Introduction
The basic definition of the term ‘syndrome’ involves a specific set of conditions that usually occur together and may point to the presence of a certain disease or an increased chance of developing that disease in the future. Therefore, the term ‘metabolic syndrome’ is exactly what its name implies – it is a constellation of metabolic factors, including insulin resistance, dyslipidemia, and hypertension, that together constitute a greater probability of future consequences, mainly type 2 diabetes mellitus and cardiovascular disease. The prevalence of metabolic syndrome increases with age with over 40% of persons older than 60 years being diagnosed with the syndrome. Up to 70 years of age it is more common in men than women Citation[1].
What is the underlying cause of insulin resistance in the geriatric population?
Metabolic syndrome has been the focus of a great amount of research for most of the twentieth century because of the major morbidity and mortality implications of cardiovascular disease. However, the recognition of a ‘hypertriglyceridemia’ syndrome can be dated back to the mid sixteenth century, to be followed by an association seen between increased visceral obesity and hypertension in the seventeenth century. Countless studies ensued throughout the following years to culminate in the identification of a risk factor clustering known as ‘syndrome X’ in the 1980s. Insulin resistance was seen to be common in not only patients with type 2 diabetes, but also in those patients with impaired glucose tolerance, as a result of the pancreatic β-cell's attempt to maintain glucose homeostasis in the face of chronic hyperglycemia and increased free-fatty acids (FFAs). Increased low-density lipoprotein (LDL), decreased high-density lipoprotein (HDL), and hypertension were noted to be concomitant variables that may be present in one individual and may be very important to the development of not only diabetes, but cardiovascular disease in that person Citation[2]. Clinical criteria were eventually established and currently exist for these components of high-risk state by the third report of the National Cholesterol Education Program's Adult Treatment Panel (NCEP-ATP III), the World Health Organization (WHO), and the International Diabetes Foundation () Citation[3-5]. As highlighted by the International Diabetes Foundation, the key component of the metabolic syndrome is central obesity as defined by waist circumference. Debate ensued with regard to the ambiguity of these criteria; therefore it should be added that, in practice, all cardiovascular risk factors should treated by clinicians without ‘syndrome’ qualification Citation[6].
Table I. National Cholesterol Education Program (NCEP), World Health Organization (WHO), and International Diabetes Foundation definitions of metabolic syndrome.
Diabetes mellitus in its very essence is a metabolic disorder. Although type 1 diabetes is thought to be autoimmune in nature, characterized by a decreased or lack of insulin secretion, and usually seen in young people, type 2 diabetes is associated with age and obesity (). The vast majority of patients seem to have a genetic risk as well that unveils an existing defect in insulin secretion Citation[7]. There are additional environmental factors that interact with genetic susceptibility in the pathogenesis of type 2 diabetes. These factors are mainly associated with a pre-existing obesity, including an elevation in free fatty acids (FFAs), as stated above, increased release of tumour necrosis factor-α (TNFα), increased synthesis of interleukin-1-β (ILβ) by the β-cell, and a deficiency of adiponectin, in addition to a multitude of other factors in the milieu [8,9].
Figure 1. The increasing incidence of obesity in conjunction with increasing age overlaps to contribute to the presence of metabolic syndrome, which in turn directly increases rates of type 2 diabetes, as well as cardiovascular disease.
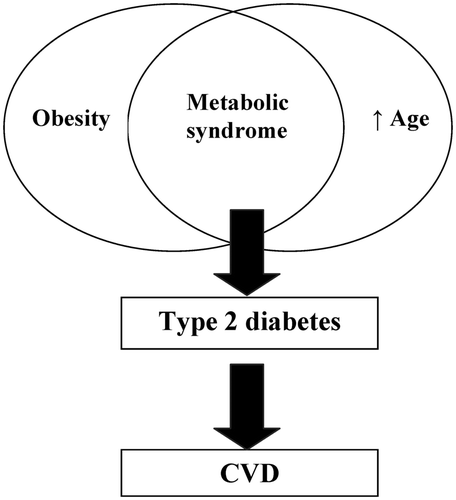
Key to the development of overt diabetes is the eventual time course of insulin resistance associated with obesity. This is not only an association in modern Western society where nutrition for many individuals is in excess, but can be observed in striking similarity, when that caloric-rich accessibility is applied to the wild. In a study looking at the effects of an abundant food supply in wild baboons, a metabolic-like syndrome evolves as is evidenced with comparative increased levels of glucose, insulin and lipid levels Citation[10].
Actual body fat distribution and ectopic fat distribution are helpful in evaluating the level of insulin sensitivity. Studies using proton magnetic resonance spectroscopy (MRS) to allow non-invasive quantifications of non-fat tissues, including muscle and liver, have shown this modality to be helpful in gaining further insight. In the muscle, MRS can differentiate between extramyocellular and intramyocellular lipid, and, in euglycemic-hyperinsulinemia clamp conditions, permits the observation that fat infiltration in muscle fibres correlates with decreased insulin sensitivity Citation[11]. Furthermore, MRS demonstrates that higher liver fat correlates with lower insulin sensitivity, adding to existing knowledge of the importance of insulin sensitivity in the suppression of endogenous hepatic glucose production Citation[12].
Insulin resistance appears to be associated with increased age, as is backed by numerous population studies; however, the exact age-associated mechanism is not definite, although defects in mitochondrial oxidative and phosphorylation may be an influence Citation[13]. In a comparison to lean young controls, elderly subjects matched for properties, including lean body mass and fat mass, not only had higher circulating insulin concentrations during glucose challenge and higher basal glucose and FFA levels, but also an increase in intramyocellular and liver fat levels by MRS. Interestingly, assessment of both mitochondrial oxidative activity and phosphorylation activity were decreased by about 40%, supporting an association with aging and related insulin resistance Citation[14].
A field of research that is further adding to our current understanding of the biochemical aspects of insulin resistance is the study of adipocytes. An adipocyte is a specialized cell that is able to store excess energy in the form of lipids and, unfortunately or not, has an almost unlimited capacity for growth in accordance with metabolic needs. The adipocyte has numerous types of receptors, including receptors for traditional endocrine hormones, such as insulin, glucagon, and thyroid-stimulating hormone (TSH), as well as nuclear hormone receptors, cytokine receptors, and catecholamine receptors, which allow adipose tissue to receive both central and peripheral signalling Citation[15]. In response, adipocytes can produce adipocyte-specific proteins known as adipokines, that have been found to have local and systemic effects important to glucose homeostasis ().
Table II. Table of adipocyte-derived proteins with significant endocrine function in metabolic syndrome.
Adiponectin is one adipokine that has been the focus of many studies within recent years. It is 30-kDa adipose-specific secreted protein that circulates in human serum at 5–30 nM concentrations. This adipokine was initially characterized in 1995/1996 by four groups using different methods and, as such, has multiple names-apM1 (adipose most abundant gene transcript 1), Acrp30 (adipocyte complement-related protein of 30 kDa), adipoQ, and GBP28 (gelatin binding protein of 28 kDa). An inverse association exists between adiponectin and insulin resistance Citation[16]. Adiponectin enhances insulin-mediated repression of glucose production in the liver. In vitro research using primary hepatocytes exposed to differing levels of insulin in adiponectin presence or absence show that adiponectin enhances even very small amounts of insulin significantly Citation[17]. Other than weight loss, the only other current approach shown to significantly improve adiponectin levels is use of activators of peroxisome proliferator-activated receptor-γ (PPAR-γ) Citation[18].
Hypogonadism and metabolic syndrome
With aging there is a decline in testosterone levels Citation[19],Citation[20]. Recently, a number of studies in men have suggested that low testosterone may be associated with insulin resistance and testosterone replacement may enhance insulin action Citation[21-25]. A recent study has shown that males with prostate cancer treated with a GnRH agonist are at increased risk of diabetes mellitus Citation[26],Citation[27]. These studies suggest that the hypogonadism associated with aging may play a role in the pathophysiology of the metabolic syndrome in older men.
Why is metabolic syndrome in the geriatric population important?
Metabolic syndrome is a high risk condition for type 2 diabetes and complications of cardiovascular disease. Diabetes is commonly referred to as an epidemic in medicine today and over 50% of persons with diabetes mellitus are over 60 years of age Citation[28]. It is a complex chronic illness that across the board affects most races and generations. Twenty million individuals have diabetes and an additional twenty-six per cent of the US population has an impaired fasting glucose. Approximately twenty per cent of patients over sixty-five years of age suffer from diabetes, with it being slightly more common in males than females Citation[29],Citation[30]. It poses a significant health burden on the basis of increases in morbidity, mortality, and cost, a fact that has been proven repeatedly Citation[31]. In addition, diabetes mellitus is associated with functional impairment in the aging male Citation[32-34]. The objective of the Third National Health and Nutrition Examination Survey 1988–1994 (NHANES III), to examine the prevalence and time trends for diagnosed and undiagnosed diabetes according to age, sex, race or ethnic groups in the US population, showed that the prevalence significantly increases with age, in addition to being higher among certain racial minority populations. The health care use, including office and inpatient visits, attributed to diabetes in an older population was also demonstrated to be substantial Citation[35],Citation[36]. NHANES 1999–2000 proceeded to show that an inability to control risk factors, such as blood pressure and cholesterol, in conjunction with a rise in the prevalence of type 2 diabetes at an earlier age leads to time-dependent vascular complications, which in turn increases as the population ages and the general lifespan increases. Further studies demonstrate that although individuals with diabetes are at increased risk for vascular disease, including macrovascular (CAD and stroke) and microvascular (retinopathy, neuropathy, nephropathy), improved glycemic control definitely and positively impacts these complications Citation[37], Citation[38]. Finally, there is emerging evidence that three central factors of the metabolic syndrome, viz. hyperglycemia, hypertriglyceridemia and hyperinsulinemia, are associated with cognitive impairment Citation[39-41]. This is due, in part, because the memory enhancing effects of leptin are blocked by hypertriglyceridemia Citation[42],Citation[43].
What are current and prospective interventions in the treatment of metabolic syndrome in the geriatric population?
Since type 2 diabetes is a disease with such a serious and expensive course, especially in the older population, the best treatment is the prevention of metabolic dysregulation to this point Citation[44]. One of the strongest and highly studied interventions to date is lifestyle modification. The Diabetes Prevention Program Research Group investigated, on a large, randomized scale, the effects of lifestyle modifications, medical treatment with metformin, or placebo in the development of diabetes in high risk individuals. Over half of the population was actually determined to have metabolic syndrome in a later study Citation[45]. However, although both lifestyle changes and biguanide therapy deterred or prevented diabetes in the overall studied population, modifications in lifestyle most strongly influenced patients over 60 years of age (DPP) Citation[46]. The importance of lifestyle modifications in this study similarly reflected prior findings in Finland and China Citation[47],Citation[48]. Metformin, which decreases insulin resistance and produces anorexia and weight loss, possibly through its effects on nitric oxide synthase Citation[49],Citation[50], was not useful in delaying the onset of diabetes in persons over 60 years of age Citation[51].
Omega-3 polyunsaturated fatty acids (PUFAs) are a group of polyunsaturated fats that can be found in fish sources in the form of eicosapentaenoic acid (EPA) and docosaheaenoic acid (DHA) as well as in some vegetables, oils, and nuts in the form of a alpha-linolenic acid (ALA) Citation[52]. Several studies have evaluated the beneficial impact of PUFAs in fish oil upon secondary prevention of cardiovascular end points. The GISSI study supported the possibility of an anti-arrhythmic effect in addition to a significant reduction in mortality early in the course of treatment with PUFAs in patients recently surviving myocardial infarction Citation[53]. A separate study found a strong association with reduced risk of sudden death in apparently healthy patients Citation[54]. In regard to dosage, three to twelve grams daily PUFAs have been shown to decrease triglycerides when used in adjunct to diet in patients with elevated triglyceride levels Citation[55]. Currently, Omacor is the only fish oil supplement approved by the FDA for the treatment of hypertriglyceridemia. Type 2 diabetes is generally associated with a dyslipidemia that is composed of low HDL, high LDL, as well as hypertriglyceridemia. Fish oil supplementation in this population has been proven statistically significant in lowering triglycerides by almost thirty per cent without significantly increasing fasting glucose or hemoglobin A1c Citation[56]. The elderly population may particularly benefit from this intervention because in addition to the obvious cardiovascular risks, elevated triglyceride levels are associated with poor cognitive performance by way of decreased verbal fluency and impaired memory tasks Citation[57].
Thiazolidinediones (TZDs) are a class of drugs that have proven to be important in the treatment of diabetes today. TZDs work via PPARγ to promote insulin sensitivity at the level of the liver as well as muscle. Increases in adiponectin with TZD administration have pointed to the importance of the adipocyte in this process Citation[18]. These effects on insulin resistance have significant implications in regard to the prevention of type 2 diabetes. The DREAM trial resulted in substantial reductions in the incidence of diabetes in high risk individuals with TZD treatment for 3 years Citation[58]. Further studies lead to the inherent vascular implications. Decreased inflammatory reaction in symptomatic carotid plaques through analysis of ubiquitin-proteasome activity, thereby meaning plaque stabilization, has been associated with TZD treatment Citation[59]. In both patients with diabetes as well as those with metabolic syndrome, improvements in homocysteinemia were recognized with the addition of this pharmacologic agent Citation[60].
Rimonabant is the first in a newly characterized group of drugs known to selectively block the cannabinoid-1 receptor. Through G protein-coupled receptor activation, the endocannabinoid system is involved in the regulation of glucose metabolism both centrally and peripherally Citation[61],Citation[62]. The RIO-North America study, a 2-year randomized, double-blind, placebo-controlled trial, looked at the effects of treatment with 20 mg/day of rimonabant in addition to diet. The selected population of both obese and overweight individuals proved to have a high dropout rate considered secondary to side effects early in the study, such as depression. However, significant reductions in waist circumference and weight, along with improvements in fasting insulin and lipid profiles were observed, thereby strongly suggesting improvement in the composite picture of metabolic syndrome and possible future complications Citation[63]. Long-term effects of rimonabant in the general obese population, including those patients who are older in age, are yet to be fully determined but may show benefits in the future.
In summary, metabolic syndrome is a condition of high risk for the consequences it imposes, especially in the aging population. In males, there is emerging evidence that hypogonadism may play a role in its pathophysiology. The study of metabolic dysregulation and the development of metabolic syndrome is vast, and ongoing research will hopefully elucidate further our current understanding. Aside from prevention, treatment remains best aimed at lifestyle modification, but pharmacologic agents may be useful in the future.
References
- Alexander C M, Landsman P B, Teutsch S M, Haffner S M. Third National Health and Nutrition Examination Survey (NHANES III); National Cholesterol Education Program (NCEP). NCEP-defined metabolic syndrome, diabetes, and prevalence of coronary heart disease among NHANES III participants age 50 years and older. Diabetes 2003; 52: 1210–1214
- Reaven G M. Role of insulin resistance in human disease. Diabetes 1988; 37: 1595–1607
- Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA 2001; 285: 2486–2497
- Alberti K G, Zimmer P Z. Definition, diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med 1998; 15: 539–553
- Athyros V G, Ganotakis E S, Elisaf M, Mikhailidis D P. The prevalence of the metabolic syndrome using the National Cholesterol Educational Program and International Diabetes Federation definitions. Curr Med Res Opin 2005; 21: 1157–1159
- Kahn R, Buse J, Ferrannini E, Stern M. The metabolic syndrome: time for a critical appraisal. Diabetes Care 2005; 28: 2289–2304
- Jackson R A. Mechanisms of age-related glucose intolerance. Diabetes Care 1990; 13(Suppl 2)9–19
- Beck-Nielsen H, Groop L C. Metabolic and genetic characterization of prediabetic states. Sequence of events leading to non-insulin-dependent diabetes mellitus. J Clin Invest 1994; 94: 1714
- Stumvoll M, Goldstein B J, van Haeften T W. Type 2 diabetes: principles of pathogenesis and therapy. Lancet 2005; 365: 1333
- Banks W A, Altmann J, Sapolsky R M, Phillips-Conroy J A, Morley J E. Serum leptin levels as a marker for a syndrome X-like condition in wild baboons. J Clin Endocrinol Metab 2003; 88: 1234–1240
- Jacob S, Machann J, Rett K, Brechtel K, Volk A, Renn W, Maerker E, Matthaei S, Schick F, Claussen C D, Haring H U. Association of increased intramyocellular lipid content with insulin resistance in lean nondiabetic offspring on type 2 diabetic subjects. Diabetes 1999; 48: 1113–1119
- Stefan N, Machann J, Schick F, Claussen C D, Thamer C, Fritsche A, Haring H. New imaging techniques of fat, muscle and liver within the context of determining insulin sensitivity. Hormone Research 2005; 64(suppl 3)38–44
- Kim M J, Rolland Y, Cepeda O, Gammack J K, Morley J E. Diabetes mellitus in older men. Aging Male 2006; 9: 139–147
- Petersen K F, Befroy D, Dufour S, Dziura J, Ariyan C, Rothman D L, DiPietro L, Cline G W, Shulman G I. Mitrochondrial dysfunction in the elderly: possible role in insulin resistance. Science 2003; 300: 1140–1142
- Kershaw E E, Flier J S. Adipose tissue as an endocrine organ. J Clin Endocrinol Metab 2004; 89: 2548–2556
- Rolland Y M, Perry H M, III, Patrick P, Banks W A, Morley J E. Leptin and adiponectin levels in middle-aged postmenopausal women: association with lifestyle habits, hormones, and inflammatory markers – a cross-sectional study. Metabolism 2006; 55: 1630–1636
- Berg A H, Combs T P, Du X, Brownlee B, Scherer P E. The adipocyte-secreted protein Acrp30 enhances hepatic insulin action. Nat Med 2001; 7: 947–953
- Combs T P, Wagner J A, Berger J, Doebber T, Wang W, Zhang B B, Tanen M, Berg A H, O'Rahilly S, Savage D B, Chatterjee K, Weiss S, Larson P J, Gottesdiener K M, Gertz B J, Charron M J, Scherer P E, Moller D E. Induction of adipocyte complement-related protein of 30 kilodaltons by PPARγ agonists: a potential mechanism of insulin sensitization. Endocrinology 2002; 143: 998–1007
- Travison T G, Morley J E, Araujo A B, O'Donnell A B, McKinlay J B. The relationship between libido and testosterone levels in aging men. J Clin Endocrinol Metab 2006; 91: 2509–2513
- Morley J E, Kaiser F E, Perry H M, III, Patrick P, Morley P M, Stauber P M, Vellas B, Baumgartner R N, Garry P J. Longitudinal changes in testosterone, luteinizing hormone, and follicle-stimulating hormone in healthy older men. Metabolism 1997; 46: 410–413
- Kohn F M. Testosterone and body functions. Aging Male 2006; 9: 183–188
- Zitzmann M, Faber S, Nieschlag E. Association of specific symptoms and metabolic risks with serum testosterone in older men. J Clin Endocrinol Metab 2006; 91: 4335–4343
- Bojesen A, Kristensen K, Birkebaek N H, Fedder J, Mosekilde L, Bennett P, Laurberg P, Frystyk J, Flyvbjerg A, Christiansen J S, Gravholt C H. The metabolic syndrome is frequent in Klinefelter's syndrome and is associated with abdominal obesity and hypogonadism. Diabetes Care 2006; 29: 1591–1598
- Kapoor D, Goodwin E, Channer K S, Jones T H. Testosterone replacement therapy improves insulin resistance, glycaemic control, visceral adiposity and hypercholesterolaemia in hypogonadal men with type 2 diabetes. Eur J Endocrinol 2006; 154: 899–906
- Lee Ch, Kuo S W, Jung Y J, Hsieh C H, He C T, Yang T C, Lian W C, Chyi-Fan S, Pei D. The effect of testosterone supplementation on insulin sensitivity, glucose effectiveness, and acute insulin response after glucose load in male type 2 diabetics. Endocr Res 2005; 31: 139–148
- Keating N L, O'Malley A J, Smith M R. Diabetes and cardiovascular disease during androgen deprivation therapy for prostate cancer. J Clin Oncol 2006; 24: 4448–4456
- Basaria S, Muller D C, Carducci M A, Egan J, Dobs A S. Hyperglycemia and insulin resistance in men with prostate carcinoma who receive androgen-deprivation therapy. Cancer 2006; 106: 581–588
- Morley J E. Diabetes mellitus: a major disease of older persons. J Gerontol A Biol Sci Med Sci 2000; 55: M255–M256
- American Diabetes Association. Standards of medical care in diabetes. Diabetes Care 2006; 29: 59–42
- LeRoith D. Diabetes in the aging male. Aging Male 2005; 8: 133–134
- American Diabetes Association. Economic consequences of diabetes mellitus in the US in 2002. Diabetes Care 2003; 26: 917–932
- Rodriguez-Saldana J, Morley J E, Reynoso M T, Medina C A, Salazar P, Cruz E, Torres A L. Diabetes mellitus in a subgroup of older Mexicans: prevalence, association with cardiovascular risk factors, functional and cognitive impairment, and mortality. J Am Geriatr Soc 2002; 50: 111–116
- Miller D K, Lui L Y, Perry H M, III, Kaiser F E, Morley J E. Reported and measured physical functioning in older inner-city diabetic African Americans. J Gerontol A Biol Sci Med Sci 1999; 54: M230–M236
- Di Fazio I, Franzoni S, Frisoni G B, Gatti S, Cornali C, Stofler P M, Trabucchi M. Predictive role of single diseases and their combination on recovery of balance and gait in disabled elderly patients. J Am Med Dir Assoc 2006; 7: 208–211
- Johnson C L, Rifkind B M, Sempos C T, Carroll M D, Bachorik P S, Breifel R R, Gordon D J, Burt V L, Brown C D, Lippel K, Cleeman J I. Declining serum total cholesterol levels among US adults: the National Health and Nutrition Examination Surveys. JAMA 1993; 269: 3002–3008
- Harris M I, Flegal K M, Cowie C C, Eberhardt M S, Goldstein D E, Little R R, Wiedmeyer H, Byrd-Holt D D. Prevalence of diabetes, impaired fasting glucose, and impaired glucose tolerance in US adults: the Third National Health and Nutrition Examination Survey, 1988–1994. Diabetes Care 1998; 21: 518–524
- UK Prospective Diabetes Study Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998; 352: 837–853
- Saydah S. Poor control of risk factors for vascular disease among adults with previously diagnosed diabetes mellitus. JAMA, 291: 335–342
- Mooradian A D, Perryman K, Fitten J, Kavonian G D, Morley J E. Cortical function in elderly non-insulin dependent diabetic patients: behavioral and electrophysiologic studies. Arch Intern Med 1988; 148: 2369–2372
- Flood J F, Mooradian A D, Morley J E. Characteristics of learning and memory in streptozocin-induced diabetic mice. Diabetes 1990; 39: 1391–1398
- Yaffe K, Kanaya A, Lindquist K, Simonsick E M, Harris T, Shorr R I, Tylavsky F A, Newman A B. The metabolic syndrome, inflammation, and risk of cognitive decline. JAMA 2004; 292: 2237–2242
- Farr S A, Banks W A, Morley J E. Effects of leptin on memory processing. Peptides 2006; 27: 1420–1425
- Banks W A, Coon A B, Robinson S M, Moinuddin A, Shultz J M, Nakaoke R, Morley J E. Triglycerides induce leptin resistance at the blood-brain barrier. Diabetes 2004; 53: 1253–1260
- Zarowitz B J, Tangalos E G, Hollenack K, O'Shea T. The application of evidence-based principles of care in older persons (Issue 3): Management of diabetes mellitus. J Am Med Dir Assoc 2006; 7: 234–240
- Orchard T J, Temprosa M, Goldberg R, Haffner S, Ratner R, Marcovina S, Fowler S. The effect of metformin and intensive lifestyle intervention on the metabolic syndrome: the Diabetes Prevention Program randomized trial. Ann Intern Med 2005; 142: 611–619
- Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002; 346: 393–403
- Tuomilehto J, Lindström J, Eriksson J G, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired gluocose tolerance. N Engl J Med 2001; 344: 1343–1350
- Pan X R, Li G W, Hu Y H, et al. Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance: the Da Qing IGT and Diabetes Study. Diabetes Care 1997; 20: 537–544
- Kumar V B, Bernardo A E, Vyas K, Franko M, Farr S, Lakshmanan L, Buddhiraju C, Morley J E. Effect of metformin on nitric oxide synthase in genetically obese (ob/ob) mice. Life Sci 2001; 69: 2789–2799
- Lee A, Morley J E. Metformin decreases food consumption and induces weight loss in subjects with obesity with type II non-insulin-dependent diabetes. Obes Res 1998; 6: 47–53
- Diabetes Prevention Program Research Group, Crandall J, Schade D, Ma Y, Fujimoto W Y, Barrett-Connor E, Fowler S, Dagogo-Jack S, Andres R. The influence of age on the effects of lifestyle modification and metformin in prevention of diabetes. J Gerontol A Biol Sci Med Sci 2006; 61: 1075–1081
- DeFillipis A P, Sperling L S. Understanding omega-3s. Am Heart J 2006; 151: 564–570
- Marchioli R, Barzi F, Bomba E, Chieffo C, DiGregorio D, DiMascio R, Franzosi M F, Geraci E, Levantesi G, Maggioni A P, Mantini L, Marfisi R M, Mastrogiuseppe G, Mininni N, Licolosi G L, Santini M, Schweiger C, Tavazzi L, Tognoni G, Tucci C, Valagussa F. Early protection against sudden death by n-3 polyunsaturated fatty acids after myocardial infarction: time-course analysis of the results of the Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto Miocardico (GISSI)-Prevenzione. Circulation 2002; 105: 1897–1903
- Albert C M, Campos H, Stamper M J, Ridker P M, Manson J E, Willett W C, Ma J. Blood levels of long-chain n-3 fatty acids and the risk of sudden death. N Eng J Med 2000; 346: 1113–1118
- Rivellese A, Maffettone A, Iovine C, DiMarino L, Annuzzi G, Mancini M, Riccardi G. Long-term effects of fish oil on insulin resistance and plasma lipoproteins in NIDDM patients with hypertriglyceridemia. Diabetes Care 1996; 19: 1207–1211
- Montori V M, Farmer A, Wollan P C, Dinneen S E. Fish oil supplementation in type 2 diabetes. Diabetes Care 2000; 23: 1407–1415
- Friedberg C, Janssen M J, Heine R J, Grobbee D E. Fish oil and glycemic control in diabetes. Diabetes Care 1998; 21: 494–500
- Gerstein H C, Yusuf S, Bosch J, Pogue J, Sheridan P, Dincaag N, Hanefeld M, Hoogwerf B, Laakso M, Mohan V, Zinman B, Holman R R. Effect of rosiglitazone on the frequency of diabetes in patients with impaired glucose tolerance or impaired fasting glucose: a randomized controlled trial. Lancet 2006; 368: 1096–1105
- Marfella R, D'Amico M, Di Filippo C, Siniscalchi A, Sasso F C, Portoghese M, Crescenzi B, Sangiuolo P, Nicoletti G F, Ferraraccio R R, Cacciapuoti F, Verza M, Coppola L, Paolisso G. Increased activity of the ubiquitin-proteasome system in patients with symptomatic carotid disease is associated with enhanced inflammation and may destabilize the atherosclerotic plaque: effects of rosiglitazone treatment. J Am Coll Cardiol 2006; 47: 2444–2455
- Cicero D G, D'Angelo A, Gaddi A, Ciccarelli L, Piccinni M N, Salvadeo S A, Ferrari I, Gravina A, Ragonesi P D. Effects of 1 year of treatment with pioglitazone or rosiglitazone added to glimepiride on lipoprotein (a) and homocysteine concentrations in patients with type 2 diabetes mellitus and metabolic syndrome: a multicenter, randomized, double-blind, controlled clinical trial. Clin Ther 2006; 28: 679–688
- Matsuda L A, Lolait S J, Brownstein M J, Young A C, Bonner T I. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature 1990; 346: 561–564
- Tangalos E G, Cota D, Fukioka K. Complex cardiometabolic risk factors: impact, assessment, and emerging therapies. J Am Med Dir Assoc 2006; 7(7 Suppl)1–10
- Pi-Sunyer F X, Aronne L J, Hesmati H M, Devin J, Rosenstock J. Effect of rimonabant, a cannabinoid-1 receptor blocker, on weight and cardiometabolic risk factors in overweight or obese patients. JAMA 2006; 295: 761–775
