Abstract
Objective: Interleukin 18 (IL-18) is an adipokine associated with obesity. Data about the relationship of IL-18 to the metabolic syndrome (MS) are still scarce. Low testosterone (T) levels are common in men with MS, but we did not find data about the levels of IL-18 in men with low T. The aim of this study was to determine the levels of IL-18 in men with MS with or without low T.
Patients and methods: A total of 251 men were included in the study. Of them 218 had MS (IDF 2005) and they were divided according to their morning total testosterone (TT) level (cutoff 10.4 nmol/l) into two groups: MS-low T (N = 84) and MS-normal T (N = 134). The control group consisted of 33 men without MS and low T. IL-18 was determined in serum using enzyme-linked immunosorbent assay. A small group of eight men with MS and low T levels received testosterone therapy for three months and physical and laboratory parameters were monitored at the end of that period.
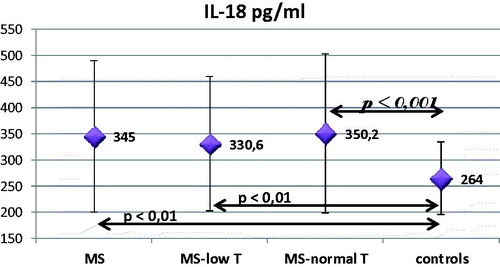
Results: MS men were at mean age (±SD) = 53.77 ± 9.59 years; body mass index (BMI) = 34.0 ± 6.3 kg/m2; and TT = 12.59 ± 5.66 nmol/l. The control group was at age = 52.12 ± 5.2 years (NS); BMI = 25.6 ± 2.4 kg/m2 (p < .001); and TT = 17.8 ± 5.68 nmol/l (p < .001), respectively. The levels of IL-18 were higher in the MS group – 345 pg/ml compared to the control one – 264 pg/ml (p < .01). There was no significant difference between MS-low T (330.6 pg/ml) and MS-normal T (350.2 pg/ml) subgroups. The MS-normal T differed more significantly from the control group (p < .001). Significant correlation of testosterone with IL-18 levels was not found. IL-18 correlated with parameters of obesity, lipids, fasting blood sugar (p < .05) and the number of criteria for MS (p < .001). Three months on T treatment showed improvement in obesity parameters and only in one patient IL-18 had clear reduction while the rest showed no change.
Conclusions: In this study, higher IL-18 levels were found in the presence of MS compared to healthy men, but they did not differ between men having MS with or without LOH.
Introduction
Interleukin 18 (IL-18)
The metabolic syndrome is a cluster of factors that gathered together significantly raise cardiovascular risk. The relation of MS to low-grade inflammation has already been observed [Citation1,Citation2]. The concentrations of some cytokines (TNF-alfa, IL-6 and IL-18) in the circulation are two- to threefold higher if MS is present. Arivazhagan et al. [Citation3] concluded that IL-17 are also elevated in benign prostatic hyperplasia.
Interleukin-18 (IL-18) is a member of the interleukin-1 family and has been discovered by Okamura et al. as an interferon-gamma – inducing factor [Citation4]. This cytokine is synthesized by several types of cells – macrophages, endothelial, smooth muscle, dendrite and Kupfer’s cells as well as by adipocytes [Citation5,Citation6] and fat tissue is considered the main source [Citation7]. IL-18 is synthesized as a precursor that is activated by the enzyme caspase-1 in the so-called inflammasome [Citation8].
After secretion, IL-18 is bound to a specific protein which results in deactivation and serves as a protective mechanism against oversecretion of IL-18 or uncontrollable pro-inflammatory activity [Citation9]. IL-18 binds to the alfa-subunit of its membrane receptor and the beta-subunit is responsible for the signal transduction in the cell [Citation10]. Both free and protein-bound form connect to the receptor but only the free form activates it [Citation9].
IL-18 is a strong pro-inflammatory cytokine potentiating the differentiation of T-lymphocytes and NK cells of the immune system and also stimulating the production and secretion in the circulation of other cytokines, chemokines and adhesion molecules [Citation8]. Interestingly, IL-18 can stimulate different type of immune reactions depending on the other cytokines involved. For example, together with IL-12, it stimulates gamma-interferon production that is of great importance for the stability of atheromatous plaque [Citation10].
IL-18 has been tightly associated with the metabolic syndrome and its complications. IL-18 is associated with obesity [Citation11,Citation12], insulin resistance [Citation13,Citation14], hypertension [Citation15] and dyslipidaemia [Citation11,Citation16]. In patients with MS higher levels of IL-18 have been established [Citation17], as well as raising its level together with the number of components of the MS [Citation18].
Paradoxically, in mice with absolute deficiency of IL-18, obesity, insulin resistance and dyslipidaemia was observed [Citation19]. Besides, significantly lower production of gamma-interferon was reported after IL-18 stimulation in blood samples of patients with type-2 diabetes and obesity. That supposes IL-18 resistance as a response to its high levels [Citation20]. The altered function of leucocytes and susceptibility to infections in diabetes support that [Citation21,Citation22]. Other authors report that IL-18 deficiency leads to hyperphagia and obesity, suggesting a role in the control of appetite [Citation23]. That reveals the complex role of IL-18 in the metabolic processes.
Testosterone and MS
Most of the components of the MS (obesity, hypertension, dislipidemia, glucose metabolism abnormalities and insulin resistance) are also found in men with hypogonadism [Citation24]. Epidemiologic studies have established connection between obesity and low serum testosterone levels in healthy men [Citation25]. Twenty percent to 64% of men with obesity have low total testosterone (TT) or free testosterone (FT) levels [Citation26]. Kupelian et al. have established that low TT and low levels of sex-hormone-binding globulin are risk factors for metabolic syndrome even in symptomless androgenic deficit [Citation27]. This confirms the data of Muller et al., that endogenous levels of sex hormones are low in MS [Citation28]. Other authors find inverse correlation of number of components of MS and levels of TT [Citation29]. It is assumed that the more parameters are abnormal, the lower levels of testosterone would be measured [Citation30]. Large longitudinal studies confirm that the frequency of MS is higher in elderly men and hypogonadism is a relevant factor [Citation27,Citation31]. In older men, gonadal status is also independently associated with determinants of physical performance – hemoglobin and muscle strength [Citation32]. Aging males’ symptoms (AMS) questionnaire shows significant associations with age and sex hormones, but when age is adjusted correlation significance disappears [Citation33]. Other authors concluded that AMS is of limited use for diagnosing hypogonadism because it has low specificity and is influenced by comorbidities that are frequent during aging [Citation34,Citation35]. Metabolic syndrome is considered to pray a key role in the pathogenesis in erectile dysfunction and lower urinary tract symptoms [Citation36].
Managing hypogonadism using testosterone replacement leads to clinical improvement of erectile dysfunction thus improving sexual health in aging [Citation37].
The importance of IL-18 for hypogonadism in men with MS is still unclear. The aim of this study was to determine the levels of IL-18 in men with MS with or without low T.
Patients and methods
Metabolic syndrome
Consecutive patients of the Clinic of endocrinology at Alexandrovska University hospital in Sofia were included in this study. The study was approved by the local ethical committee and all men signed informed consent entering this study. At first, they were clinically analyzed. Data about medical history, physical examination, height, weight, waist and hip circumferences, blood pressure were gathered. Body mass index (BMI) was calculated (kg/m2) = weight/height2. For the evaluation of body composition (fat and nonfat tissue, body water) was used bioimpedance analyzer Tanita TBF-215 (Tanita, Japan). Standard metabolic markers as well as the hormonal status of the gonadal axis were measured in the Central laboratory of the hospital which is the referent one for the country.
According to the criteria of International Diabetes Federation 2005 [Citation38] abdominal obesity plus two of the following criteria are needed for the diagnosis of the metabolic syndrome. We analyzed:
abdominal obesity (waist circumference ≥94 cm or BMI >30 kg/m2);
carbohydrate metabolism disturbances – fasting blood glucose ≥5.6 mmol/l or previously diagnosed type-2 diabetes;
blood pressure (BP) – systolic ≥130 or diastolic ≥85 mmHg, or treatment for high blood pressure;
high density lipoproteins level (HDL) < 1.03 mmol/l, or present lipid-lowering treatment;
triglycerides level (TG) ≥ 1.7 mmol/l, or present lipid-lowering treatment.
That’s how the group of patients with metabolic syndrome (MS) was formed.
Late-onset hypogonadism – LOH
By definition to set this diagnosis low serum testosterone and clinical signs of testosterone deficiency are needed. Total testosterone (TT) was measured twice in different days in the morning in fasting state. Of the two results mean arithmetic was calculated and used as TT level for the purposes of the study. We accepted the level 10.4 nmol/l as a cutoff level for the testosterone deficiency as used by other authors [Citation39].
For analysis of clinical symptoms and signs, the men filled out internationally approved questionnaires – ADAM (Androgen Deficiency in Aging Males) [Citation40] and AMS (Aging Male’s Symptoms) [Citation41]. AMS is also influenced by comorbidities, which lowers its specificity [Citation34]. The probability (risk) for LOH was thus defined. Other reasons for hypogonadism were excluded - hyperprolactinemia, pituitary tumors, operative interventions and others.
The men with metabolic syndrome and low testosterone levels who were to be treated with testosterone were additionally examined and those with contraindications for testosterone treatment were excluded. Only the patients on stable antidiabetic, antihypertensive and lipid-lowering treatment for at least 6 months before the treatment period and during it were included. For the treatment, either testosterone undecanoat (Nebido, Bayer-Schering) – 1 ampoule of 1000 mg/4 ml for intramuscular application, or testosterone gel (Androgel, Laboratoires Besins International, Abbot) was used – 50 mg in daily sachets. After starting this therapy, the men were monitored for a period of three months, and blood samples were collected for further analysis.
The control group of men was formed by healthy volunteers – without diabetes and/or obesity, which were analyzed by the same protocol. They were asked to come in the clinic in fasting state in the morning for blood drawing for laboratory analysis and routine physical examination and anthropometric measurements. After getting the results, some of the men were excluded because of previously unrecognized disturbance of the carbohydrate metabolism, or when fulfilling the criteria for MS, or if low serum testosterone levels were found.
Blood samples’ analysis
Blood samples were taken using the forearm’s veins. Routine laboratory analyses were done in the same day. For measuring the interleukin-18 levels, after centrifugation (4000 r/min) serum samples were frozen at −80 °C. TT levels were measured by electrohemiluminiscent method (Elecsys 2010, Roche Diagnostics). For measuring the interleukin-18 levels, the enzyme-linked immunosorbent method (MBL Co., Ltd, Japan) was used.
Statistical analysis
Statistical analysis of the information was done using appropriate methods for every analysis in the computer program SPSS 13.0 (SPSS Inc., Chicago, IL). Defining the type of variable distribution, we used Kolmogorov–Smirnoff test. Descriptive analyses were made using mean and standard deviation. Comparing the groups, we applied the ANOVA or t-test, confirmed afterwards by Mann–Whitney test. Correlations between variables are searched using the correlation tests of Pearson or Spearman. Results are accepted as statistically significant if p < .05.
Results
Hypogonadism in MS
In this study, 251 men were included, 218 of which with MS and the control group consisted of 33 clinically healthy men without DM, MS or LOH. The two groups are at similar age, but they differ significantly in their weight (p < .001), BMI (p < .001), waist circumference (p < .001) and waist-to-hip ratio (p < .001) ().
Table 1. Baseline characteristics of the study groups.
In the group of patients with MS, 87% had been diagnosed with type-2 diabetes and their glycated hemoglobin A1c level (HbA1c) was 8.3 ± 1.85%; mean duration of diabetes was 7.5 ± 7.5 years. In 76.8% of the patients with type-2 diabetes, polyneuropathy was established; retinopathy had 20.1% of them (18% proliferative form); nephropathy had been diagnosed in 9% of the patients.
Arterial hypertension was present in 81.6% of the patients with MS, as 90% of them had already been on some antihypertensive medication. Mean systolic blood pressure was 133.3 ± 15.9 mmHg; and diastolic – 83.3 ± 8.8 mmHg. In the control group, 33.3% of men also had hypertension.
Lipid disorders were present in 75% of the patients with MS and in 39% of the men in the control group.
In the group of patients with MS, 84 (38.5%) had low levels of TT and the rest 134 (60.5%) had testosterone levels higher than the threshold of 10.4 nmol/l. Patients with MS and low TT had significantly higher weight (p < .001), BMI (p < .001), waist circumference (p < .01), fat % (p < .01) and a higher HbA1c ().
In the group of patients with MS the TT level correlated significantly with the BMI, weight, waist circumference, fat %, HbA1c, TG (negatively) and HDL (positively) (). A correlation with the number of components of the MS was also found.
Table 2. Correlations of testosterone with basic parameters.
Interleukin-18 (IL-18)
The level of IL-18 was measured in 251 men 33 of which were controls. The group of patients included 218 men 84 of which were with TT below 10.4 nmol/l, and 134 – above that cutoff. Patients with MS had significantly higher levels of IL-18 compared to the control group (p < .01), especially the group with normal T-levels (p < .001). In patients with MS and low testosterone, IL-18 was lower than in patients with MS and normal T but not statistically significant ().
A correlation of IL-18 and testosterone was not established. The AMS questionnaire correlated with the IL-18 levels (Spearman’s rho = 0.186, p < .01), especially its somatic domain (Spearman’s rho = 0.204, p < .01), and the relation is weaker for the sexual domain (Spearman’s rho = 0.146, p < .05).
Some anthropometric and laboratory parameters also showed correlation with IL-18 ().
Table 3. Correlations of IL-18 with basic parameters.
Table 4. Patients characteristics before and after testosterone–replacement therapy (TRT) (data presented as mean ± SD).
Testosterone replacement therapy (TRT)
From the patients assigned to TRT, only eight were treated for a period of three months and seven of them finished the study period. The rest were not treated due to personal, mostly financial reasons. All patients on TRT had type-2 diabetes except for one that had impaired fasting glucose levels. One of the type-2 diabetes patients was treated only with diet with stable glycemia and the others were on metformin in a stable dose for more than six months as a monotherapy or in combination with basal insulin, incretin-based therapy or sulfonylurea. Glycemic control varied from glycated hemoglobin levels of 5.5% to 7.8% initially, mean value 6.67% (). Mean-daily blood sugar levels were 6.43 ± 1.67 mmol/l. Hypertension was present in six patients, controlled on medication. There was no significant impairment of the lipid profile parameters (two of them were on fibrate and one on statin therapy).
Six of the eight patients were treated with i.m. injection (Nebido) and the other two - with transdermal gel (Androgel). Basic parameters were analyzed after three months of therapy (). Improvement in the obesity indicators was established without substantial changes in the glycemic or lipid profile and prostate-specific antigen (PSA). The results of self-reported symptoms of androgen deficiency were improved (ADAM, AMS).
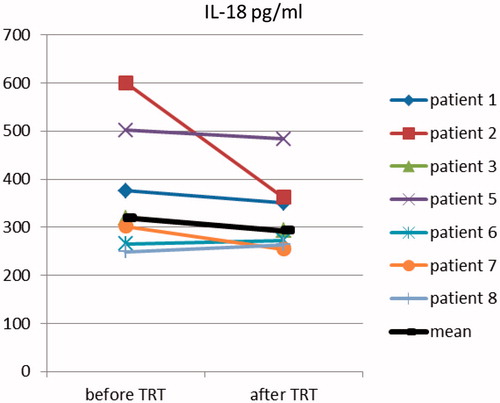
No change of the level of IL-18 was found after three months of therapy- mean IL-18 before treatment is 319.06 pg/ml and after treatment – 293.105 pg/ml (NS, p = .366) ().
Discussion
Based on the anti-inflammatory effects of androgens, our hypothesis was that because of its tight link with MS, testosterone would influence IL-18 levels (or vice versa), which are related to MS in the literature too. Additionally, we assumed that testosterone substitution would influence IL-18 levels. As far as we know, this is the first study analyzing IL-18, metabolic syndrome and testosterone levels.
The prevalence of hypogonadism in our patients with MS is high – 39%, similar to the data in the literature [Citation28]. Our data confirmed the negative correlation of TT with the number of the MS components () already reported by other authors [Citation29].
Interleukin-18 is a pleiotropic cytokine that regulates both innate and acquired immune response and plays an important role in inflammation [Citation42]. The levels of IL-18 in patients with insulin resistance, obesity and metabolic syndrome are reported to be increased [Citation12,Citation14]. Our data support that finding in patients with the MS compared to controls and the correlation of IL-18 to the components of the syndrome. In our study, hypertension is the only component of the metabolic syndrome that has no statistically significant association with the level of IL-18, although other authors have described it [Citation43]. The reason for this could be the antihypertensive medication. The strongest correlation of the other components of MS is shown for hypertriglyceridemia. This association has been reported by others in healthy population [Citation44]. An antilipolytic effect of IL-18 has also been discussed in HIV-positive patients with lipodystrophy [Citation45]. The correlation with the number of components for defining MS has been reported in other studies [Citation43,Citation46] and IL-18 is presumed a predictor for developing MS.
The relation to type-2 diabetes is still unclear despite all reports for their association [Citation20,Citation47–49], even to development of complications such as nephropathy, cardiovascular diseases [Citation50] and retinopathy [Citation51]. Our data shows association of IL-18 to the fasting blood glucose level, but no association was found to other parameters or complications of diabetes. Negi et al. [Citation52] have reported the incidence of developing diabetes type 2 in a group of almost 10,000 men for 9 years and the importance of some anthropometric and basic biochemical parameters. Higher levels of IL-18 have been associated with the development of diabetes. After adjustment for other factors - age, BMI, family history for diabetes, the relation is still significant. But after adjusting for blood sugar levels – it is weaker. The authors suppose that fasting blood sugar itself regulates IL-18 levels. This is supported in other studies too, in which hyperglycemia promotes the synthesis of IL-18 and other pro-inflammatory cytokines [Citation53]. On the other hand, inflammatory cytokines lead to insulin resistance and diabetes type 2 [Citation54] and further higher blood sugar levels. Vicious circle with a positive feedback mechanism is thus formed. In a study, Dezayee et al. establish significant association of IL-18 and HbA1c, diabetes duration and lipid parameters [Citation55]. Our data support the relation of high levels of IL-18 with the atherogenic dyslipidemia.
Some authors report a correlation with the age of the patients [Citation52], but such was not found in our study.
The correlation of IL-18 to the parameters of obesity is moderate, so it provokes the question if adipose tissue is the main source of IL-18. In experimental models some authors assume that IL-18 is released mainly by the nonfat cells in the adipose tissue [Citation7]. Correlation with the obesity parameters is reported by others too, but only in Caucasians, not in Afro–Americans [Citation52]. IL-18 is a stress regulator at cellular level rather than systemic levels. IL-18 is shown to promote survival of immune cells by augmenting autophagy, calcium influx, protein translation and energy metabolism [Citation56]. However, true biological roles of IL-18 are still to be clarified.
Low levels of testosterone in our patients with MS did not additionally influence the levels of IL-18. An association of IL-18 with testosterone was not found. In a polish study evaluating the prevalence of MS, some androgens and IL-18 were tested in 160 men [Citation46]. Higher level of IL-18 was reported in MS patients and also its association with the number of criteria defining the syndrome, which is confirmed by our data too with a higher strength and level of significance. The authors assume that inflammatory markers are better predictors for MS than the androgens (adrenal androgens and free testosterone). Anyway, association in-between IL-18 and androgens was not reported.
After three months of testosterone treatment obesity improvement was found. In longer trials, it has been observed that sustaining the weight loss is predicted by initial BMI and waist circumference [Citation57]. We did not found any significant change in the levels of IL-18 in our patients that underwent TRT for three months, but the small number of treated men and the short period of treatment seriously limits any further more generalized conclusion and the treatment arm of this study may be considered only as a pilot one. However, in patient 2, there is a very clear reduction in the level of IL-18. If there is some kind of effect, it might need longer period of treatment and larger group of patients to be manifested. Anyway, there is data confirming gender difference for the levels of IL-18 [Citation58]. An experimental study with mice also established that higher IL-18 lead to higher mortality only in male animals [Citation59].
A possible explanation of the unchanged levels of IL-18 is that high cholesterol levels could lead to that as a metabolic regulatory mechanism.
Conclusions
In this study, higher IL-18 levels were found in the presence of MS compared to healthy men, but they do not differ between men having MS with low or normal T. IL-18 may be concerned with metabolic syndrome, but the role of IL-18 and testosterone levels and their relation to each other and to the metabolic syndrome are to be investigated in future studies.
Disclosure statement
The authors declare that they have no conflict of interest.
References
- Grundy SM, Cleeman JI, Daniels SR, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute scientific statement: Executive Summary. Crit Pathw Cardiol. 2005;4:198–203.
- Glass CK, Olefsky JM. Inflammation and lipid signaling in the etiology of insulin resistance. Cell Metab. 2012;15:635–645.
- Arivazhagan J, Nandeesha H, Dorairajan LN, et al. Association of elevated interleukin-17 and angiopoietin-2 with prostate size in benign prostatic hyperplasia. Aging Male. 2017;20:115–118.
- Okamura H, Tsutsi H, Komatsu T, et al. Cloning of a new cytokine that induces IFN-gamma production by T cells. Nature. 1995;378:88–91.
- Garcia VE, Uyemura K, Sieling PA, et al. IL-18 promotes type 1 cytokine production from NK cells and T cells in human intracellular infection. J Immunol. 1999;162:6114–6121.
- Dinarello CA. Interleukin-18 and the pathogenesis of inflammatory diseases. Semin Nephrol. 2007;27:98–114.
- Fain JN, Tichansky DS, Madan AK. Most of the interleukin 1 receptor antagonist, cathepsin S, macrophage migration inhibitory factor, nerve growth factor, and interleukin 18 release by explants of human adipose tissue is by the non-fat cells, not by the adipocytes. Metab Clin Exp. 2006;55:1113–1121.
- Dinarello CA. Interleukin 1 and interleukin 18 as mediators of inflammation and the aging process. Am J Clin Nutr. 2006;83:447S–455S.
- Novick D, Schwartsburd B, Pinkus R, et al. A novel IL-18BP ELISA shows elevated serum IL-18BP in sepsis and extensive decrease of free IL-18. Cytokine. 2001;14:334–342.
- Nakanishi K, Yoshimoto T, Tsutsui H, et al. Interleukin-18 is a unique cytokine that stimulates both Th1 and Th2 responses depending on its cytokine milieu. Cytokine Growth Factor Rev. 2001;12:53–72.
- Hung J, McQuillan BM, Chapman CM, et al. Elevated interleukin-18 levels are associated with the metabolic syndrome independent of obesity and insulin resistance. Arterioscler Thromb Vasc Biol. 2005;25:1268–1273.
- Bruun JM, Stallknecht B, Helge JW, et al. Interleukin-18 in plasma and adipose tissue: effects of obesity, insulin resistance, and weight loss. Eur J Endocrinol. 2007;157:465–471.
- Bosch M, Lopez-Bermejo A, Vendrell J, et al. Circulating IL-18 concentration is associated with insulin sensitivity and glucose tolerance through increased fat-free mass. Diabetologia. 2005;48:1841–1843.
- Fischer CP, Perstrup LB, Berntsen A, et al. Elevated plasma interleukin-18 is a marker of insulin-resistance in type 2 diabetic and non-diabetic humans. Clin Immunol. 2005;117:152–160.
- Rabkin SW. The role of interleukin 18 in the pathogenesis of hypertension-induced vascular disease. Nat Clin Pract Cardiovasc Med. 2009;6:192–199.
- Evans J, Collins M, Jennings C, et al. The association of interleukin-18 genotype and serum levels with metabolic risk factors for cardiovascular disease. Eur J Endocrinol. 2007;157:633–640.
- Van Guilder GP, Hoetzer GL, Greiner JJ, et al. Influence of metabolic syndrome on biomarkers of oxidative stress and inflammation in obese adults. Obesity. 2006;14:2127–2131.
- Zirlik A, Abdullah SM, Gerdes N, et al. Interleukin-18, the metabolic syndrome, and subclinical atherosclerosis: results from the Dallas Heart Study. Arterioscler Thromb Vasc Biol. 2007;27:2043–2049.
- Netea MG, Joosten LA, Lewis E, et al. Deficiency of interleukin-18 in mice leads to hyperphagia, obesity and insulin resistance. Nat Med. 2006;12:650–656.
- Zilverschoon GR, Tack CJ, Joosten LA, et al. Interleukin-18 resistance in patients with obesity and type 2 diabetes mellitus. Int J Obes (Lond). 2008;32:1407–1414.
- Delamaire M, Maugendre D, Moreno M, et al. Impaired leucocyte functions in diabetic patients. Diabet Med. 1997;14:29–34.
- Gallacher SJ, Thomson G, Fraser WD, et al. Neutrophil bactericidal function in diabetes mellitus: evidence for association with blood glucose control. Diabet Med. 1995;12:916–920.
- Zorrilla EP, Sanchez-Alavez M, Sugama S, et al. Interleukin-18 controls energy homeostasis by suppressing appetite and feed efficiency. Proc Natl Acad Sci USA. 2007;104:11097–11102.
- Lunenfeld B, Mskhalaya G, Zitzmann M, et al. Recommendations on the diagnosis, treatment and monitoring of hypogonadism in men. Aging Male. 2015;18:5–15.
- Allen NE, Appleby PN, Davey GK, et al. Lifestyle and nutritional determinants of bioavailable androgens and related hormones in British men. Cancer Causes Control. 2002;13:353–363.
- Kalyani RR, Dobs AS. Androgen deficiency, diabetes, and the metabolic syndrome in men. Curr Opin Endocrinol Diabetes Obes. 2007;14:226–234.
- Kupelian V, Page ST, Araujo AB, et al. Low sex hormone-binding globulin, total testosterone, and symptomatic androgen deficiency are associated with development of the metabolic syndrome in nonobese men. J Clin Endocrinol Metab. 2006;91:843–850.
- Muller M, Grobbee DE, den Tonkelaar I, et al. Endogenous sex hormones and metabolic syndrome in aging men. J Clin Endocrinol Metab. 2005;90:2618–2623.
- Kaplan SA, Meehan AG, Shah A. The age related decrease in testosterone is significantly exacerbated in obese men with the metabolic syndrome. What are the implications for the relatively high incidence of erectile dysfunction observed in these men?. J Urol. 2006;176:1524–1527. discussion 1527–1528.
- Guay AT. The emerging link between hypogonadism and metabolic syndrome. J Androl. 2009;30:370–376.
- Rodriguez A, Muller DC, Metter EJ, et al. Aging, androgens, and the metabolic syndrome in a longitudinal study of aging. J Clin Endocrinol Metab. 2007;92:3568–3572.
- Maggio M, Ceda GP, Lauretani F, et al. Gonadal status and physical performance in older men. Aging Male. 2011;14:42–47.
- Yu XH, Zhao J, Zhang SC, et al. The impact of age, BMI and sex hormone on aging males' symptoms and the international index of erectile function scores. Aging Male. 2017;20:235–240.
- Panach-Navarrete J, Martinez-Jabaloyas JM, DE-SDT study group. The influence of comorbidities on the aging males' symptoms scale in patients with erectile dysfunction. Aging Male. 2017;20:146–152.
- Almehmadi Y, Yassin DJ, Yassin AA. Erectile dysfunction is a prognostic indicator of comorbidities in men with late onset hypogonadism. Aging Male. 2015;18:186–194.
- Demir O, Akgul K, Akar Z, et al. Association between severity of lower urinary tract symptoms, erectile dysfunction and metabolic syndrome. Aging Male. 2009;12:29–34.
- Kaya E, Sikka SC, Kadowitz PJ, et al. Aging and sexual health: getting to the problem. Aging Male. 2017;20:65–80.
- Alberti KG, Zimmet P, Shaw J, et al. The metabolic syndrome-a new worldwide definition. Lancet. 2005;366:1059–1062.
- Mulligan T, Frick MF, Zuraw QC, et al. Prevalence of hypogonadism in males aged at least 45 years: the HIM study. Int J Clin Pract. 2006;60:762–769.
- Morley JE, Charlton E, Patrick P, et al. Validation of a screening questionnaire for androgen deficiency in aging males. Metab Clin Exp. 2000;49:1239–1242.
- Heinemann LAJZT, Vermeulen A, Thiel C. A new ‘Aging Male’s Symptoms’ (AMS) Rating Scale. Aging Male. 1999;2:105–114.
- Ghayur T, Banerjee S, Hugunin M, et al. Caspase-1 processes IFN-gamma-inducing factor and regulates LPS-induced IFN-gamma production. Nature. 1997;386:619–623.
- Troseid M, Seljeflot I, Weiss TW, et al. Arterial stiffness is independently associated with interleukin-18 and components of the metabolic syndrome. Atherosclerosis. 2010;209:337–339.
- Olusi SO, Al-Awadhi A, Abraham M. Relations of serum interleukin 18 levels to serum lipid and glucose concentrations in an apparently healthy adult population. Horm Res Paediatr. 2003;60:29–33.
- Lindegaard B, Ditlevsen S, Plomgaard P, et al. Acute reduction of lipolysis reduces adiponectin and IL-18: evidence from an intervention study with acipimox and insulin. Diabetologia. 2013;56:2034–2043.
- Herman WA, Lacka K, Kaufman E, et al. The associations between iL-18 serum levels and the prevalence of metabolic syndrome in Polish men over the age of 40 according to other selected inflammatory indices and androgens: comparison of NCEP with IDF criteria. Exp Clin Endocrinol Diabetes. 2011;119:423–430.
- Esposito K, Marfella R, Giugliano D. Plasma interleukin-18 concentrations are elevated in type 2 diabetes. Diabetes Care. 2004;27:272
- Thorand B, Kolb H, Baumert J, et al. Elevated levels of interleukin-18 predict the development of type 2 diabetes: results from the MONICA/KORA Augsburg Study, 1984–2002. Diabetes. 2005;54:2932–2938.
- Suchanek H, Mysliwska J, Siebert J, et al. High serum interleukin-18 concentrations in patients with coronary artery disease and type 2 diabetes mellitus. Eur Cytokine Netw. 2005;16:177–185.
- Araki S, Haneda M, Koya D, et al. Predictive impact of elevated serum level of IL-18 for early renal dysfunction in type 2 diabetes: an observational follow-up study. Diabetologia. 2007;50:867–873.
- Nagai N, Ito Y, Okamoto N, et al. Changes in interleukin 18 in the retinas of Otsuka long-evans Tokushima fatty rats, a model of human type 2 diabetes. J Oleo Sci. 2013;62:513–523.
- Negi SI, Pankow JS, Fernstrom K, et al. Racial differences in association of elevated interleukin-18 levels with type 2 diabetes: the Atherosclerosis Risk in Communities study. Diabetes Care. 2012;35:1513–1518.
- Esposito K, Nappo F, Marfella R, et al. Inflammatory cytokine concentrations are acutely increased by hyperglycemia in humans: role of oxidative stress. Circulation. 2002;106:2067–2072.
- Bloch-Damti A, Bashan N. Proposed mechanisms for the induction of insulin resistance by oxidative stress. Antioxid Redox Signal. 2005;7:1553–1567.
- Dezayee ZM. Interleukin-18 can predict pre-clinical atherosclerosis and poor glycemic control in type 2 diabetes mellitus. Int J Appl Basic Med Res. 2011;1:109–112.
- Ma Z, Li W, Yoshiya S, et al. Augmentation of Immune Checkpoint Cancer Immunotherapy with IL18. Clin Cancer Res. 2016;22:2969–2980.
- Salman M, Yassin DJ, Shoukfeh H, et al. Early weight loss predicts the reduction of obesity in men with erectile dysfunction and hypogonadism undergoing long-term testosterone replacement therapy. Aging Male. 2017;20:45–48.
- Opstad TB, Pettersen AA, Arnesen H, et al. Circulating levels of IL-18 are significantly influenced by the IL-18 + 183 A/G polymorphism in coronary artery disease patients with diabetes type 2 and the metabolic syndrome: an observational study. Cardiovasc Diabetol. 2011;10:110.
- Aoyama M, Kotani J, Usami M. Gender difference in granulocyte dynamics and apoptosis and the role of IL-18 during endotoxin-induced systemic inflammation. Shock. 2009;32:401–409.