Abstract
Purpose: We aimed at evaluating androgen status (serum testosterone [TT] and estimated free testosterone [eFT]) and its determinants in non-diabetic elderly men with heart failure (HF). Additionally, we investigated its associations with body composition and long-term survival.
Methods: Seventy three non-diabetic men with HF and 20 healthy men aged over 55 years were studied. Echocardiography, 6-min walk test, grip strength, body composition measurement by DEXA method were performed. TT, sex hormone binding globulin, NT-proBNP, and adipokines (adiponectin and leptin) were measured. All-cause mortality was evaluated at six years of follow-up.
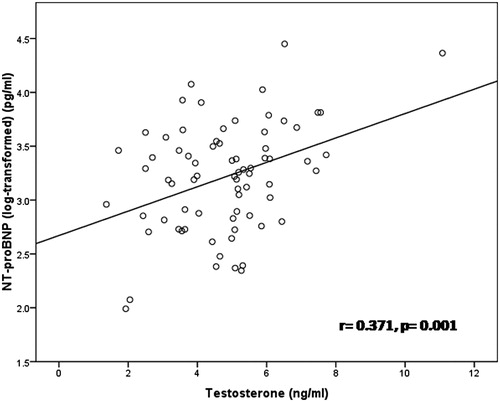
Results: Androgen status (TT, eFT) was similar in elderly men with HF compared to healthy controls (4.79 ± 1.65 vs. 4.45 ± 1.68 ng/ml and 0.409 ± 0.277 vs. 0.350 ± 0.204 nmol/l, respectively). In HF patients, TT was positively associated with NT-proBNP (r= 0.371, p = 0.001) and adiponectin levels (r = 0.349, p = 0.002), while inverse association was noted with fat mass (r = −0.413, p < 0.001). TT and eFT were independently determined by age, total fat mass and adiponectin levels in elderly men with HF (p < 0.05 for all). Androgen status was not predictor for all-cause mortality at six years of follow-up.
Conclusions: In non-diabetic men with HF, androgen status is not altered and is not predictive of long-term outcome.
Introduction
Heart failure (HF) is the only cardiovascular disease of increasing incidence and prevalence in contemporary populations and is the consequence of worldwide aging of modern societies, as HF occurs commonly in elderly people [Citation1]. The prevalence of HF is approximately 1–2% of the adult population in developed countries, rising to ≥10% among people >70 years of age [Citation1]. In healthy men, anabolic deficiency constitutes an important element of the normal aging process [Citation2].
Deterioration of cardiac muscle function leads to numerous neurohormonal and metabolic disorders, including imbalance between anabolic and catabolic hormones in favor of the latter [Citation3]. In an unselected cohort of men with HF, Jankowska et al. [Citation4] have shown decreased serum levels of androgen hormones (testosterone [TT], estimated free testosterone [eFT]) which were markers of poor survival. Circulating levels of TT strongly determine muscle mass and strength in healthy subjects [Citation5]. Morphological and functional anomalies in the muscle of HF patients have been involved not only in symptom development, but even in the pathophysiology and worsening of the HF [Citation6,Citation7]. To date, no study evaluated direct association between androgen status and skeletal muscle mass in elderly men with HF.
Adipose tissue contributes to endocrine signaling via the secretion of bioactive molecules, termed adipokines [Citation8]. Adiponectin and leptin are the most abundant adipokines. They are involved in the regulation of whole-body energy metabolism [Citation9]. To our knowledge, this is the first study evaluating the relationship of androgen hormones (TT and eFT) with leptin and adiponectin in men with HF.
Numerous studies indicated diabetes as an independent risk factor of male hypogonadism [Citation10,Citation11]. On the other hand, low TT concentrations are associated with insulin resistance and diabetes [Citation12]. In summary, the current evidence suggests a bidirectional relationship between serum TT and diabetes in men initiating a self-perpetuating cycle. Thus, we wanted to exclude diabetes as a strong determinant of androgen status in men in this study. Diabetes is very common in HF and is associated with poorer functional status and worse prognosis [Citation1]. More recent data from various registries show that the prevalence of diabetes in patients with HF ranges from approximately 25% to 40%, depending on the population studied [Citation13,Citation14].
The purpose of the study was to evaluate androgen status (TT, eFT) in non-cachectic men with HF and its association with HF severity, body composition, muscle strength, and long-term survival. Additionally, we aimed at evaluating determinants of androgen status in this population.
Methods
One hundred fifty two men, aged 55 years and older, with chronic HF due to ischemic or idiopathic dilated cardiomyopathy were screened. Inclusion criteria were: (1) Duration of chronic HF longer than one year; (2) Echocardiographically assessed left ventricular ejection fraction <40%; (3) New York Heart Association (NYHA) functional class II or III; (4) Stable medication regimen within the previous six weeks; (5) Clinically stable condition with no clinical evidence of decompensated HF. Exclusion criteria were: (1) Diabetes mellitus determined by either self-report or evidence in the hospital case record; (2) Primary lung disease including chronic obstructive pulmonary disease; (3) Musculoskeletal diseases; (4) Uncontrolled hypertension of more than 170/110 mm Hg; (5) Myocardial infarction or unstable angina within the previous three months; (6) Acute or chronic infection, inflammatory diseases such as systemic connective tissue disease; (7) Symptomatic peripheral vascular disease; (8) Chronic alcohol abuse; (9) Chronic kidney disease defined as serum creatinine ≥200 μmol/l; (10) Valvular cardiomyopathy or artificial heart valve; (11) Malignant disease, significant liver, thyroid, suprarenal gland or pituitary disease; (12) Cardiac cachexia defined as unintentional weight loss of ≥7.5% body weight in the preceding six months [Citation3,Citation15].
At baseline visit, 73 patients met the study inclusion and exclusion criteria and were selected for participation. The control group consisted of 20 male volunteers aged 55 years and older, without history of any chronic disease and without any regular medication regimen. Written informed consent was obtained from all patients with chronic HF and healthy subjects prior to inclusion into the study. The study was conducted according to the principles outlined in the Declaration of Helsinki. The Ethics Committee of the Clinical Hospital Zvezdara approved the study protocol.
Clinical and cardiovascular assessment
After the patient had given written informed consent, the medical history was reviewed. A physical examination was performed to assess current clinical status. All patients underwent two-dimensional Doppler echocardiography examination (GE Vivid 7) by the same sonographer. Left ventricular ejection fraction (LVEF) was measured using the Simpson biplane method. The 6-min walk test was performed according to the standard protocol.
Measurement of body composition, bone mineral density and grip strength
A dual energy X-ray absorptiometry machine (Lunar Prodigy Advance, GE. Healthcare, Madison, WI, USA) was used, according to standard protocol, to measure bone mineral density (BMD) (g/cm2), bone mineral content (BMC) and body composition (lean and fat mass) [Citation16]. Grip strength, as a measure of muscle strength, was determined by Jamar dynamometer (Lafayette Instrument, Lafayette, IN, USA) using standard protocols.
Laboratory analysis
Basal blood samples were taken at 8 am from an antecubital vein. Participants were asked to refrain from smoking at least 8 h before blood sampling, to fast from 9 pm the previous evening as well as to withhold vasoactive medication for at least 12 h before their appointment. Serum samples were immediately deep frozen and kept at −70 °C until assay. Total adiponectin and leptin levels in serum were measured by RIA (Linco Diagnostics, Inc., St. Charles, MO, USA). Serum levels of TT and N-terminal pro B-type natriuretic peptide (NT-proBNP) were measured with a fully automated “sandwich” electrochemiluminescence method by using Elecsys analyzer (Roche Diagnostics; GmbH, Mannheim, Germany). The measurement of NT-proBNP is helpful in screening to identify or exclude cardiovascular disease, for the differential diagnosis of symptoms that might be due to HF and are astonishingly powerful prognostic tools [Citation17]. NT-proBNP is now widely used in clinical practice and cardiovascular research throughout the world and have been incorporated into most national and international cardiovascular guidelines for HF [Citation1,Citation18]. Sex hormone-binding globulin was analyzed by immunoassay using an Immulite 1000 (EURO/DPC, Gwynedd, UK). Serum level of eFT was calculated with the validated equation of Vermeulen et al. [Citation19]. Cutoff value for TT deficiency has been taken to be below 3.26 ng/ml [Citation20,Citation21]. Estimated creatinine clearance was calculated from serum creatinine values using the Cockroft–Gault formula.
Clinical follow-up
Duration of follow-up was six years. Information regarding survival was obtained from patients or their relatives by phone calls. No patient was lost to follow-up. The primary end point for the analysis was all-cause mortality.
Statistics
Evaluation of normality was performed using the Kolmogorov–Smirnov test. Results are expressed as mean ± SD or median with interquartile range (IQR). The log10-transformations were performed for NT-proBNP and hsCRP, which follow exponential distribution. Student t-test was used to calculate differences between mean values. Mann–Whitney U-test was used to determine differences between median values. χ2-Test evaluated differences in frequencies between studied groups. The Pearson coefficient was used for measuring linear correlation between variables. Partial correlation analysis was performed to adjust for total fat mass. The variables showing a correlation with a p < 0.05 were then entered into a multivariable linear regression model, with TT and eFT serving as the dependent variables. Multivariate analysis was performed to assess factors independently associated with TT and eFT. Kaplan–Meier curves and the log-rank test were used to evaluate differences in death rates between patients with vs. without TT deficiency. Univariate and multivariate Cox regression analysis were used to evaluate determinants for all-cause mortality. All statistical analyzes were performed using the Statistical Package for the Social Sciences (SPSS) for Windows Version 17.0 (Chicago, IL). A value of p ≤ 0.05 was considered statistically significant.
Results
Baseline patient characteristics
Baseline patient characteristics are presented in . Patients with HF and healthy controls were similar of age and body mass index, while 6-min walking distance and grip strength were inferior in the first group. Body composition analysis did not find a difference in fat mass and lean mass between study groups, while BMD and BMC were significantly decreased in HF patients. Androgen status (TT, eFT) was similar in elderly men with HF compared to healthy controls. TT deficiency was noted in 13 (18%) patients with HF, while in 5 (25%) of healthy controls (p > 0.05). Serum adiponectin and NT-proBNP concentrations were significantly increased in HF patients. TT and eFT levels were found not to correlate with severity of HF symptoms expressed by NYHA class (4.67 ± 1.30 vs. 5.12 ± 2.40 ng/ml, p = 0.318 and 0.38 ± 0.18 vs. 0.50 ± 0.45 nmol/l, p = 0.094, respectively in NYHA II vs. NYHA class III).
Table 1. Baseline characteristics of elderly men with HF and healthy controls.
Univariate and multivariate logistic regression analysis with TT and eFT as dependent variables
In HF patients, serum TT and eFT were inversely correlated with age and total fat mass (), while positive associations were noted between eFT with impaired left ventricule contractile function (expressed by LVEF, dp/dt or Tei index) (). Additionally, serum TT and eFT positively correlated with serum adiponectin () even after adjustement for total fat mass (r = 0.281, p = 0.017 and r= 0.313, p = 0.007, respectively) and neurohormonal activation (NT-proBNP) (). On the other hand, an inverse association was noted between TT with serum leptin levels, which was abolished after correction for total fat mass. No association were found between TT and eFT with lean mass, muscle strength, and bone status. Multivariate linear regression analysis with TT and eFT as the dependent variables was performed (). The independent variables which entered in the model were the following: age, total fat mass, NT-proBNP (log-transformed), LVEF, hsCRP (log-transformed), creatinine clearance, serum leptin, and adiponectin. Age, total fat mass, and serum adiponectin were independent determinants of both TT and eFT.
Figure 1. Relationship between serum TT with total fat mass (A) and adiponectin (B) in elderly men with HF.
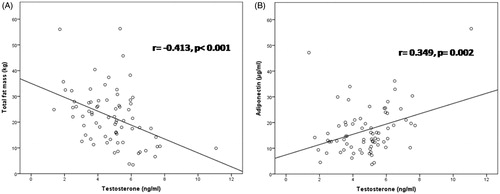
Table 2. Correlation of TT and eFT with other investigated variables in elderly men with HF.
Table 3. Multivariate regression analysis (stepwise model) with TT and eFT as dependent variables in elderly men with HF.
Survival analysis
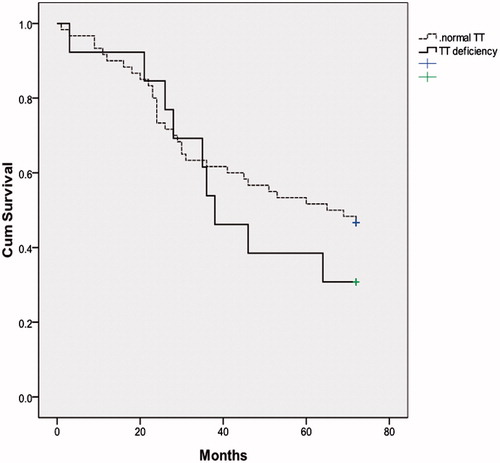
Kaplan–Meier survival analysis showed similar survival in HF patients with normal TT vs. patients with TT deficiency (p = 0.384) (). Patients with TT deficiency showed just a trend of impaired survival at six years of follow-up. Unlike to TT and eFT, univariate Cox regression analysis demonstrated that age, creatinine clearance, NT-proBNP, 6-min walking distance, LVEF, serum adiponectin and treatment with loop diuretics were associated with impaired survival (). In multivariate Cox regression analysis, NT-proBNP and 6-min walking distance remained independent predictors of all-cause death at six years follow-up.
Table 4. Univariate and multivariate Cox regression analysis on predictors of all-cause mortality in elderly men with HF.
Discussion
In this study, there are several important findings. In a selected cohort of elderly men with HF without diabetes and cardiac cachexia, we found similar levels of TT and eFT compared to healthy controls. Serum TT and eFT were independently determined by age, total fat mass, and serum adiponectin levels. Serum TT and eFT were positively associated with HF severity expressed by neurohormone activation (NT-proBNP), while eFT correlated with echocardiographic parameters of impaired left ventricular contractile function. We have also found no association between androgen status neither with skeletal muscle mass/function nor with bone status. Finally, but not less important, TT and eFT were not predictors for all-cause mortality at six years of follow-up in this selected population.
Literature data on androgen status in men with HF, assessed on the basis of serum levels of TT and eFT are equivocal. Some authors found deficiencies of TT [Citation4], whereas others have not [Citation22,Citation23]. In line with the results of Anker et al. [Citation22,Citation23] we have found no difference in serum levels of TT and eFT between elderly men with HF and healthy controls. Similar to previous results, we found that approximately 20% of men with HF have biochemical evidence of TT deficiency [Citation24]. TT deficiency presents one aspect of anabolic insufficiency that induces a metabolic shift favoring catabolism, a major underlying mechanism for tissue wasting seen in HF [Citation3,Citation6]. In another study, which included 382 men with systolic HF, TT and eFT deficiency (defined as blood levels ≤ 10th percentile in a population of healthy men of the same age) was found in 23% and 30% of men with HF aged < 60 years, and 23% and 34% of men aged ≥ 60 years, respectively [Citation10]. Difference in patients populations may be explanation of discrepancy between study results. Unlike to our study, Jankowska et al. [Citation4] included younger patients with more impaired renal function, while almost one third of study patients were diabetics. In general, diabetes may be considered as the most prominent potential confounding variable that can lead to this discrepancy between studies.
The present study showed that TT and eFT were independently determined by age, total fat mass and adiponectin levels in elderly men with HF. This is the first study evaluating the relationship of TT and eFT with leptin and adiponectin adjusted for total fat mass in men with HF. Circulating TT and eFT inversely correlated with total fat mass, while positive association was noted with serum adiponectin. Additonally, serum TT was inversely associated with serum leptin levels. A significant inverse association between TT and the degree of obesity was reported in many studies [Citation25,Citation26]. Fat mass can directly impact on TT levels [Citation25]. Adipocytes have high expression of aromatase that converts TT to estradiol and thus lower its circulating levels. Additionally, estrogens act upon hypothalamo-pituitary axis (HPT) as a negative feedback mechanism to suppress gonadotropin-releasing hormone and subsequent luteinizing hormone, and ultimately contribute to a reduction in gonadal TT release. Adipose tissue is an endocrine organ producing many factors such as leptin and adiponectin. Leptin is known to regulate body weight and food intake but also stimulates gonadotropin-releasing hormone neurons in the hypothalamus to induce luteinizing hormone release and subsequent testicular stimulation of TT release under normal conditions. Plasma leptin is thought to reflect adiposity, and obesity is associated with elevated leptin. In HF syndrome hypothalamus can become leptin resistant and thus resulting with a loss of this feedback stimulation of TT production [Citation27]. Additionally, serum leptin can directly inhibit Leydig cell TT production to further diminish androgen status [Citation27]. A positive association between adiponectin with TT and eFT in our sample is intriguing. Serum adiponectin levels were elevated in our HF patients, as it was previously reported [Citation28]. Unlike other adipose tissue-derived peptides like leptin that increase with increase of fat mass, adiponectin levels inversely correlate with fat mass [Citation29]. Adiponectin, an adipokine with insulin sensitizing effects increases AMP kinase signaling, inhibits cardiac hypertrophy and protects the heart from ischemia-reperfusion injury [Citation30]. Paradoxically, serum adiponectin was associated with severity of HF and poor outcome [Citation28]. Similarly we demonstrated significant association between increased serum adiponectin and long-term survival in univariate analysis. TT replacement therapy has been shown to decrease serum levels of adiponectin in hypogonadal men [Citation31]. Actually, TT would be expected to elevate total adiponectin levels by decreasing fat mass. Importantly, circulating adiponectin is present as several characteristic multimers known as low molecular weight (LMW), middle molecular weight (MMW) and high molecular weight (HMW) adiponectin capable of activating different signal transduction pathways involved in metabolism [Citation32]. Castration of male mice was shown to induce a dramatic elevation of the HMW form but had no effect on either the MMW or the LMW form in the circulation [Citation32]. TT treatment reversed this effect by selectively lowering HMW adiponectin. The specific roles of adiponectin isoforms and the long-term effect of TT on adiponectin has to be evaluated in future studies.
In the present study, serum TT and eFT paradoxically increased with a disease severity in elderly men with HF. Serum TT and eFT were positively associated with neurohormone activation (NT-proBNP), while eFT increased with deterioration of left ventricular contractile function (LVEF, dp/dt, Tei index). Additionally, we found a trend of higher values of TT and eFT in patients with more advanced symptoms assessed by NYHA class. These results were very surprising and in contrast with literature data so far despite the fact that all relationships were rather weak in magnitude. Jankowska et al. [Citation4] did not find any association between TT and eFT with echocardiographic (LVEF) or humoral (NT-proBNP) indices of left ventricular function, while low TT and eFT levels were found to correlate with higher severity of HF symptoms (higher NYHA class). Kalicinska et al. [Citation10] did not find associations between circulating TT and eFT with measures of HF severity (NYHA class, LVEF, NT-proBNP) in multivariable models. As further comparisons, in patients with systolic and diastolic HF, Guder et al. [Citation33] found that eFT, but not TT, was inversely associated with NYHA class and plasma NT-pro-BNP. The equivocal results, as well as the rather weak, although statistically significant correlations, suggest that serum androgen levels are not good surrogate of HF severity.
Direct association between androgen status with skeletal muscle mass has not been previously studied in men with HF. In this study, we found no correlation between TT and eFT neither with skeletal muscle mass (total lean mass) nor with muscle function (grip strength or 6-min walk distance). Previous studies have been hypothesized that a relative hypotestosteronaemia could be involved in the impairment of skeletal muscle function and exercise capacity which characterize HF patients [Citation34,Citation35]. Jankowska et al. [Citation36] found that circulating TT levels were independently related to peak oxygen consumption in men with HF which is the most objective parameters of exercise capacity. Iellamo et al. [Citation37] reported that TT therapy in men with systolic HF was related to an improvement in exercise capacity, as indicated by the increased distance walked in an incremental shuttle walk test, and in forearm muscle strength. Similarly, Caminiti et al. [Citation38] also found a significant amelioration of leg muscular performance in TT treated patients and the increase in muscle strength was positively related to the increase in TT levels in elderly men with HF. These findings imply an improvement in peripheral muscles function, particularly of large, weightbearing, muscles which are the more important to the early fatigue and effort intolerance experienced by HF patients. In line with these findings, Malkin et al. [Citation39] demonstrated that TT replacement therapy improves functional capacity and symptoms in men with moderately severe HF. Another study provides evidence for a superior effect of combined exercise training and TT replacement therapies on muscle sympathetic nerve activity, muscle wasting, and functional capacity in patients with HF with TT deficiency [Citation40]. For whatever reason, TT replacement therapy has always been controversial. The publication of two weak studies with questionable methodology and interpretation appear to rise concerns regarding TT replacement therapy [Citation41]. Importantly, two high-quality recent studies demonstrated reduced mortality, by half, in TT-deficient men who received TT compared with men who did not [Citation42,Citation43]. Subsequently, the real experts in TT replacement therapy underline its beneficial efficacy/safety profile in every day clinical life [Citation44,Citation45].
We found no association of androgen status (TT, eFT) and all-cause mortality in HF males without diabetes. An association between low TT level and long-term mortality in men with HF remains unclear. In three published large studies which included men with HF, in univariate analyzes both TT and eFT deficiency were found to be adverse long-term prognostic factors in this group of patients [Citation4,Citation33,Citation46]. After adjustment for additional clinical prognostic factors, results became less evident and in only one of these studies (in 208 men with systolic HF, median age 63 years, three-year follow-up) low TT and eFT levels were shown to be independent adverse prognosticators [Citation4]. These disparate findings can be explained by differences in the HF populations. The population evaluated by Guder et al. [Citation33] included almost 50% of patients with preserved ejection fraction, unlike those in the study of Jankowska et al. [Citation4]. Additionally, they speculated that their results differed from those of Jankowska et al. because they included additional prognostic variables, such as inflammatory markers, medication use and others. Specificity of our study population has been previously outlined.
Study limitations
There are some limitations that need to be acknowledged. Our observational study was based on an older male HF population with reduced ejection fraction without diabetes, so that the results cannot be generalized directly to overall HF population. In older adults, HF with preserved ejection fraction is a common type of HF. However, in this study we included only patients with reduced ejection fraction. The sample size is relatively small, however rather homogenous with long and complete follow-up. There is no consensus regarding which fraction of serum TT should be routinely measured. Free TT reflects the most active fraction of TT which exerts direct biological effects on tissues. Thus, we calculated eFT using a well-established equation of Vermeulen et al. [Citation19]. We are aware that correlation analyzes, in particular these in cross-sectional models, do not allow conclusions about cause-and-effect relationships. Adiponectin isoforms were not measured in this study.
Conclusions
Our study suggest that androgen deficiency may not be overt in elderly men with HF without diabetes. Serum TT and eFT were determined by age, total fat mass, and serum adiponectin levels independently of HF severity. Androgen status in our study did not predict long-term survival. In fact, the notion of absence of decreased TT in non-diabetic and non-cachectic elderly men with HF and the lack of its association with prognosis may question the concept of TT replacement in these selected patients.
Acknowledgements
This work was financially supported by Serbian Ministry of Science (grant 175033).
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2016;18:891–975.
- Feldman HA, Longcope C, Derby CA, et al. Age trends in the level of serum testosterone and other hormones in middle-aged men: longitudinal results from the Massachusetts Male Aging Study. J Clin Endocrinol Metab. 2002;87:589– 598.
- Loncar G, Springer J, Anker M, et al. Cardiac cachexia: hic et nunc. J Cachexia Sarcopenia Muscle. 2016;7:246–260.
- Jankowska EA, Biel B, Majda J, et al. Anabolic deficiency in men with chronic heart failure: prevalence and detrimental impact on survival. Circulation. 2006;114:1829–1837.
- Ottenbacher KJ, Ottenbacher ME, Ottenbacher AJ, et al. Androgen treatment and muscle strength in elderly men: a meta-analysis. J Am Geriatr Soc. 2006;54:1666.
- Loncar G, Fülster S, von Haehling S, et al. Metabolism and the heart: an overview of muscle, fat, and bone metabolism in heart failure. Int J Cardiol. 2013;162:77–85.
- Piepoli MF, Kaczmarek A, Francis DP, et al. Reduced peripheral skeletal muscle mass and abnormal reflex physiology in chronic heart failure. Circulation. 2006;114:126–134.
- Kershaw EE, Flier JS. Adipose tissue as an endocrine organ. J Clin Endocrinol Metab. 2004;89:2548–2556.
- Lafontan M, Viguerie N. Role of adipokines in the control of energy metabolism: focus on adiponectin. Curr Opin Pharmacol. 2006;6:580–585.
- Kalicińska E, Wojtas K, Majda J, et al. Anabolic deficiencies in men with systolic heart failure: do co-morbidities and therapies really contribute significantly?. Aging Male. 2013;16:123–131.
- Wang C, Jackson G, Jones TH, et al. Low testosterone associated with obesity and the metabolic syndrome contributes to sexual dysfunction and cardiovascular disease risk in men with type 2 diabetes. Diabetes Care. 2011;34:1669–1675.
- Basaria S. Male hypogonadism. Lancet. 2014;383:1250–1263.
- Matsue Y, Suzuki M, Nakamura R, et al. Prevalence and prognostic implications of pre-diabetic state in patients with heart failure. Circ J. 2011;75:2833e2839.
- Lehrke M, Marx N. Diabetes mellitus and heart failure. Am J Cardiol. 2017;120:S37–S47.
- Anker SD, Ponikowski P, Varney S, et al. Wasting as independent risk factor for mortality in chronic heart failure. Lancet. 1997;349:1050–1053.
- Trippel T, Lenk J, Gunga HC, et al. Estimating fat mass in heart failure patient. Arch Med Sci Atheroscler Dis. 2016;1:e1–e12.
- Maisel A, Mueller C, Adams K, Jr et al. State of the art: using natriuretic peptide levels in clinical practice. Eur J Heart Fail. 2008;10:824–839.
- Yancy CW, Jessup M, Bozkurt B, et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Am Coll Cardiol. 2017. pii: S0735-1097(17)37087-0. [Epub ahead of print]. doi:10.1016/j.jacc.2017.04.025
- Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab. 1999;84:3666–3672.
- Harman SM, Metter EJ, Tobin JD, et al. Longitudinal effects of aging on serum total and free testosterone levels in healthy men. Baltimore Longitudinal Study of Aging. J Clin Endocrinol Metab. 2001;86:724–731.
- Wu FC, EMAS Group, et al. Identification of late-onset hypogonadism in middle-aged and elderly men. N Engl J Med. 2010;363:123–135.
- Anker SD, Chua TP, Ponikowski P, et al. Hormonal changes and catabolic/anabolic imbalance in chronic heart failure and their importance for cardiac cachexia. Circulation. 1997;96:526–534.
- Anker SD, Clark AL, Kemp M, et al. Tumor necrosis factor and steroid metabolism in chronic heart failure: possible relation to muscle wasting. J Am Coll Cardiol. 1997;30:997–1001.
- Malkin CJ, Jones TH, Channer KS. Testosterone in chronic heart failure. Front Horm Res. 2009;37:183–196.
- Kelly DM, Jones TH. Testosterone and obesity. Obes Rev. 2015;16:581–606.
- Pasquali R, Casimirri F, Cantobelli S, et al. Effect of obesity and body fat distribution on sex hormones and insulin in men. Metab Clin Exp. 1991;40:101–104.
- Isidori AM, Caprio M, Strollo F, et al. Leptin and androgens in male obesity: evidence for leptin contribution to reduced androgen levels. J Clin Endocrinol Metab. 1999;84:3673–3680.
- Kistorp C, Faber J, Galatius S, et al. Plasma adiponectin, body mass index, and mortality in patients with chronic heart failure. Circulation. 2005;112:1756–1762.
- Spranger J, Kroke A, Möhlig M, et al. Adiponectin and protection against type 2 diabetes mellitus. Lancet. 2003;361:226–228.
- Shibata R, Sato K, Pimentel DR, et al. Adiponectin protects against myocardial ischemia-reperfusion injury through AMPK- and Cox-2-dependent mechanisms. Nat Med. 2005;11:1096–1103.
- Kapoor D, Clarke S, Channer KS, et al. The effect of testosterone replacement therapy on adipocytokines and C-reactive protein in hypogonadal men with type 2 diabetes. Eur J Endocrinol. 2007;156:595–602.
- Xu A, Chan KW, Hoo RL, et al. Testosterone selectively reduces the high molecular weight form of adiponectin by inhibiting its secretion from adipocytes. J Biol Chem. 2005;280:18073–18080.
- Güder G, Frantz S, Bauersachs J, et al. Low circulating androgens and mortality risk in heart failure. Heart. 2010; 96:504–509.
- Volterrani M, Rosano G, Iellamo F. Testosterone and heart failure. Endocrine. 2012;42:272–277.
- Josiak K, Jankowska EA, Piepoli MF, et al. Skeletal myopathy in patients with chronic heart failure: significance of anabolic-androgenic hormones. J Cachexia Sarcopenia Muscle. 2014;5:287–296.
- Jankowska EA, Filippatos G, Ponikowska B, et al. Reduction in circulating testosterone relates to exercise capacity in men with chronic heart failure. J Cardiac Fail. 2009;15:442–450.
- Iellamo F, Sala-Mercado JA, Ichinose M, et al. Spontaneous baroreflex control of heart rate during exercise and muscle metaboreflex activation in heart failure. Am J Physiol Heart Circ Physiol. 2007;293:H1929–H1936.
- Caminiti G, Volterrani M, Iellamo F, et al. Effect of long-acting testosterone treatment on functional exercise capacity, skeletal muscle performance, insulin resistance, and baroreflex sensitivity in elderly patients with chronic heart failure a double-blind, placebo-controlled, randomized study. J Am Coll Cardiol. 2009;54:919–927.
- Malkin CJ, Pugh PJ, West JN, et al. Testosterone therapy in men with moderate severity heart failure: a double-blind randomized placebo controlled trial. Eur Heart J. 2006;27:57–64.
- Dos Santos MR, Sayegh AL, Bacurau AV, et al. Effect of exercise training and testosterone replacement on skeletal muscle wasting in patients with heart failure with testosterone deficiency. Mayo Clin Proc. 2016;91:575–586.
- Morgentaler A, Lunenfeld B. Testosterone and cardiovascular risk: world's experts take unprecedented action to correct misinformation. Aging Male. 2014;17:63–65.
- Shores MM, Smith NL, Forsberg CW, et al. Testosterone treatment and mortality in men with low testosterone levels. J Clin Endocrinol Metab. 2012;97:2050–2058.
- Muraleedharan V, Marsh H, Kapoor D, et al. Testosterone deficiency is associated with increased risk of mortality and testosterone replacement improves survival in men with type 2 diabetes. Eur J Endocrinol. 2013;169:725–733.
- Lunenfeld B, Mskhalaya G, Zitzmann M, et al. Recommendations on the diagnosis, treatment and monitoring of hypogonadism in men. Aging Male. 2015;18:5–15.
- Morgentaler A, Zitzmann M, Traish AM, et al. Fundamental concepts regarding testosterone deficiency and treatment: international expert consensus resolutions. Mayo Clin Proc. 2016;91:881–896.
- Wu HY, Wang XF, Wang JH, et al. Testosterone level and mortality in elderly men with systolic chronic heart failure. Asian J Androl. 2011;13:759–763.