Abstract
Background: The associations between serum vitamin D (VD), serum testosterone (TT) and metabolic syndrome are complex and with limited published research, particularly on the effects of VD treatment on sexual hormones, erectile function and the metabolic syndrome.
Objectives: This study assessed whether a monthly high dose VD treatment for 12 months in VD deficient middle-aged men was associated with: changes in levels of sexual hormones, improvement of diabetes control and metabolic syndrome components, better erectile function [International Index of Erectile Function (IIEF)-5 questionnaire]; and changes in a prostate marker.
Materials and methods: Descriptive research of a prospective study, conducted between October 2014 and September 2015, 102 male patients ≥35 [(±SD: 53.2 ± 10.5), (range 35–64)] years with deficient serum VD level (<30 ng/mL) were included in the study. Participants were followed up for one year, with monitoring at 3-, 6-, 9- and 12-months. At the initial baseline visit, a complete medical examination was conducted, and blood was drawn for laboratory tests for above biochemical and hormonal variables under examination. Participants received an initial VD (Ergocalciferol; oral solution 600 000 IU/1.5 ml), and followed a VD treatment regime thereafter. At the four follow up visits (3, 6, 9 and 12 months), blood was collected, and patients’ erectile function was evaluated by IIEF-5 questionnaire.
Main outcome measures: During the follow up visits, all the biochemical and hormonal (TT, estradiol and luteinizing hormones, HbA1c, serum lipids profile) were assessed, and patients’ erectile function was evaluated by IIEF-5 questionnaire.
Results: Patients’ mean age was 53.2 ± 10.4 years. Serum VD exhibited significant increments (p <0.001) from baseline (15.16 ± 4.64 ng/mL), to 3 (31.90 ± 15.99 ng/mL), 6 (37.23 ± 12.42 ng/mL), 9 (44.88 ± 14.49 ng/mL) and 12 months (48.54 ± 11.62 ng/mL), and there was significant stepladder increases in both serum TT level (12.46 ± 3.30 to 15.99 ± 1.84 nmol/L) and erectile function scores (13.88 ± 3.96 to 20.25 ± 3.24) (p <0.001 for both). We also observed significant stepladder decreases in estradiol (87.90 ± 27.16 to 69.85 ± 14.80 pmol/L, p = 0.001), PTH (from 58.52 ± 28.99 to 38.33 ± 19.44 pg/mL, p <0.001) and HbA1c levels (7.41 ± 2.85 to 6.66 ± 1.67%, p = 0.001). Mean BMI significantly decreased from 33.91 ± 6.67 to 33.14 ± 6.35 kg/m2 (p = 0.001); and PSA values significantly increased from 0.59 ± 0.30 to 0.82 ± 0.39 ng/mL (p <0.001) at the end of the 12 months’ follow-up. There were no changes in LH levels.
Conclusion: This study demonstrated that VD treatment improves testosterone levels, metabolic syndrome and erectile function in middle-aged men. More randomized placebo-controlled interventional trials of VD treatment in patients with the metabolic syndrome and low TT could assist in uncovering the putative roles of VD.
Introduction
In an ageing male population, low serum testosterone (TT) (41.4% prevalence in men > 60 years) and suboptimal vitamin D (VD) status are associated with atherosclerotic cardiovascular conditions (ACC) and risks (e.g. atherogenic lipid profile, coronary heart disease, insulin resistance and diabetes mellitus (DM), obesity and endothelial dysfunction) [Citation1,Citation2]. In parallel, obesity and ACC are also significantly positively associated with erectile dysfunction (ED) [Citation3]. Given that patients with low TT often have low VD levels (impaired hydroxylating enzyme CYP2R1 in the testis) [Citation4], collectively, research suggests a relationship between low VD and low TT [Citation5,Citation6], and increased risk of type 2 DM [Citation7]. Although VD and TT are both related to ACC, there is limited information on the beneficial effect of VD treatment in men with VD deficiency on sexual hormones, erectile function and the metabolic syndrome.
Whilst patients with low TT can receive replacement therapy, there are instances where such therapy, with old preparations, is not feasible due to side effects and/or cost effectiveness considerations or alternatively due to inconsistent evidence of effectiveness. For example, Testosterone treatment with old preparations can: stimulate erythropoiesis (increase hematocrit > 51% in elderly patients) [Citation8], however, meta-analysis does not support a causal role between TT and adverse cardiovascular events [Citation9,Citation10]. Likewise, in terms of prostate health, some studies reported significant increase in prostate specific antigen (PSA) from a 0.50 ng/mL mean baseline to a mean of 0.80 ng/mL following therapy [Citation11,Citation12], while others found that there is a substantial evidence that TRT does not affect prostate health [Citation13]. Furthermore, while injectable and transdermal TT delivery systems avoid first-pass hepatic metabolism, there remains concerns regarding lipid profiles during testosterone treatment [Citation14], although some randomized controlled trials observed no problems on lipid profile [Citation15]. Multiple recent studies confirmed the favorable effects on all components of metabolic syndrome and corrections of the deteriorations of cardiovascular risk parameters [Citation16–18].
Serum VD (potent steroid hormone) is positively correlated with TT, exhibits a concordant seasonal fluctuation [Citation19], and elevates when TT was supplemented in androgen-deficient men [Citation20] and vice versa, suggesting that VD treatment might increase TT levels [Citation21]. Such possible association between serum VD and TT have been reported; however, conflicting findings endure [Citation22].
On the one hand, evidence for a positive effect of VD treatment on semen quality and TT exists [Citation23], where a systematic review found that VD was positively associated with semen characteristics and androgen status in men [Citation24]. Moreover, VD treatment might increase TT levels, as there were significant increases in TT in a VD supplemented group, but none in the placebo group [Citation21]. In contrast, in men without significant VD deficiency, there was no increase in serum TT after high dose VD treatment; and in subjects with moderately low serum TT, substitution with TT did not increase serum VD [Citation25].
Given such inconsistencies, this study assessed whether a monthly high dose VD treatment for 12 months in VD deficient middle-aged men was associated with: changes in levels of sexual hormones, improvement of diabetes control and metabolic syndrome components, better erectile function.
The specific objectives included:
Changes in levels of sex hormones (TT, Estradiol), related gonadotropin hormone (Luteinising Hormone) and parathyroid hormone (PTH);
Improvement of the components of metabolic syndrome: BMI, Serum Lipids [total cholesterol (TChol), high-density lipoprotein cholesterol (HDL-C), Low-Density Lipoprotein Cholesterol (LDL-C), triglycerides (TG)] and DM control (Glycated Hemoglobin: HbA1c);
Better erectile function; and,
Changes in a prostate (an end organ of TT) cancer marker using Prostate Specific Antigen (PSA) as may be associated with prostate cancer.
Materials and methods
Ethics and patient sample
The Hospital Research Ethics Committee (Institutional Review Board) approved this study (protocol # 13294/13). Participants were randomly selected from the greater population of male patients (age ≥35 years) treated in the Urology clinics and enrolment in the study was between October 2014 and September 2015 at a tertiary academic hospital. This age threshold was selected because late onset hypogonadism starts in men around age 40 years [Citation1], the estimated age-related decline in serum testosterone on average varied from −0.7 to −1.0% per year of age with the steepest decline from 30 to 40 year of age [Citation26].
The main inclusion criterion was a 25(OH)D concentration in the deficiency range (<30 ng/mL) [Normal 25(OH)D range = 30–80 ng/mL; mild to moderate deficiency as 21–29 ng/mL; severe deficiency <20 ng/mL] [Citation27]. Hence patients were eligible to be invited to participate in the study if during routine outpatient care, they had serum 25(OH)D was <30 ng/mL and erectile dysfunction (International Index of Erectile Function (IIEF)-5 questionnaire score ≤ 20). The exclusion criteria included patients who were: using any medication for VD, ED or TT deficiency; patients with history of malignancy within the past 5 years; any acute illness other than urology; and any predominant andrology disease/s. Patients presenting with liver and/or kidney diseases or secondary hypogonadism were also excluded. Participation in the study was voluntary; respondents signed an informed written consent upon inclusion in the study, and could withdraw from the study if they wished to do so. All data were confidential and protected. Clinical evaluation comprised general, genital, neurologic and urologic examinations. Our sample (123 patients) enroled in the study during October 2014 and September 2015, and was then followed up for one year; 102 patients completed the regular 12-month follow-up in line with the study protocol, and were therefore included in the current analysis.
Procedures, VD supplement regime and data collection
We treated our patients with Ergocalciferol (Sterogyl 15A:(Oral solution 600 000 IU/1.5 ml, Desma Pharma, Paris, France) via oral administration for the duration of 1 year (continued thereafter according to patients’ symptoms/signs). Ergocalciferol (vitamin D2) is a form of VD, and it was previously shown that vitamin D2 form of VD restores, not the vitamin D3 administration, serum 25-hydroxyvitamin D levels in subjects with hypogonadism [Citation28]. We monitored patients every 3 months after commencing treatment in order to assess response and evaluate any adverse effects.
Data were collected at baseline, and at the four follow-ups (3, 6, 9 and 12 months). During the initial baseline visit, medical history, baseline safety and complete physical examination assessments were undertaken, weight and height were measured (to calculate body mass index, BMI), and blood was drawn for laboratory tests [25(OH)D, TT, PTH, Estradiol, LH, HbA1c, serum lipid profile, total PSA]. Once the laboratory results arrived (≈1 week later), they were recorded and screened for VD deficiency.
Only patients who exhibited VD deficiency at this stage were invited to participate in the study. If they agreed and signed a written informed consent, we started the first oral dose of 600 000 IU/month. We then monitored VD at 3, 6, 9 and 12 months, and once the patients’ serum VD level reached ≥ 30 ng/mL, we switched the dose to 600 000 IU/2 months and still monitored the patients. Our target 25(OH)D serum level range was 30–80 ng/mL during the study, and if any level reached close to 80 ng/mL during the monitoring, we further decreased the dose (by increasing the time interval between doses) to 600 000 IU/3 months to prevent any VD toxicity.
At each follow up, the blood samples were drawn in the morning (between 7 and 8 a.m.), with patients fasting overnight (≥ 8 h) before the blood samples were taken for the laboratory tests). Serum TT level was verified using chemiluminescence (ADVIA Centaur, Siemens Healthcare Diagnostics, Germany). Parathyroid hormone (PTH) and 25(OH)D levels were assayed using sandwiched enzyme-linked immunoassay by IBL (International, Hamburg, Germany) and IDS (Tyne and Wear, UK), respectively.
Participants’ erectile function was evaluated using the IIEF-5 questionnaire [Citation29]. IIEF-5 is a simple, patient-administered ED diagnostic tool for easy use by physicians in clinical settings; and based on IIEF-5 scores, ED severity was classified into five categories: severe (5–7), moderate (8–11), mild to moderate (12–16), mild (17–21) and no ED (22–25) [Citation29].
Statistical analysis
Categorical and continuous values were presented as frequency (percentage) and mean ± SD or median and range as appropriate. Descriptive statistics were used to summarize demographic, laboratory, clinical and other characteristics of the patients. The primary aim of the study is to assess and compare the changes in the outcome measured for different parameters over one-year time duration (baseline, 3 months, 6 months, 9 months and 12 months). To assess changes over time in different outcomes measured, separate repeated-measures ANOVA procedure with linear models were fitted. And, where an overall group difference was found to be statistically significant, pair-wise comparisons were made using Bonferroni correction method. Relationships between two quantitative variables were examined using Pearson’s correlation coefficients. Pictorial presentations of the key results were made using appropriate statistical graphs. All p values presented were two-tailed, and p values <0.05 was considered as statistically significant. All Statistical analyses were done using statistical packages SPSS 22.0 (SPSS Inc., Chicago, IL).
We used ng/mL as unit in this study to express 25(OH)D values. For consistency, we converted other studies’ 25(OH)D units i.e. nmol/L into ng/mL by dividing it by ∼2.5.
Results
One hundred and twenty-three patients were initially enroled, and we had 21 dropouts due to non-completion of the follow up period. Hence, the current analysis included 102 participants who completed the regular follow-up in line with the study protocol.
depicts the hormonal, biochemical and metabolic syndrome components including extent of diabetes control, and prostate health indicator of the sample at baseline, 3, 6, 9 and 12-months. Patients’ mean age (±SD) was 53.2 ± 10.5 years. Serum VD levels exhibited significant increments with each follow up to range between 31.90 ± 15.99 and 48.54 ± 11.62 ng/mL (p < 0.001) (), indicating that the VD doses were working in the expected manner and the achieved serum levels were away from VD toxicity levels.
Figure 1. Monitoring during vitamin D supplementation: mean values at baseline, and at 3, 6, 9 and 12 months follow-up. (1A) Vitamin D, (1B) Parathormone, (1C) Testosterone, (1D) Luteinizing Hormone, (1E) Estradiol, (1F) Prostate Specific Antigen, (1G) Total Cholesterol, (1H) Glycated Hemoglobin, (1I) Erectile function.
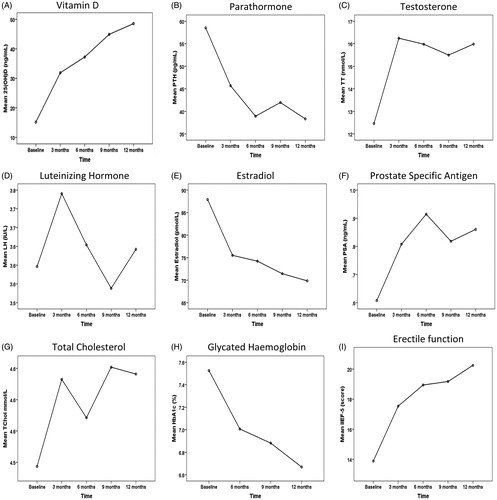
Table 1. Participant characteristics at baseline, and at 3, 6, 9 and 12 months follow-up.
Strikingly, treatment with ergocalciferol was associated with significant stepladder increases in both serum TT level (12.46 ± 3.30 to 15.99 ± 1.84 nmol/L) and erectile function scores (13.88 ± 3.96 to 20.25 ± 3.24) from the initial baseline values to 12 months (p < 0.001 for both) (). In addition, mean BMI significantly decreased at 12 months (from 33.91 ± 6.67 to 33.14 ± 6.35 kg/m2, p = 0.001) (); and patients’ diabetes control improved as evidenced by a significant steady stepladder HbA1c decrease (from 7.41 ± 2.85 to 6.66 ± 1.67%, p = 0.001). In terms of the lipid profile, there were significant decreases in low-density lipoprotein cholesterol (from 2.91 ± 0.86 to 2.68 ± 0.42 mmol/L, p = 0.001) and triglycerides (from 1.61 ± 0.51 to 1.44 ± 0.46 mmol/L, p = 0.035), whilst total cholesterol exhibited a decrease (from 4.49 ± 2.11 to 4.64 ± 1.58 mmol/L) that did not reach statistical significance. We also observed significant stepladder decreases in estradiol (from 87.90 ± 27.16 to 69.85 ± 14.80 pmol/L, p = 0.001) and PTH (from 58.52 ± 28.99 to 38.33 ± 19.44 pg/mL, p < 0.001); and a significant increase in PSA (from 0.59 ± 0.30 to 0.82 ± 0.39 ng/mL, p < 0.001) at the end of the 12-month follow-up period. There were no changes in LH levels.
Discussion
The discussion below considers the association of VD treatment with each parameter under examination individually. However, in reality, these parameters are all connected, linked and interlaced.
As for the objective of the relationship of VD treatment with hormones, our sample exhibited a significant steady increase in TT (mean ≈3.55 nmol/L) and decrease in PTH and estradiol levels across the 12 months. Our findings support earlier research where serum TT effectively increased after VD treatment [Citation21,Citation30]. Preclinical studies (rats) found a reduced TT in the Diabetic compared to normal rats; after VD treatment (12 weeks), serum TT level was effectively increased in both the high and low VD supplemented groups [Citation30]. Our findings are also in agreement with research among 54 patients, where compared to baseline values, there were significant increases in TT levels in the VD (3332 IU daily for 1 year) supplemented group [Citation21]. Likewise, other research has demonstrated that androgen levels were associated with VD levels in 2299 older men [Citation19]; and a cross-sectional analysis of men in a National Survey found that increasing VD ends with higher TT levels [Citation31].
Nevertheless, our findings contrast with others. Research showed a significant increase in serum 25OHD level following VD treatment compared to baseline, but there was no effect on mean TT concentrations at the end of the study [Citation22]. However, Heijboer’s findings [Citation22] were based on three small clinical trials (42, 49, 92 patients, respectively) with short treatment duration (3–16 weeks), of individuals with normal baseline TT, who received a seemingly low VD treatment (600, 1200, 2000 IU daily). Thus, patients received a maximum of 2000 IU cholecalciferol daily, and the median increase was 14 ng/mL [Citation22]; however, their median serum 25OHD following VD treatment was between 22 and 29 ng/mL, clearly falling in the internationally accepted in VD deficiency range [Citation27].
Certainly, it has been advocated that the most advantageous serum VD concentrations begin at 30 ng/mL, and the best are between 36 and 40 ng/mL [Citation27]. Perhaps it is such considerations that may contribute to explain why the evidence on the association of VD and TT levels in men seems inconsistent [Citation5,6,31–34]. Besides TT, we observed a significant stepladder decrease in estradiol and no changes in LH levels across the one year follow up, in partial agreement with others, where serum VD was not related to estradiol or gonadotropin levels [Citation5]. Future research should examine the optimum dosage and duration of treatment and identify if VD also improves TT levels in hypogonadic patients.
As regards the components of metabolic syndrome, in terms of anthropometric features (e.g. BMI), our sample exhibited a steady BMI decrease (mean ≈−0.77 kg/m2) after VD treatment. Interestingly, others have reported a BMI reductions of bigger magnitude (−1.9 kg/m2) after VD treatment compared to baseline levels [Citation21]. Several explanations might account for the differences observed in our BMI reductions as compared with Pilz et al. patients’ baseline TT was 10.7 ± 3.9 nmol/L, a level that can be accepted as hypogonadic [Citation21]. Low TT levels inversely correlate with insulin sensitivity and obesity in men; Testosterone treatment therapy decreases leptin and adiponectin that contribute to the development of insulin resistance; and there is also a significant reduction in visceral adiposity in hypogonadal men [Citation35]. These aspects of Pilz et al.’s study [Citation21] design and sample features might contribute to explain the higher BMI decrease among Pilz et al. hypogonadic patients [Citation21], as compared with the BMI decrease we observed in this study.
For the lipid metabolism objectives (association of VD treatment with serum lipid profile), our sample exhibited a steady decrease in both LDL-C and in TGs across the 12 months. It was not straightforward to directly contrast our lipid metabolism findings with other published studies [Citation12,Citation21,Citation22,Citation36], as much research of the effect of VD on TT concentrations in male human subjects seem to have not examined the patients’ lipid profile despite the importance of this parameter in hypogonadic and also VD deficient men. Future research would benefit from the inclusion of such variables in order to develop and expand the evidence base.
Nevertheless, as for glucose homeostasis (association of VD treatment with DM control), the stepladder decrease of HbA1c across the 12-months follow-up reflected a steady progress of our patients’ glucose tolerance and a consistent improvement in their DM. We are in support of others, where a 4-week program of high-dose VD treatment (10 000 IU daily) in subjects with impaired fasting glucose was associated with an improved insulin sensitivity and a decreased acute insulin response to glucose, both of which are risk factors for DM [Citation37]. Despite the importance of DM, nevertheless, some studies e.g. [Citation22] did not report any HbA1c data. Future research tackling the VD treatment–TT concentration dilemma should attempt to correct such omissions.
In connection with the prostate health objective (association of VD treatment with PSA), we observed a PSA increase from 0.59 ± 0.30 ng/mL (baseline) to 0.82 ± 0.39 ng/mL (12 months) (p < 0.001). VD has tissue-specific effects on estrogen and androgen production/metabolism [Citation38]. A recent meta-analysis (21 studies) indicated that higher VD levels (ranged from 28.8 to 48.0 ng/mL across studies) were associated with a 17% increase in risk of overall prostate cancer [Citation39]. Of note that none of our sample required a prostate biopsy or was diagnosed with prostate cancer.
Across our sample, erectile function significantly improved as confirmed by patients’ subjective reporting (IIEF-5 questionnaire). Earlier research across the general population in the USA showed that low serum VD was associated with higher prevalence of peripheral arterial disease [Citation40]. Likewise, very recent cross-sectional analyses (3390 men aged >20 years, free of ACC disease) also reported that VD deficiency was associated with an increased ED prevalence [Citation41]. In support, deficient levels of VD < 20 ng/mL were associated with increased ED risk, where a decreased prevalence of ED was associated with VD levels >35 ng/mL [Citation41]. When researchers restricted their analysis to the 562 men (among 3390 men) with serum levels of sex hormones and adjusted for sex hormone levels, the association of VD with ED became even stronger [Citation41].
VD’s biological activities are arbitrated through the VD receptor (VDR). VDR and enzymes that metabolize VD are present in the testes (Sertoli cells, germ cells, Leydig cells, spermatozoa) and epithelial cells that line the male reproductive tract [Citation42,Citation43]. The biological link between VD deficiency and ED exhibits several interlaced mechanisms that could suggest that the link of VD with ED appears to be independent of sex hormones [Citation41].
The first mechanism seems to work via endothelial integrity. Vascular ED stems from endothelial dysfunction and/or atherosclerosis; and low VD is correlated to endothelial dysfunction [Citation44]. Certainly, VD may directly protect endothelial cells against oxidative stress; and deficient VD levels may contribute to ED through inflammation. Many ED patients are VD deficient, particularly patients with arteriogenic ED [Citation45]. Arterial calcification indicates bigger ED risk, and is inversely associated with serum VD levels [Citation41].
The second mechanism seems to function via nitric oxide (NO) mediated vascular dilation. The penis is a very vascular organ, and erections are vascular occurrences. Sexual stimulation releases neurotransmitters from the corpus cavernosa as well as NO (a relaxing factor) from the endothelial cells of the penis [Citation46]. Activated VD stimulates the production of NO in endothelial cells and NO synthases (catalyze the production of NO from L-arginine) [Citation47], key to vascular dilation and thereby critical to preventing ED. This may clarify why endothelium derived, NO-evoked dilation is halved in arteries from VD–deficient male rats [Citation48]. Unsurprisingly, there exists a higher prevalence of ED among VD deficient patients compared to those with optimal levels [Citation41].
The third mechanism seems to operate via binding to androgen receptors. Computer (in silico) modeling shows that besides activating the VDR, 1,25-D displays high affinity for some of the body’s other nuclear receptors. This suggests that when 1,25-D rises above its normal range, it binds the α/β thyroid receptors, the glucocorticoid receptor, and the androgen receptor, displacing their native ligands [Citation49]. During Marshall et al.’s molecular modeling of the actions of angiotensin receptor blockers upon the nuclear receptors, they showed the symmetry with which endogenous ligands exhibited very similar affinities across some members of the type 1 nuclear receptor family [Citation50]. For example, 1,25-D docked into the VDR with a (nanomolar) Kd of 8.48, but also exhibited a Kd of 8.05 into the androgen receptor [Citation50]. VDR is also a member of the thyroid hormone and retinoic acid receptor subfamily of nuclear hormone receptors that heterodimerizes with retinoid X-receptor (RXR) isoforms to adjust the expression of genes encoding factors which, in a variety of cell types, control functions such as proliferation, differentiation, metabolism, ion transport and apoptosis [Citation51].
Despite its strengths and practical value, this study has limitations and generalizations should be cautious. We did not assess participants’ blood pressure, an important component of metabolic syndrome. Data on the seasonal fluctuation of TT and VD would have been beneficial, as such information would have allowed the analysis to adjust for such relationships should they exist. The cross-sectional nature of the current study does not enable any determination of the etiology of ED (psychogenic versus organic, arteriogenic versus cavernosal insufficiency). ED was measured subjectively (self-reported questionnaire), and a more objective measurement of ED using penile Doppler USG would have been beneficial. Although, the changes in, for instance, mean TT, estradiol and PSA etc. levels were statistically significant, nevertheless the magnitude of such changes were sometimes modest (and within the normal range). Hence, the clinical significance of such statistically significant changes will need to be treated with caution e.g. the meaning of a small change in mean PSA in an otherwise healthy population. Whilst we are unable to fully ensure that our sample did not take any erectogenic medications after starting vitamin D treatment and hence was the reason why they improved their erectile function, there were no erectogenic medications either prescribed by the attending physicians or appeared electronic medical records. For improvements in metabolic syndrome, we did not collect data on any diet and/or exercise program that patients might have self-initiated. We are unable to confirm whether improvements in erectile function were due to a direct effect of vitamin D; or alternatively as an indirect effect of the VD on increased T levels which improves metabolic syndrome and insulin resistance and has favorable effects on erectile function and sexual health. Finally, the inclusion of a placebo group would have been useful. Future research should address these limitations.
Conclusion
This study demonstrated that, in middle-aged VD-deficient men, VD treatment improves sexual hormones, metabolic syndrome and ED. More randomized placebo-controlled interventional trials of VD treatment in ED patients with metabolic syndrome could assist in uncovering the putative roles of VD and TT in the interruption of the vicious cycle underwritten by metabolic imbalances. During VD treatment, monitoring should include assessment of improvement of symptoms, glycemic control, lipid levels and potential adverse effects including prostate diseases in older men.
Disclosure of interest
The authors report no conflicts of interest
References
- Araujo AB, O'Donnell AB, Brambilla DJ, et al. Prevalence and incidence of androgen deficiency in middle-aged and older men: estimates from the Massachusetts Male Aging Study. J Clin Endocrinol Metab 2004;89:5920–6
- Lutsey PL, Michos ED. Vitamin D, calcium, and atherosclerotic risk: evidence from serum levels and supplementation studies. Curr Atheroscler Rep 2013;15:293
- Ahmed A, Alnaama A, Shams K, Salem M. Prevalence and risk factors of erectile dysfunction among patients attending primary health care centres in Qatar. East Mediterr Health J 2011;17:587–92
- Foresta C, Selice R, De Toni L, et al. Altered bone status in unilateral testicular cancer survivors: Role of CYP2R1 and its luteinizing hormone-dependency. J Endocrinol Invest 2013;36:379–84
- Rafiq R, van Schoor NM, Sohl E, et al. Associations of vitamin D status and vitamin D-related polymorphisms with sex hormones in older men. J Steroid Biochem Mol Biol 2016;164:11–17
- Wang N, Han B, Li Q, et al. Vitamin D is associated with testosterone and hypogonadism in Chinese men: Results from a cross-sectional SPECT-China study. Reprod Biol Endocrinol 2015;13:74
- Pittas AG, Lau J, Hu FB, Dawson-Hughes B. The role of vitamin D and calcium in type 2 diabetes: a systematic review and meta-analysis. J Clin Endocrinol Metab 2007;92:2017–29
- Hajjar RR, Kaiser FE, Morley JE. Outcomes of long-term testosterone replacement in older hypogonadal males: a retrospective analysis. J Clin Endocrinol Metab 1997;82:3793–6
- Haider A, Yassin A, Haider KS, et al. Men with testosterone deficiency and a history of cardiovascular diseases benefit from long-term testosterone therapy: observational, real-life data from a registry study. Vasc Health Risk Manag 2016;12:251–61
- Corona G, Maseroli E, Rastrelli G, et al. Cardiovascular risk associated with testosterone-boosting medications: a systematic review and meta-analysis. Expert Opin Drug Saf 2014;13:1327–51
- Raynaud JP, Gardette J, Rollet J, Legros JJ. Prostate-specific antigen (PSA) concentrations in hypogonadal men during 6 years of transdermal testosterone treatment. BJU Int 2013;111:880–90
- Yassin A, Nettleship JE, Talib RA, et al. Effects of testosterone replacement therapy withdrawal and re-treatment in hypogonadal elderly men upon obesity, voiding function and prostate safety parameters. Aging Male 2016;19:64–9
- Boyle P, Koechlin A, Bota M, et al. Endogenous and exogenous testosterone and the risk of prostate cancer and increased prostate-specific antigen (PSA) level: a meta-analysis. BJU Int 2016;118:731–41
- Bassil N, Alkaade S, Morley JE. The benefits and risks of testosterone replacement therapy: a review. Ther Clin Risk Manag 2009;5:427–48
- Corona G, Giagulli VA, Maseroli E, et al. Therapy of endocrine disease: Testosterone supplementation and body composition: results from a meta-analysis study. Eur J Endocrinol 2016;174:R99–116
- Morales A, Nieschlag E, Schubert M, et al. Clinical experience with the new long-acting injectable testosterone undecanoate. Report on the educational symposium on the occasion of the 5th World Congress on. The Aging Male, 9–12 February 2006, Salzburg, Austria. Aging Male 2006;9:221–7
- Yassin AA, Nettleship J, Almehmadi Y, et al. Effects of continuous long-term testosterone therapy (TTh) on anthropometric, endocrine and metabolic parameters for up to 10 years in 115 hypogonadal elderly men: real-life experience from an observational registry study. Andrologia 2016;48:793–9
- Grosman H, Rosales M, Fabre B, et al. Association between testosterone levels and the metabolic syndrome in adult men. Aging Male 2014;17:161–5
- Wehr E, Pilz S, Boehm BO, et al. Association of vitamin D status with serum androgen levels in men. Clin Endocrinol (Oxf) 2010;73:243–8
- Francis RM, Peacock M, Aaron JE, et al. Osteoporosis in hypogonadal men: role of decreased plasma 1,25-dihydroxyvitamin D, calcium malabsorption, and low bone formation. Bone 1986;7:261–8
- Pilz S, Frisch S, Koertke H, et al. Effect of vitamin D supplementation on testosterone levels in men. Horm Metab Res 2011;43:223–5
- Heijboer AC, Oosterwerff M, Schroten NF, et al. Vitamin D supplementation and testosterone concentrations in male human subjects. Clin Endocrinol (Oxf) 2015;83:105–10
- Anagnostis P, Karras S, Goulis DG. Vitamin D in human reproduction: a narrative review. Int J Clin Pract 2013;67:225–35
- Lerchbaum E, Obermayer-Pietsch B. Vitamin D and fertility: a systematic review. Eur J Endocrinol 2012;166:765–78
- Jorde R, Grimnes G, Hutchinson MS, et al. Supplementation with vitamin D does not increase serum testosterone levels in healthy males. Horm Metab Res 2013;45:675–81
- Andersson AM, Jensen TK, Juul A, et al. Secular decline in male testosterone and sex hormone binding globulin serum levels in Danish population surveys. J Clin Endocrinol Metab 2007;92:4696–705
- Bischoff-Ferrari HA, Giovannucci E, Willett WC, et al. Estimation of optimal serum concentrations of 25-hydroxyvitamin D for multiple health outcomes. Am J Clin Nutr 2006;84:18–28
- Foresta C, Calogero AE, Lombardo F, et al. Late-onset hypogonadism: beyond testosterone. Asian J Androl 2015;17:236–8
- Rosen RC, Cappelleri JC, Smith MD, et al. Development and evaluation of an abridged, 5-item version of the International Index of Erectile Function (IIEF-5) as a diagnostic tool for erectile dysfunction. Int J Impot Res 1999;11:319–26
- Ding C, Wang Q, Hao Y, et al. Vitamin D supplement improved testicular function in diabetic rats. Biochem Biophys Res Commun 2016;473:161–7
- Anic GM, Albanes D, Rohrmann S, et al. Association between serum 25-hydroxyvitamin D and serum sex steroid hormones among men in NHANES. Clin Endocrinol (Oxf) 2016;85:258–66
- Tak YJ, Lee JG, Kim YJ, et al. Serum 25-hydroxyvitamin D levels and testosterone deficiency in middle-aged Korean men: a cross-sectional study. Asian J Androl 2015;17:324–8
- Chin KY, Ima-Nirwana S, Wan Ngah WZ. Vitamin D is significantly associated with total testosterone and sex hormone-binding globulin in Malaysian men. Aging Male 2015;18:175–9
- Nimptsch K, Platz EA, Willett WC, Giovannucci E. Association between plasma 25-OH vitamin D and testosterone levels in men. Clin Endocrinol (Oxf) 2012;77:106–12
- Blaya R, Thomaz LD, Guilhermano F, et al. Total testosterone levels are correlated to metabolic syndrome components. Aging Male 2016;19:85–9
- Saad F, Yassin A, Doros G, Haider A. Effects of long-term treatment with testosterone on weight and waist size in 411 hypogonadal men with obesity classes I-III: observational data from two registry studies. Int J Obes (Lond) 2016;40:162–70
- Nazarian S, St Peter JV, Boston RC, et al. Vitamin D3 supplementation improves insulin sensitivity in subjects with impaired fasting glucose. Transl Res 2011;158:276–81
- Lundqvist J, Norlin M, Wikvall K. 1α,25-Dihydroxyvitamin D3 exerts tissue-specific effects on estrogen and androgen metabolism. Biochim Biophys Acta 2011;1811:263–70
- Xu Y, Shao X, Yao Y, et al. Positive association between circulating 25-hydroxyvitamin D levels and prostate cancer risk: new findings from an updated meta-analysis. J Cancer Res Clin Oncol 2014;140:1465–77
- Melamed ML, Muntner P, Michos ED, et al. Serum 25-hydroxyvitamin D levels and the prevalence of peripheral arterial disease: results from NHANES 2001 to 2004. Arterioscler Thromb Vasc Biol 2008;28:1179–85
- Farag YM, Guallar E, Zhao D, et al. Vitamin D deficiency is independently associated with greater prevalence of erectile dysfunction: the National Health and Nutrition Examination Survey (NHANES) 2001–2004. Atherosclerosis 2016;252:61–7
- Blomberg Jensen M, Nielsen JE, Jørgensen A, et al. Vitamin D receptor and vitamin D metabolizing enzymes are expressed in the human male reproductive tract. Hum Reprod 2010;25:1303–11
- Blomberg Jensen M. Vitamin D and male reproduction. Nat Rev Endocrinol 2014;10:175–86
- Reis JP, von Muhlen D, Michos ED, et al. Serum vitamin D, parathyroid hormone levels, and carotid atherosclerosis. Atherosclerosis 2009;207:585–90
- Barassi A, Pezzilli R, Colpi GM, et al. Vitamin D and erectile dysfunction. J Sex Med 2014;11:2792–800
- Sorenson M, Grant WB. Does vitamin D deficiency contribute to erectile dysfunction? Dermatoendocrinol 2012;4:128–36
- Molinari C, Uberti F, Grossini E, et al. 1alpha,25-dihydroxycholecalciferol induces nitric oxide production in cultured endothelial cells. Cell Physiol Biochem 2011;27:661–8
- Tare M, Emmett SJ, Coleman HA, et al. Vitamin D insufficiency is associated with impaired vascular endothelial and smooth muscle function and hypertension in young rats. J Physiol 2011;589:4777–86
- Proal AD, Albert PJ, Marshall TG. Dysregulation of the vitamin D nuclear receptor may contribute to the higher prevalence of some autoimmune diseases in women. Ann N Y Acad Sci 2009;1173:252–9
- Marshall TG. Vitamin D discovery outpaces FDA decision making. Bioessays 2008;30:173–82
- Haussler MR, Haussler CA, Whitfield GK, et al. The nuclear vitamin D receptor controls the expression of genes encoding factors which feed the “Fountain of Youth” to mediate healthful aging. J Steroid Biochem Mol Biol 2010;121:88–97

