Abstract
Introduction: We investigated if “thermobalancing” therapy (TT), using Dr Allen’s therapeutic device (DATD) in men with benign prostatic hyperplasia (BPH), can aid in understanding the etiology and pathophysiology of BPH.
Methods: We compared urinary and other parameters of BPH patients who received TT over 6 months (treatment group) with those of healthy volunteers who had not received the treatment (control group). Dynamics of symptoms and indicators in each group were evaluated in comparison with their data at the beginning and end of the study. Parameters were the International Prostate Symptom Score (IPSS) for urinary symptoms and quality of life (QoL), ultrasound measurement of prostate volume (PV) and uroflowmetry (maximum flow rate, Qmax). TT effectiveness was examined in 124 men with BPH and PV <60 mL. We also investigated the data of five patients with BPH and PV >60 mL.
Results: TT decreased urinary symptoms and PV, increased Qmax and improved QoL in men with BPH, PV <60 mL, and in men with BPH, PV >60 mL.
Conclusions: The present study demonstrated that TT is effective for BPH, suggesting that blood circulation plays a crucial role in its cause. The continuous heat exposure that does not exceed the normal body temperature terminates the trigger of BPH development, “micro-focus” of hypothermia, and the following spontaneous expansion of capillaries. TT could be considered to be a useful tool in BPH treatment.
Introduction
Benign prostatic hyperplasia (BPH) used to be considered as a consequence of aging. Treatment centered on medical/surgical intervention to counteract lower urinary tract symptoms (LUTS). Some medications, such as amsulosin tablets, have shown efficacy with no adverse events [Citation1], and dutasteride has reduced prostate volume with the link to improvement of bone mineral density and elevation of serum testosterone in men with LUTS secondary to BPH [Citation2]. However, medical treatment of BPH is not efficacious, as Fourcade et al. found that 52.8% of men with BPH were dissatisfied with the results of medical treatment conducted according to current international guidelines for BPH [Citation3].
As men get into their 50s and older, they may start to have different signs, such as lower sex drive and sense of vitality, erectile dysfunction, decreased energy, reduced muscle mass and bone density, and anemia, which are usually symptoms of low testosterone that require testosterone replacement therapy (TRT) [Citation4]. As TRT interruption results in worsening of symptoms lifelong TRT may be needed [Citation5]. Investigations of TRT safety have shown that the long-term oral therapy had no harmful effects on IPSS total score and did not change PV and PSA in aging men [Citation6]. In addition to clinical and biochemical parameters, prostate histology and apoptotic index has suggested that TRT does not cause the risk for prostate cancer development [Citation7].
Discovering the cause of prostate gland (PG) enlargement is crucial for BPH treatment. Therefore, it was thought that hormones have a fundamental role in BPH/LUTS development, suggesting that androgens must be present for BPH to occur [Citation8]. However, castrated boys do not develop BPH as they get older [Citation9]. So hormones may be initial triggers to the process of prostate enlargement.
PG inflammation is a frequent histologic finding in PG biopsies, even if clinical signs of prostatitis are absent [Citation10]. Inflammation may contribute to cytokine production via inflammatory cells driving production of local growth factors and angiogenesis in PG tissue [Citation11]. DiBello et al. found a significant association between clinical BPH and the metabolic syndrome (MetS) [Citation12]. The main risk factors for the development of severe LUTS were obesity, high plasma level of fasting blood glucose, hypertension and presence of erectile dysfunction [Citation13]. The impact of the MetS on LUTS is most significant in men with an enlarged PG and/or high levels of prostate-specific antigen [Citation14].
In the last decade, investigations of BPH pathogenesis have focused on vascular dysfunction. For instance, aging could activate risk factors for systemic vascular disease, resulting in disturbed blood flow [Citation15]. Development of prostatic hyperplasia could be associated with prostatic hypoxia [Citation16]. Also, a correlation between pelvic ischemia and LUTS in elderly males [Citation17], and increased pressure in the PG have been suggested [Citation18].
One decade ago, I suggested that spontaneous expansion of capillaries is the basis of PG enlargement, chronic prostatitis and kidney stones [Citation19,Citation20]. Constriction of capillaries in response to an irritating trigger leads to local “micro-hypothermia” which, in turn, becomes a constant irritant and makes the disease chronic. To eliminate this “hub” of micro-hypothermia, blood flow increases through spontaneous expansion of the capillary network locally. Formation of new capillaries is, essentially, the growth of excess tissue. Hence, long-term hypothermia in the PG leads to its continuous enlargement. “Thermobalancing” therapy (TT) was invented to stop such spontaneous tissue growth [Citation21].
TT using Dr Allen’s therapeutic device (DATD) aims to improve blood circulation in the PG. DATD provides a method of treating an affected PG by the application of a special mixture of waxes (“thermoelement”) topically to the coccyx area upon its projection. TT was used as monotherapy for 6 months in a trial involving 124 men with BPH, and confirmed the effectiveness and safety of TT [Citation22]. Here, we investigated the effect of long-term use of DATD on patients with an enlarged PG to provide information on the etiology and pathophysiology of BPH.
Materials and methods
Study design
The Ethics Committee of Yerevan State Medical University (Yerevan, Armenia) approved the protocol of this clinical observational study based on TT using DATD. We compared urinary and other parameters of BPH patients who received TT over 6 months (treatment group) with those of healthy volunteers who had not received the treatment (control group). Dynamics of symptoms and indicators in each group were evaluated in comparison with their data at the beginning and the end of the study.
Evaluation
Baseline evaluations were a full physical examination, medical history, digital rectal examination, serum biochemistry, measurement of prostate-specific antigen and electrolytes, urinalysis and renal function tests. Evaluations were made at baseline and 6 months after treatment. International Prostate Symptom Score – Quality of Life (IPSS) scores for urinary symptoms (UrS) and quality of life (QoL) were used in men with BPH. Prostate volume (PV) was measured at baseline and 6 months after treatment by a US-9000E2 ultrasound scanner (Rising Medical Equipment, Beijing, China). The standard ellipsoid formula (length × width × height × 0.52) was used to determine prostate volume. Uroflowmetry (maximum urinary flow rate (Qmax, mL/s) reflected the rate of urinary flow (Sanuro2UL, Santron Meditronic, Maharashtra, India). We have not used a bladder wall thickness test (BWT), which is an effective tool to evaluate response to medical treatment in patients with LUTS [Citation23], as changes in Qmax, we have investigated the significant correlation with BWT [Citation24]. There are no diagnostic tools for the investigation, a sexual function such as overnight erection test, blood test on low testosterone levels or others were methodically used during this study.
Participants and interventions
Of 226 men with BPH with PV <60 mL, we selected 124 patients. 80 men were excluded because their PV was >60 mL or they had severe comorbidities; 10 preferred to undergo surgery for BPH; 4 had suspected PG cancer; 8 were lost to follow-up.
DATD
Men who met the inclusion criteria of the study had treatment for BPH using DATD. The latter was attached to the coccyx area of the patient.
Results
Baseline characteristics of the study cohort
The baseline characteristics of the study cohort are shown in Supplementary Table 1.
Urinary symptoms
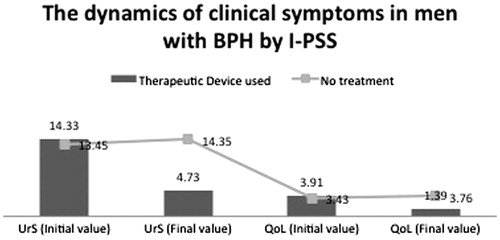
In the control group, the mean IPSS–UrS score increased from 13.45 ± 3.254 to 14.35 ± 3.396 whereas, in the treatment group, it decreased from 14.33 ± 3.399 to 4.73 ± 2.754 at the end of the observation period (). For the control group, the z value was −6.018 at a significance level of 0.000 (i.e. p < 0.001). For the treatment group, the z value was −9.674 at a significance level of 0.000 (i.e. p < 0.001). Hence, DATD decreased urinary symptoms significantly whereas, in the absence of treatment, urinary symptoms worsened significantly.
QoL
In the control group, the mean IPSS − QoL score increased from 3.43 ± 0.956 to 3.76 ± 0.983 whereas, in the treatment group, it decreased from 3.91 ± 0.755 to 1.39 ± 1.110 (). For the control group, the z value was −5.286 at a significance level of 0.000 (i.e. p < 0.001). For the treatment group, the z value was −9.672 at a significance level of 0.000 (i.e., p < 0.001). These results suggested that DATD treatment improved QoL whereas, in the control group, QoL worsened.
PV
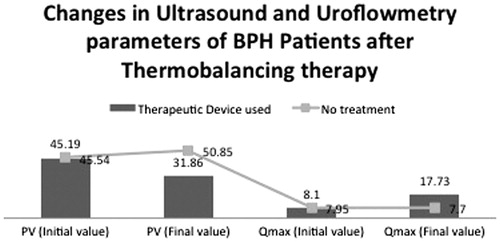
In the control group, the mean PV increased from 45.54 mL to 50.85 mL whereas, in the treatment group, it decreased from 45.19 mL to 31.86 mL (). For the control group, the z value was –8.727 at a significance level of 0.000 (i.e. p < 0.001). Hence, there was a significant increase in PV in the control group. For the treatment group, the z value was –9.669 at a significance level of 0.000 (i.e. p < 0.001). Hence, DATD reduced PV significantly whereas, in the control group, PV increased.
Qmax
In the control group, the mean Qmax decreased from 7.95 ± 2.871 to 7.7 ± 2.695 mL/s whereas, in the treatment group, the mean Qmax increased from 8.10 ± 3.041 to 17.73 ± 4.392 mL/s (). For the control group, the z value was −1.929 at a significance level of 0.054 (i.e. p > 0.05), suggesting no significant difference. For the treatment group, the z value was −9.621 at a significance level of 0.000 (i.e., p < 0.001), suggesting a significant increase in Qmax. These results suggested that DATD increased Qmax significantly whereas, in the control-group, a significant difference in Qmax was not observed.
Symptoms and parameters in men with BPH and PV >60 mL
The age of all men with BPH and PV >60 mL was >70 years (). DATD used as long-term monotherapy decreased PV and increased Qmax, which led to an improvement in QoL. Even though PV decreased significantly in all cases, PG size remained large. Hence, patients with PV >60 mL should use DATD for a longer period. The exact time period must be tailored to each patient because PG size may vary considerably. Also, no side effects were associated with DATD use.
Table 1. Changes in prostate volume (PV, mL) and uroflowmetry (maximum urinary flow rate, Qmax, mL/s), and International Prostate Symptom Score (IPSS) for urinary symptoms (UrS) and quality of life (QoL), in men with BPH (PV >60 mL) upon thermobalancing therapy.
Discussion
The present study demonstrated that TT decreased UrS and PV, increased Qmax and improved QoL in men with BPH, PV <60 mL, and in men with BPH, PV >60 mL. These outcomes suggest that TT is effective for BPH.
This was not a randomized clinical study. Having a “placebo” or “sham” group as controls could have provided more statistical rigor with regard to outcomes. However, most men with BPH suffer from depression and anxiety, their health-related QoL is considered poor, and their psychological wellbeing affected severely [Citation25,Citation26]. Six month may be considered as a short period of time for taking tablets/placebo but long for using something attached to the body. Therefore, proposing that men with BPH should wear a “placebo belt” for 6 months that does not alleviate symptoms would be very difficult. Usually, patients with BPH felt symptom relief within weeks of wearing DATD, and used the device as required.
Results in the control group showed that after 6 months they were almost the same, except increased PV. These data are in accordance with other studies confirming the progressive nature of BPH, with symptom progression being the most common manifestation [Citation27].
Investigations on BPH pathogenesis have looked at inflammation [Citation28]. Recently, Jack revealed that reducing chronic inflammation is a target for BPH treatment [Citation29]. Other scholars have studied the association between the MetS and LUTS in patients with BPH. The MetS is associated with an increased risk of storage symptoms in patients with benign prostate enlargement (BPE). The MetS and its metabolic components could be involved in LUTS − BPE pathogenesis [Citation30]. Also, the MetS with an increase in number of risk factors for the MetS (especially hypertension and hypertriglyceridemia) increase the likelihood of having moderate-to-severe LUTS in middle-aged men with high PV [Citation31].
Yeh et al.[Citation32], in univariate analysis, age, cigarette smoking, alcohol consumption, physical activity and PV significantly correlated with the severity of LUTS, but the presence or any components of MS did not. Results of multivariate analysis showed that aging, cigarette smoking, lack of regular exercise and larger PV were independent predictors for moderate/severe LUTS [Citation32]. Hence, inflammation and the MetS could be triggers for the constriction of capillaries with the development of a focus of micro-hypothermia. Continuous expansion of the capillary network could lead to PG growth and cause/worsen urinary symptoms.
Using DATD, the thermoelement is applied tightly to the skin. This approach overcomes the skin barrier and spreads heat energy toward the PG, terminates the focus of micro-hypothermia, and reverses PG enlargement gradually. TT is the only noninvasive treatment that targets the underlying cause of BPH continuously for a prolonged period of time (days, months or years) to reduce PV and relieve symptoms [Citation33]. It was also found that TT helps men to recover from chronic prostatitis [Citation34] confirming its positive impact on the prostate tissue, which is important as chronic prostatic inflammation can correlate with prostate volume and weight [Citation35].
Thus, TT can play a crucial part in the prevention of the development and progression of BPH, particularly for moderate-to-low LUTS secondary to PG enlargement. TT is different from using heating treatments because the energy source is the body, so the temperature does not exceed the normal range of body temperature. Exposure to heating and cooling even for a short period of time are stressful and dangerous to health [Citation36].
Based on the role of inflammation in BPH development, nonsteroidal anti-inflammatory drugs (NSAIDs) are prescribed sometimes. However, NSAIDs are associated with side effects in the gastrointestinal tract. Furthermore, these drugs can damage gastric and duodenal mucosa, and even the esophagus [Citation37]. Certain NSAIDs can increase the risk of heart attack and stroke, especially if used long-term [Citation38].
In the last decade, the necessity of medical/surgical treatment of BPH has been challenged. BPH/LUTS should not be viewed as an inevitable disease of older people but as part of the aging process that can be prevented [Citation39]. Therefore, recommendations on the evaluation and treatment of LUTS in older men are in demand. BPH treatment should be holistic, and may include conservative measures, lifestyle interventions and behavioral modifications, as well as medication and surgery [Citation40].
Russo et al say [Citation41], BPH/LUTS may be considered as a complex disorder that can also be discovered in the earlier stage. Primum non- “nocere” (first do no harm) should always be kept in mind, and therefore the counteraction of previous metabolic alterations should be the milestone of BPH/LUTS treatment. The next challenges of urologists should be the contrast of early onset of BPH/LUTS and the development of new target therapies in men at risk [Citation41].
The BPH/LUTS management currently programed on the medicine/surgical base: mild or nonbothersome symptoms of BPH do not require treatment; bothersome symptoms are managed with lifestyle modifications, medications, and surgery; and dietary supplements, such as saw palmetto, pygeum, cernilton and beta sitosterols, and acupuncture are not recommended for the management of BPH [Citation42].
The effectiveness of TT allows us to suggest that blood circulation has a primary role in the etiology and pathophysiology of BPE/BPH. DATD spreads energy to the PG. Hence, the PG shrank because TT could reverse pathologic angiogenesis (i.e. terminating spontaneous expansion of capillaries). DATD was free from side effects, so it can have an important role in prevention of the development and progression of BPH. Hence, TT could be considered to be a useful tool in BPH treatment.
Conclusions
We considered 6-month period is long for using “placebo belt” attached to the body, therefore observational study was used. The present research demonstrated that TT decreased UrS and PV, increased Qmax and improved QoL in men with BPH, PV <60 mL, and in men with BPH, PV >60 mL. These outcomes suggest that TT is effective for BPH affecting its cause by improving blood flow in the prostate due to continuous heat exposure that does not exceed the normal body temperature. TT could be considered to be a useful tool in BPH treatment.
Declaration of interest
The author declares that there are no competing interests in writing of this article. Simon Allen applied to the US Patent and Trademark Office (USPTO) in 2009 “Therapeutic Device and Method”, and patent was granted by USPTO in 2016. Dr Allen has not received reimbursements, fees, funding or salary relating to the content of this manuscript.
Supplementary material available online.
Supplementary_Table_baseline_characteristics.docx
Download MS Word (12.5 KB)Acknowledgements
We are grateful to the staff of the Department of Urology of the Yerevan State Medical University and the Mikaelyan Institute of Surgery (Yerevan, Armenia) for their help in supervision of patients during the study; and to reviewers/editors of the Ageing Male Journal for their constructive suggestions that improved the article.
References
- Lin KH, Lin YW, Wen YC, Lee LM. Efficacy and safety of orally disintegrating tamsulosin tablets in Taiwanese patients with benign prostatic hyperplasia. Aging Male 2012;15:246–52
- Wada N, Hashizume K, Matsumoto S, Kakizaki H. Dutasteride improves bone mineral density in male patients with lower urinary tract symptoms and prostatic enlargement: a preliminary study. Aging Male 2016;19:12–14
- Fourcade RO, Lacoin F, Rouprêt М, et al. Outcomes and general health-related quality of life among patients medically treated in general daily practice for lower urinary tract symptoms due to benign prostatic hyperplasia. World J Urol 2012;30:419–26
- Yassin AA, El-Sakka AI, Saad F, Gooren LJ. Lower urinary-tract symptoms and testosterone in elderly men. World J Urol 2008;26:359–64
- Yassin A, Nettleship JE, Talib R, et al. Effects of testosterone replacement therapy withdrawal and re-treatment in hypogonadal elderly men upon obesity, voiding function and prostate safety parameters. Aging Male 2016;19:64–9
- Meuleman EJ, Legros JJ, Bouloux PM, et al. Study 43203 Investigators., Effects of long-term oral testosterone undecanoate therapy on urinary symptoms: data from a 1-year, placebo-controlled, dose-ranging trial in aging men with symptomatic hypogonadism. Aging Male 2015;18:157–63
- Efesoy O, Apa D, Tek M, Çayan S. The effect of testosterone treatment on prostate histology and apoptosis in men with late-onset hypogonadism. Aging Male 2016;19:79–84
- Bhasin S, Singh AB, Mac RP, et al. Managing the risks of prostate disease during testosterone replacement therapy in older men: recommendations for a standardized monitoring plan. J Androl 2003;24:299–311
- Wu CP, Gu FL. The prostate in eunuchs. Prog Clin Biol Res 1991;370:249–55
- Schatteman PH, Hoekx L, Wyndaele JJ. Inflammation in prostate biopsies of men without prostatic malignancy or clinical prostatitis: correlation with total serum PSA and PSA density. Eur Urol 2000;37:404–12
- Bostanci Y, Kazzazi A, Momtahen S, et al. Correlation between benign prostatic hyperplasia and inflammation. Curr Opin Urol 2013;23:5–10
- DiBello JR, Ioannou C, Rees J, et al. Prevalence of metabolic syndrome and its components among men with and without clinical benign prostatic hyperplasia: a large, cross-sectional, UK epidemiological study. BJU Int 2016;117:801–8
- Demir O, Akgul K, Akar Z, et al. Association between severity of lower urinary tract symptoms, erectile dysfunction and metabolic syndrome. The Aging Male 2009;12:29–34
- Yang TK, Hsieh JT, Chen SC, et al. Metabolic syndrome associated with reduced lower urinary tract symptoms in middle-aged men receiving health checkup. Urology 2012;80:1093–7
- Shimizu S, Tsounapi P, Shimizu T, et al. Lower urinary tract symptoms, benign prostatic hyperplasia/benign prostatic enlargement and erectile dysfunction: are these conditions related to vascular dysfunction? Int J Urol 2014;21:856–64
- Saito M, Tsounapi P, Oikawa R, et al. Prostatic ischemia induces ventral prostatic hyperplasia in the SHR; possible mechanism of development of BPH. Sci Rep 2014;4:3822. doi: 10.1038/srep03822
- Thurmond P, Yang JH, Azadzoi KM. LUTS in pelvic ischemia: a new concept in voiding dysfunction. Am J Physiol Renal Physiol 2016. doi: 10.1152/ajprenal.00333.2015
- Cohen PG. Abdominal obesity and intra-abdominal pressure: a new paradigm for the pathogenesis of the hypogonadal-obesity-BPH-LUTS connection. Horm Mol Biol Clin Investig 2012;11:317–20
- Aghajanyan IG, Allen S. Positive response to thermobalancing therapy enabled by therapeutic device in men with non-malignant prostate diseases: BPH and chronic prostatitis. Diseases 2016;4:18
- Allen S, Aghajanyan I. Thermobalancing therapy can improve the quality of life of patients with urological diseases: chronic prostatitis, BPH and kidney stones. Kidney Urol 2016;Res 1:004
- Allen S, Adjani A. Therapeutic Device and Method, United States Patent and Trademark Office, 2016, 14 pages, Patent No: US 9,408,744 B2. Available from: https://www.google.com/patents/US9408744
- Allen S, Aghajanyan IG. Benign prostatic hyperplasia treatment with new physiotherapeutic device. Urol J 2015;12:2371–6
- Salah Azab S, Elsheikh MG. The impact of the bladder wall thickness on the outcome of the medical treatment using alpha-blocker of BPH patients with LUTS. Aging Male 2015;18:89–92
- Karakose A, Aydogdu O, Yusuf Ziya Atesci YZ. The relationship between bladder wall thickness and lower urinary tract symptoms: does bladder wall thickness change after alpha-blocker therapy with alfuzosin? Can Urol Assoc J 2014;8:E26–9
- Martin S, Vincent A, Taylor AW, et al. Lower urinary tract symptoms, depression, anxiety and systemic inflammatory factors in men: a population-based cohort study. PLoS One 2015;10:e0137903
- Pinto JD, He HG, Chan SW, Wang W. Health-related quality of life and psychological well-being in men with benign prostatic hyperplasia: an integrative review. Jpn J Nurs Sci 2016;13:309–23
- Wiygul J, Babayan RK. Watchful waiting in benign prostatic hyperplasia. Curr Opin Urol 2009;19:3–6
- Füllhase C, Hakenberg O. New concepts for the treatment of male lower urinary tract symptoms. Curr Opin Urol 2015;25:19–26
- Jack AS. Inflammation in the pathophysiology of benign prostatic hypertrophy. Eur Urol Suppl 2015;14:1455–8
- De Nunzio C, Cindolo L, Gacci M, et al. Metabolic syndrome and lower urinary tract symptoms in patients with benign prostatic enlargement: a possible link to storage symptoms. Urology 2014;84:1181–7
- Kim JH, Doo SW, Yun JH, et al. Lower likelihood of having moderate-to-severe lower urinary tract symptoms in middle-aged healthy korean men with metabolic syndrome. Urology 2014;84:665–9
- Yeh HC, Liu CC, Lee YC, et al. Associations of the lower urinary tract symptoms with the lifestyle, prostate volume, and metabolic syndrome in the elderly males. Aging Male 2012;15:166–72
- Allen S, Aghajanyan I. Thermobalancing conservative treatment for moderate-to-low-degree lower urinary tract symptoms (LUTS) secondary to prostate enlargement. Cogent Med 2016;3:1195067. doi: http://dx.doi.org/10.1080/2331205X.2016.1195067
- Allen S, Aghajanyan I. Effect of thermobalancing therapy on chronic prostatitis and chronic pelvic pain syndrome. J Clin Urol 2016;1–8. doi: 10.1177/2051415816671036
- Gandaglia G, Briganti A, Gontero P, et al. The role of chronic prostatic inflammation in the pathogenesis and progression of benign prostatic hyperplasia (BPH). BJU Int 2013;112:432–41
- Solonin YG, Katsyuba EA. Thermoregulation and blood circulation in adults during short-term exposure to extreme temperatures. Fiziol Cheloveka 2003;29:67–74
- Sostres C, Gargallo CJ, Lanas A. Nonsteroidal anti-inflammatory drugs and upper and lower gastrointestinal mucosal damage. Arthritis Res. Ther 2013;15:S3
- McGettigan P, Henry D. Use of non-steroidal anti-inflammatory drugs that elevate cardiovascular risk: an examination of sales and essential medicines lists in low-, middle-, and high-income countries. PLoS Med 2013;10:e1001388
- Corona G, Vignozzi L, Rastrelli G, et al. Benign prostatic hyperplasia: a new metabolic disease of the aging male and its correlation with sexual dysfunctions. Int J Endocrinol 2014;2014:329456
- Abrams P, Chapple C, Khoury S, et al. Evaluation and treatment of lower urinary tract symptoms in older men. J Urol 2009;181:1779–87
- Russo GI, Cimino S, Morgia G. Benign prostatic hyperplasia and metabolic syndrome: the expanding evidences of a new disease of aging male. Aging Male 2015;18:133–4
- Pearson R, Williams PM. Common questions about the diagnosis and management of benign prostatic hyperplasia. Am Fam Physician 2014;90:769–74