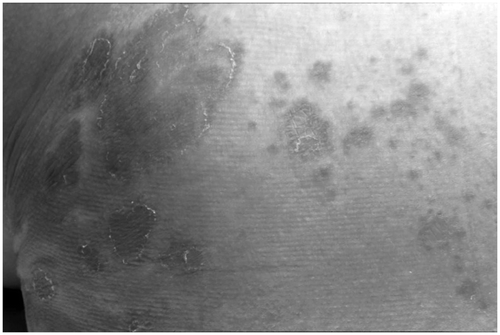
Patients also suffer from weight loss and diabetes mellitus (75%), and normochromic anemia (90%). Further symptoms are thromboembolism (30%) and neuropsychiatric disorders. Gastrointestinal symptoms such as diarrhea or obstipation, which may lead to vitamin B deficiencies, develop, further aggravating the dermatosis Citation[47]. Finally, glucagonomas may produce multiple hormones in addition to glucagon, including VIP, gastrin, serotonin, insulin, calcitonin, pancreatic polypeptide, and ACTH.
The diagnosis is established by typical clinical findings in association with hyperglucagonemia Citation[48]. However, a serum glucagon concentration below 500 pg/mL does not exclude a glucagonoma, because pathological molecular weight forms of glucagon may not be measured by the immunoassays used. On the other hand, concentrations above 1000 pg/mL are virtually diagnostic of glucagonoma. The underlying tumor has to be diagnosed by specific imaging procedures.
Therapy consists in surgical removal of the pancreatic tumor. Near-complete resolution of the skin disease is seen often only one week after surgery. However, since the glucagonoma is a malignant tumor, further growth of the tumor and recurrence of the necrolytic migratory erythema is not unlikely Citation[49].
Growth hormone
Growth hormone (GH)-regulated gene expression contributes to many of the effects of GH on cellular metabolism, growth, and differentiation. Study of model systems has revealed several mechanisms by which GH regulates gene expression and transcription factors. Several genes are also sexually dimorphic expressed. GH-regulated STAT 5 has also been implicated in regulation of other physiologically important genes, including those encoding components of the IGF-I axis, and insulin Citation[50].
The insulin-like growth factors (IGF) form a family of ligands with specific binding proteins and receptors. They are important in both the development of the organism and the maintenance of normal function of many cells. The system also has powerful anti-apoptotic effects. Specific signalling pathways emanating from the IGF-I receptor affect cancer cell proliferation, adhesion, migration, and cell death Citation[51].
The hypersecretion of growth hormone induces disease acromegaly (; ). The pasty edema of the skin is a consequence of an increasing amount of glycosaminoglycans and collagens in the dermis. Also, the water storage is increased due to the increase in hydrophil compounds of the dermal matrix. The epidermis and the skin appendages become hyperplastic. Collagen-bundle coarsening and acidic glycosaminoglycan deposits are found irrespective of the current endocrine status. Dermal dendrocytes were often markedly reduced in numbers, but those present were plump with few dendrites. Collagen, glycosaminoglycans and DDs are affected in acromegaly, even when the endocrine situation is controlled Citation[52].
Figure 10. Acromegaly. Thickening, pasty consistence, thickening of skin folds, thickening of lips and eyelids, hypertrichosis, bushy eyebrows, growth of bones, enlargment of the ears, protrusion of zygoma, mandible and chin.
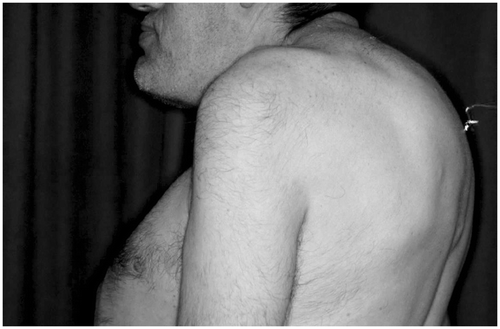
Table IV. Clinical signs of acromegaly.
Hyperpigmentation occurs as a consequence of the concomitant hypersecretion of MSH. Sweating in heat and physical stress is reduced. Patients with acromegaly describe decreased wellbeing in controlled studies; the questionnaires concerned indicate decreased quality of life. After substitution of growth hormone, mood and energy increased again Citation[53].
The secretion of growth hormone also declines with age and the concentration of IGF, which is produced under the influence of GH. A substitution of GH in aging men therefore appears to be plausible. This was already performed in several studies, and a regulation of dysfunctions, to a certain extent, was observed. However, uncontrolled treatment was often started without proving a lack of GH secretion. In these cases, the physiological levels were often exceeded, and side effects were observed. These were, particularly in elderly and obese patients, hyperinsulinemia, sodium and water retention, weight gain, edema in lower extremities, carpal tunnel syndrome, increase of intracranial pressure, papillary edema, arthralgia and myalgia. As the most severe side effect, the growth enhancement of malignant tumors was observed Citation[53]. All of these side effects were reversible after cessation of growth hormone application.
Due to the side effects described, an indiscriminate use of growth hormone as a ‘youth hormone’ should be strongly discouraged.
Merkel cells
Merkel cells exist in the normal epidermis, but at a considerably number in connection to the hair follicles and to the Haarscheiben Citation[54]. They are difficult to identify in a hematoxylin-eosin stained skin section. Their specific appearance is observed by electron microscopy, by which the cells show neurosecretory granules. These granules contain neuropeptides and biogenic amines, which denominate these cells as neuroendocrine cells. In the cytoskeleton, Merkel cells express cytokeratins of low-molecular weight (CK 8, 18, 19, 20).
As a function of Merkel cells, the action as a mechanoreceptor is established. Their association to the bulge region of the hair follicles may suggest paracrine trophic functions during hair regrowth. Their intimate contact with Langerhans cells also argues for a functional role in the neuroimmunological system of the skin. A specific endocrine product of the Merkel cells could not be identified until recently Citation[55].
Clinically most relevant are Merkel cells by reason of the Merkel cell carcinoma (MCC), which is a highly aggressive malignant tumor, giving rise to metastasis in lymph nodes and distant organs in most cases. They are to diagnose histopathologically due to the neuroendocrine phenotype and the expression of cytokeratin, CK 20. The treatment of Merkel cell carcinoma requires aggressive surgical and chemotherapeutical regimens, but the overall prognosis is poor. Low levels of neuroendocrine differentiation in MCC associates with poor prognosis Citation[56].
Thyroid hormones
Hypothyrosis (Myxedema)
The thyroid hormones thyroxine (T4) and triiodothyronine (T3) are important for growth and function of most tissues. The transport to the hormone target organs requires binding to the thyroid hormone-binding globulin (TBG) in blood. In the cell, there are binding proteins for T4 and T3 in the cytosol, and these then bind to the DNA-bound thyroid hormone receptor. Receptors of α- and a β-type were identified in many cells. They express three major functional domains, one binding DNA, one binding ligand, and two major domains regulating RNA transcription Citation[57].
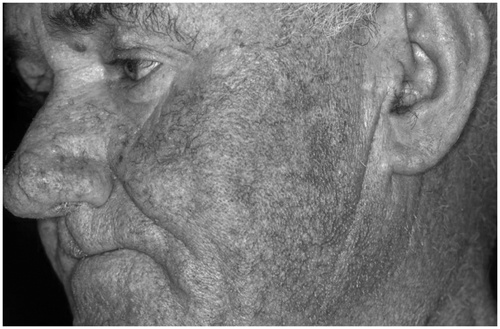
General deficiency of thyroid hormones leads to skin changes characteristic of myxedema, in particular in Graves' disease and occasionally in Hashimoto's thyroiditis. The skin is pasty and voluminous and shows a non-impressible edema, which is not position dependent (). The skin is dry, cool, and pale. This appearance is the consequence of decreased vascular flow and decreased reaction to heat. Also, the mucous membranes become dry; the tongue becomes fissured. The epidermis is thin, raspy and hyperkeratotic (shark skin). The texture of the cornea is altered. The hairs are dry, fragile and raspy. Sometimes localized alopecia or a diffuse effluvium occur. The nails grow slowly and become brittle. Wound healing is decelerated.
Myxedema is a consequence of the storage of large amounts of proteoglycans, which are also responsible for the yellowish color of the skin. After substitution of the thyroid hormones, the pathologic proteoglycan deposits are quickly mobilized again Citation[58].
Hyperthyreosis
In hyperthyrosis, which may result from autochtone hyperfunction of the gland or from increased stimulation by pituitary TSH, the skin is warm, tender, and wet with a soft turgor, resembling to the infantile skin. This originates from a peripheral vasodilation, with increased blood flow, particularly in the face. The patients tend to experience increased sweating, especially in plantae and palmae. The hairs are thin and soft; the nails have a normal appearance. Pigmentation is also unaltered.
Hyperthyrosis may also be caused by the stimulation of the TSH receptor by antibody-like proteins (Graves' disease, Basedow's disease). Autoimmune thyroid diseases may be associated with other diseases of the immune system, e.g. with chronic urticaria Citation[58]. Its prevalence is quoted to be between 12% and 29% of urticaria cases. In 182 patients with chronic urticaria, of whom 90 had a positive autologous intradermal serum test, 18 of the skin test-positive patients and 4 skin test-negative patients had thyroid microsomal antibodies Citation[59]. TSH outside the normal range was found in 13 skin test-positive patients and 1 skin test-negative patient. In the overall study group, an elevated TSH was present in seven patients comparable to the 5% expected prevalence in the community. Thyroglobulin antibodies (TGA) were present in two of 182 patients.
Most of the urticaria patients are euthyroid. If hypothyrosis is diagnosed, a hormone substitution should be performed. Improvement of chronic urticaria is achieved best with L-thyroxin in euthyreot patients. The blood levels of thyroxin should be monitored in order to avoid hyperthyroidism, particularly in elderly patients Citation[60].
Vitamin D
Vitamin D (‘soltriol’) Citation[61] is a phylogenetic, ancient steroid hormone which impacts on multiple organs and organ systems. The effect on bone metabolism and the maintenance of calcium homeostasis of the body is its most important function, but it is also indispensable in reproduction, in the parathyroid gland, in the skin, in the differentiation of muscle cells, and in promoting cancer growth.
Provitamin D is formed from 7-dehydrocholsterol in the skin, under the influence of sunlight. In particular effective are the wavelengths of the UV spectrum at about 300 nm Citation[62]. Once formed, vitamin D3 is metabolized in the liver to 25-hydroxyvitamin D3, and then in the kidney, to its biologically active form, 1,25-dihydroxyvitamin D3. The melanocytes of the skin play an important role in the synthesis of vitamin D. It is well known that men with dark skin color have a higher bone mineral density than Caucasians, and they have a lower calcium excretion.
1,25-dihidroxy-vitamin D3 binds, similar to other steroid hormones, to the so-called vitamin D-response elements (VDRE) of the DNA, thus influencing the transcription of certain genes. Among others, the activity of the phospholipase D1 is specifically enhanced. This leads to the hydrolysation of phospholipids, whereby lipids as second messengers are formed, e.g. diacylglycerol (DAG). DAG again activates several protein kinases, which are of relevance in the terminal differentiation of the keratinocytes Citation[63]. In this way, the effects of vitamin D in psoriasis may be explained Citation[64]. The metabolite calcipotriol is less effective in the calcium metabolism than the original vitamin D. Calcipotriol is possibly effective in all skin diseases with abnormalities of the keratinocyte differentiation.
Vitamin D deficiency is a consequence of decreased uptake and exposure to nature light. Vitamin D deficiency has been associated with increased risks of deadly cancers, cardiovascular disease, multiple sclerosis, rheumatoid arthritis, and type 1 diabetes mellitus. Although chronic excessive exposure to sunlight increases the risk of nonmelanoma skin cancer, the avoidance of all direct sun exposure increases the risk of vitamin D deficiency, which can have serious consequences Citation[65].
Vitiligo and melanocytes
Vitiligo describes a local loss of skin pigmentation due to the malfunction or loss of melanocytes. Vitiligo is a common disease; worldwide, about 0.5% of men suffer from vitiligo. The causes are unclear: genetic factors, autoimmunity to melanocytes, toxic compounds, nerve disorders, and defective melanocyte growth factors are discussed Citation[66]. Huang et al. Citation[67] interpreted vitiligo as a manifestation of apoptosis. This is more likely than the necrosis of melanocytes, which is rarely observed. The apoptosis is induced by UV light, by cytokines from the keratinocytes, or by environmental pollutants, e.g. hydroquinone. Since corticosteroids and other immune suppressants are able to influence apoptosis, it is plausible that these compounds have a certain effect on vitiligo.
A specific treatment of vitiligo, however, is not known. Currently available literature examined trials using UV B (narrowband 311 nm), UV A in combination with 5-methoxypsoralen, topical corticoids, and topical calcineurin antagonists (tacrolimus and pimecrolimus). Randomized controlled studies are not yet available; mostly, the application was not controlled and used in only a few cases (). Surgical methods were also applied; these included the auto transplantation of blister skin or cultivated melanocytes. In expanded vitiligo, the decoloring of the pigment residues is advisable. After unsuccessful therapies, a camouflage (make-up) should be recommended. In each case a reliable sun protection is essential.
Figure 12. Vitiligo. Local loss of skin pigmentation due to the malfunction or loss of melanocytes. After three months' treatment with tacrolimus, the size of lesions is decreased.
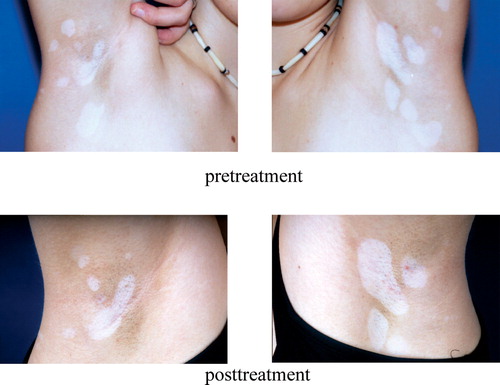
A similar mechanism as in vitiligo is assumed to be the basis of depigmentation in malignant melanoma, which is suggested to be a good prognostic sign. Insights in humoral and cellular immune reactions affecting normal and malignant melanocytes could lead to strategies for therapeutic modalities in vitiligo as well as in melanoma Citation[68].
α-melanocyte-stimulating hormone (α-MSH) stimulates the production of melanin, predominantly the eumelanin as well as the dendricity of melanocytes. The hormone is secreted by the intermediate lobe of the pituitary gland. Also, the melanocytes themselves and other epidermal cells of the skin produce MSH. It is part of the so-called proopiomelanocortin peptide (POMC). Melanocytes express the melanocortin receptor 1 (MC1-R). Mutations of MRC-1 are not infrequent; these melanocytes do not respond to α-MSH stimulation, and are highly sensitive to UV irradiation. The loss-of-function mutations are associated with red hair, and an increased risk of skin cancer Citation[69].
Melanocytes secrete a number of compounds into their cellular environment, such as signalling molecules and cytokines, POMC peptides, catecholamines, and nitric oxide (NO). By the secretions, they influence the function of keratinocytes, lymphocytes, fibroblasts, mast cells, and vascular endothelial cells. This may, in part, explain the suppression of immune reaction by UV irradiation Citation[70]. The application of α-MSH or related peptides could offer a treatment option of inflammatory, auto-immune, or allergic diseases Citation[71].
References
- Grandhe N P, Bhansali A, Dogra S, Kumar B. Acanthosis nigricans: Relation with type 2 diabetes mellitus, anthropometric variables, and body mass in Indians. Postgrad Med J 2005; 81(958)541–544
- Selkin B A, Reynolds R V, Selkin G. Cutaneous manifestations of internal malignancy, UptoDate® 2005. Available at: http://www.utdol.com
- Mekhail T M, Markman M. Acanthosis nigricans with endometrial carcinoma: Case report and review of the literature. Gynecol Oncol 2002; 84(2)332–334
- Torley D, Bellus G A, Munro C S. Genes, growth factors and acanthosis nigricans. Br J Dermatol 2002; 147(6)1096–1101
- Garcia Hidalgo L. Dermatological complications of obesity. Am J Clin Dermatol 2002; 3(7)497–506
- McPhaul M J, Young M. Complexities of androgen action. J Am Acad Dermatol 2001; 45(Suppl)S87–S94
- Oh B R, Kim S J, Moon J D, Kim H N, Kwon D D, Won Y H, Ryu S B, Park Y I. Association of benign prostatic hyperplasia with male pattern baldness. Urology 1998; 51(5)744–748
- Gruber D, Sator M, Frigo P, Knogler W, Huber J C. Korrelation der Hautfaltendicke mit der Knochendichte, Östradiol, FSH und Prolactinspiegel bci 231 Frauen. Wien Klin Wochenschr 1995; 107(20)622–625
- Denti L, Pasolini G, Sanfelici L, Benedetti R, Cecchetti A, Ceda G P, Ablondi F, Valenti G. Aging-related decline of gonadal function in healthy men: Correlation with body composition and lipoproteins. J Am Geriatr Soc 2000; 48(1)51–58
- Hoffmann R. Male androgenetic alopecia. Clin Exp Derm 2002; 27: 373–382
- Kasten R, Pfirrmann G, Voigtlander V. Klinefelter's syndrome associated with mixed connective tissue disease (Sharp's syndrome) and thrombophilia with postthrombotic syndrome. J Dtsch Dermatol Ges 2005; 3(8)623–626
- Swerdlow A J, Schoemaker M J, Higgins C D, Wright A F, Jacobs P A. UK Clinical Cytogenetics Group. Cancer incidence and mortality in men with Klinefelter syndrome: A cohort study. J Natl Cancer Inst 2005; 97(16)1204–1210
- Hoffmann R. TrichoScan: A novel tool for the analysis of hair growth in vivo. J Investig Dermatol Symp Proc 2003; 8(1)109–115
- Öberg K. Carcinoid tumors, carcinoid syndrome, and related disorders. Williams Textbook of Endocrinology. 10th ed, P R Larsen, H M Kronenberg, S Melmed, K S Polonsky. Saunders, Philadelphia 2003; 1857–1870
- Rapaport M J. Follow-up of 1 mg finasteride treatment of male pattern baldness – difference between clinical trials and private office follow-up: Influences on prescribing habits evaluated. Dermatol Surg 2004; 30(5)761–763
- Modlin I M, Shapiro M D, Kidd M. An analysis of rare carcinoid tumors: Clarifying these clinical conundrums. World J Surg 2005; 29(1)92–101
- Bell H K, Poston G J, Vora J, Wilson N JE. Cutaneous manifestations of the malignant carcinoid syndrome. Br J Dermatol, 152: 71–75
- Sitaraman S V, Goldfinger S T. The carcinoid syndrome, UpToDate (13.2) June 2005. Available at: http://www.utdol. com
- Freinkel R K, Freinkel N. Cutaneous manifestations of endocrine disorders. Dermatology in general medicine, T B Fitzpatrick, A Z Eisen, K Wolff, J M Freedberg, K F Austen. McGraw Hill, New York 1987, Chapter 174
- Stewart P M. The adrenal cortex. Williams textbook of endocrinology, 10th ed, P R Larsen, H M Kronenberg, S Melmed, K S Polonsky. Saunders, Philadelphia 2003; 491–551
- Ponec M. Hormone receptors in the skin. Dermatology in general medicine, T B Fitzpatrick, A Z Eisen, K Wolff, J M Freedberg, K F Austen. McGraw Hill, New York 1987, Chapter 31
- Payne D N, Adcock I M. Molecular mechanisms of corticosteroid actions. Paediatr Respir Rev 2001; 2(2)145–150
- Rosmond R. The glucocorticoid receptor gene and its association to metabolic syndrome. Obes Res 2002; 10(10)1078–1086
- Brazzini B, Pimpinelli N. New and established topical corticosteroids in dermatology: Clinical pharmacology and therapeutic use. Am J Clin Dermatol 2002; 3(1)47–58
- Wozniak K D, Bar M. Zur bedeutung von hautveränderungen beim diabetes mellitus. Z Gesamte Inn Med 1990; 45(22)669–673
- Richardson T, Kerr D. Skin-related complications of insulin therapy: Epidemiology and emerging management strategies. Am J Clin Dermatol 2003; 4(10)661–667
- Greenman R L, Panasyuk S, Wang X, Lyons T E, Dinh T, Longoria L, Giurini J M, Freeman J, Khaodhiar L, Veves A. Early changes in the skin microcirculation and muscle metabolism of the diabetic foot. Lancet 2005; 366(9498)1711–1717
- Freinkel R K, Freinkel N. Cutaneous manifestations of endocrine disorders. Dermatology in general medicine, T B Fitzpatrick, A Z Eisen, K Wolff, J M Freedberg, K F Austen. McGraw Hill, New York 1987, Chapter 174
- Pinzur M, Freeland R, Juknelis D. The association between body mass index and foot disorders in diabetic patients. Foot Ankle Int 2005; 26(5)375–377
- Mendonca D A, Cosker T, Makwana N K. Vacuum-assisted closure to aid wound healing in foot and ankle surgery. Foot Ankle Int 2005; 26(9)761–766
- Tom W L, Peng D H, Allaei A, Hsu D, Hata T R. The effect of short-contact topical tretinoin therapy for foot ulcers in patients with diabetes. Arch Dermatol 2005; 141(11)1373–1377
- Brownlee M, Aiello L P, Friedman E, Vinik A I, Nesto R W, Boulton J M. Complications of diabetes mellitus. Williams textbook of endocrinology, 10th ed, P R Larsen, H M Kronenberg, S Melmed, K S Polonsky. Saunders, Philadelphia 2003; 1509–1584
- Frykberg R G. An evidence-based approach to diabetic foot infections. Am J Surg 2003; 186(5A)S44–S54
- Robbins J M. Treatment of onychomycosis in the diabetic patient population. J Diabetes Complications 2003; 17(2)98–104
- McCulloch D K. Evaluation of the diabetic foot, UptoDate® 2005. Available at: http://www.utdol.com
- Chuang T Y, Lewis D A, Spandau D F. Decreased incidence of nonmelanoma skin cancer in patients with type 2 diabetes mellitus using insulin: A pilot study. Br J Dermatol 2005; 153(3)552–557
- Raine-Fenning N J, Brincat M P, Muscat-Baron Y. Skin aging and menopause: Implications for treatment. Am J Clin Dermatol 2003; 4(6)371–378
- Wildt L, Sir-Peterman T. Oestrogen and age estimations of perimenopausal women. Lancet 1999; 354: 224
- Niewöhner C B, Nuttall R Q. Gynecomastia in a hospitalized male population. Amer J Med 1984; 77: 633–638
- Carney J A, Hruska L S, Beauchamp G D, Gordon H. Dominant inheritance of the complex of myxomas, spotty pigmentation, and endocrine overactivity. Mayo Clin Proc 1986; 61(3)165–172
- Carney J A. Carney complex: The complex of myxomas, spotty pigmentation, endocrine overactivity and schwannomas. Semin Dermatol 1995; 14: 90
- Nieman L K. Cushing's syndrome due to ACTH-independent micronodular and macronodular adrenal hyperplasia, Available at: UpToDate Version 13.3, September 8, 2004
- Kyle R A. POEMS syndrome (osteosclerotic myeloma), Available at: UpToDate September 12, 2005
- Dispenzieri A, Kyle R A, Lacy M Q, et al. POEMS syndrome. Definitions and long-term outcome. Blood 2003; 101: 2496
- Perniciaro C. POEMS syndrome. Semin Dermatol 1995; 14: 162
- Rosenberg P M, Goldfinger S T. Glucagonoma and the glucagonoma syndrome, UptoDate® 2005. Available at: http://www.utdol.com
- van Beek A P, de Haas E R, van Vloten W A, Lips C J, Roijers J F, Canninga-van Dijk M R. The glucagonoma syndrome and necrolytic migratory erythema: A clinical review. Eur J Endocrinol 2004; 151(5)531–537
- Chastain M A. The glucagonoma syndrome: A review of its features and discussion of new perspectives. Am J Med Sci 2001; 321(5)306–320
- Zhang M, Xu X, Shen Y, Hu Z H, Wu L M, Zheng S S. Clinical experience in diagnosis and treatment of glucagonoma syndrome. Hepatobiliary Pancreat Dis Int 2004; 3(3)473–475
- Quatresooz P, Hermanns-Le T, Ciccarelli A, Beckers A, Pierard G E. Tensegrity and type 1 dermal dendrocytes in acromegaly. Eur J Clin Invest 2005; 35(2)133–139
- LeRoith D, Roberts C T, Jr. The insulin-like growth factor system and cancer. Cancer Lett 2003; 195(2)127–137
- Renehan A G, Zwahlen M, Minder C, O'Dwyer S T, Shalet S M, Egger M. Insulin-like growth factor (IGF)-I, IGF binding protein-3, and cancer risk: Systematic review and meta-regression analysis. Lancet 2004; 363(9418)1346–1353
- Carroll P V, Christ E R, Bengtsson B A, Carlsson L, Christiansen J S, Clemmons D, Hintz R, Ho K, Laron Z, Sizonenko P, et al. Growth hormone deficiency in adulthood and the effects of growth hormone replacement: A review. Growth Hormone Research Society Scientific Committee. J Clin Endocrinol Metab 1998; 83(2)382–395
- Pinkus. 1902, (cited in Moll et al., 2005)
- Moll I, Roessler M, Brandner J M, Eispert A C, Houdek P, Moll R. Human Merkel cells – aspects of cell biology, distribution and functions. Eur J Cell Biol 2005; 84(2–3)259–271
- Koljonen V, Haglund C, Tukiainen E, Bohling T. Neuroendocrine differentiation in primary Merkel cell carcinoma – possible prognostic significance. Anticancer Res 2005; 25(2A)853–858
- Larsen P R, Davies T F, Schlumberger M J, Hay I D. Thyroid physiology and diagnostic evalulation of patients with thyroid disorders. Williams textbook of endocrinology. 10th ed, P R Larsen, H M Kronenberg, S Melmed, K S Polonsky. Saunders, Philadelphia 2003; 33–37
- O'Donnell B F, Francis D M, Swana G T, Seed P T, Kobza Black A, Greaves M W. Thyroid autoimmunity in chronic urticaria. Br J Dermatol 2005; 153(2)331–335
- Fatourechi V. Pretibial myxedema: Pathophysiology and treatment options. Am J Clin Dermatol 2005; 6(5)295–309
- Davies T F, Larsen R P. Thyrotoxicos. Williams textbook of endocrinology. 10th ed, P R Larsen, H M Kronenberg, S Melmed, K S Polonsky. Saunders, Philadelphia 2003; 374–422
- Stumpf W E. The endocrinology of sunlight and darkness. Complementary roles of vitamin D and pineal hormones. Naturwissenschaften 1988; 75(5)247–251
- Scarlett W L. Ultraviolet radiation: Sun exposure, tanning beds, and vitamin D levels. What you need to know and how to decrease the risk of skin cancer. J Am Osteopath Assoc 2003; 103(8)371–375
- Bollinger Bollag W, Bollag R J. 1,25-Dihydroxyvitamin D(3), phospholipase D and protein kinase C in keratinocyte differentiation. Mol Cell Endocrinol 2001; 177(1–2)173–182
- Kira M, Kobayashi T, Yoshikawa K. Vitamin D and the skin. J Dermatol 2003; 30(6)429–437
- Holick M F. Sunlight and vitamin D for bone health and prevention of autoimmune diseases, cancers, and cardiovascular disease. Am J Clin Nutr 2004; 80(6 Suppl)S1678–S88
- Njoo M D, Westerhof W. Vitiligo. Pathogenesis and treatment. Am J Clin Dermatol 2001; 2(3)167–181
- Huang C L, Nordlund J J, Boissy R. Vitiligo: A manifestation of apoptosis?. Am J Clin Dermatol 2002; 3(5)301–308
- Kadekaro A L, Kanto H, Kavanagh R, Abdel-Malek Z A. Significance of the melanocortin 1 receptor in regulating human melanocyte pigmentation, proliferation, and survival. Ann NY Acad Sci 2003; 994: 359–365
- Wankowicz-Kalinska A, Le Poole C, van den Wijngaard R, Storkus W J, Das P K. Melanocyte-specific immune response in melanoma and vitiligo: Two faces of the same coin?. Pigment Cell Res 2003; 16(3)254–260
- Luger T A, Schwarz T, Kalden H, Scholzen T, Schwarz A, Brzoska T. Role of epidermal cell-derived α-melanocyte stimulating hormone in ultraviolet light mediated local immunosuppression. Ann NY Acad Sci 1999; 20(885)209–216
- Luger T A, Scholzen T E, Brzoska T, Bohm M. New insights into the functions of α-MSH and related peptides in the immune system. Ann NY Acad Sci 2003; 994: 133–140